Cell-to-cell trafficking of RNAs plays an important role in coordinating gene expression at the whole-plant level as well as in virus/viroid infection and host defense response. This work identifies a three-dimensional RNA structure motif in a viroid that mediates trafficking between the leaf mesophyll tissues, providing mechanistic insights into trafficking regulation.
Abstract
Cell-to-cell trafficking of RNA is an emerging biological principle that integrates systemic gene regulation, viral infection, antiviral response, and cell-to-cell communication. A key mechanistic question is how an RNA is specifically selected for trafficking from one type of cell into another type. Here, we report the identification of an RNA motif in Potato spindle tuber viroid (PSTVd) required for trafficking from palisade mesophyll to spongy mesophyll in Nicotiana benthamiana leaves. This motif, called loop 6, has the sequence 5′-CGA-3′...5′-GAC-3′ flanked on both sides by cis Watson-Crick G/C and G/U wobble base pairs. We present a three-dimensional (3D) structural model of loop 6 that specifies all non-Watson-Crick base pair interactions, derived by isostericity-based sequence comparisons with 3D RNA motifs from the RNA x-ray crystal structure database. The model is supported by available chemical modification patterns, natural sequence conservation/variations in PSTVd isolates and related species, and functional characterization of all possible mutants for each of the loop 6 base pairs. Our findings and approaches have broad implications for studying the 3D RNA structural motifs mediating trafficking of diverse RNA species across specific cellular boundaries and for studying the structure-function relationships of RNA motifs in other biological processes.
INTRODUCTION
A central question in current biology concerns how basic processes in individual cells are integrated to support development and function of the whole multicellular organism. Cellular boundaries play a pivotal role in this integration by maintaining a certain level of cellular autonomy while enabling communication between cells to achieve coordinated gene expression and metabolism within an organism. Cell-to-cell trafficking of specific RNA and protein molecules is emerging as a new paradigm of gene regulation at the whole-organism level in plants, in addition to its fundamental role in the systemic spread of viral infection and defense responses (Giakountis and Coupland, 2008; Kehr and Buhtz, 2008; Lucas et al., 2009; Turgeon and Wolf, 2009; Chitwood and Timmermans, 2010; Chuck and O’Connor, 2010; Hannapel, 2010; Lehesranta et al., 2010). Such trafficking requires rethinking of RNAs and proteins as functioning solely within the cells in which they are produced.
Infectious agents, such as viroids and viruses, must move from cell to cell and throughout a plant to establish systemic infections. All viroids and many plant viruses have RNA genomes, so that their systemic infections represent classical examples of cell-to-cell and long-distance RNA trafficking (Flores et al., 2005; Scholthof, 2005; Lucas, 2006; Ding and Itaya, 2007; Ding, 2009). Many cellular RNAs also traffic between cells and between organs. Analyses of phloem sap collected from various plant species revealed many different mRNAs (Sasaki et al., 1998; Ruiz-Medrano et al., 1999; Doering-Saad et al., 2006; Omid et al., 2007; Deeken et al., 2008), small RNAs, including microRNAs (miRNAs) and short interfering RNAs (siRNAs) (Yoo et al., 2004; Buhtz et al., 2008, 2010; Varkonyi-Gasic et al., 2010), and some other types of RNAs (Zhang et al., 2009). Grafting experiments have shown that some mRNA species are transported over long distances in the phloem to regulate developmental processes, such as leaf morphogenesis in tomato (Solanum lycopersicum; Kim et al., 2001; Haywood et al., 2005) and tuber formation in potato (Solanum tuberosum; Banerjee et al., 2006). Trafficking of a miRNA from shoot to root has been implicated in the regulation of a gene involved in phosphate homeostasis in Arabidopsis thaliana (Lin et al., 2008; Pant et al., 2008). Intercellular trafficking of some miRNAs contributes to regulation of root vascular development (Carlsbecker et al., 2010). siRNAs can act as mobile signals that traffic from cell to cell and from organ to organ to mediate gene silencing including RNA-dependent DNA methylation (Chitwood et al., 2009; Schwab et al., 2009; Dunoyer et al., 2010a, 2010b; Molnar et al., 2010). Gene silencing signals containing siRNAs also traffic within a plant to mediate systemic RNA silencing as a means of antiviral defense (Ding and Voinnet, 2007; Kalantidis et al., 2008). RNA trafficking also occurs between some parasitic plants and their hosts, with biological functions yet to be understood (Roney et al., 2007; David-Schwartz et al., 2008).
Cell-to-cell RNA trafficking also occurs in animals. Numerous circulating nucleic acids, including RNAs, have been found in human plasma and serum under healthy and diseased conditions (Fleischhacker and Schmidt, 2007; Vlassov et al., 2007). Gene silencing signals in animals traffic intercellularly as a means of gene regulation and antiviral defense (Ding and Voinnet, 2007; Jose and Hunter, 2007). Exosomes, which are membrane vesicles released into extracellular spaces by many types of mammalian cells, enclose and mobilize mRNAs and miRNAs between different cells (Valadi et al., 2007; Kosaka et al., 2010). Viral miRNAs can also be transfered via exosomes between certain cells of the immune system and function therein (Pegtel et al., 2010). In addition to this secretory pathway, cell contact also enables intercellular transport of small RNAs between mammalian immune cells (Rechavi et al., 2009). These and other examples support the idea that interecellular RNA trafficking plays a role in signaling (Vlassov et al., 2007; Dinger et al., 2008; Simons and Raposo, 2009).
An outstanding question is how different RNAs are recognized for transport between distinct types of cells, which is essential for controlling the specificity of trafficking. We have been using Potato spindle tuber viroid (PSTVd) infection as a model system to test the hypothesis that unique RNA structural motifs mediate trafficking across different cellular boundaries. Viroids are single-stranded, circular, and noncoding RNAs that infect plants. They are the smallest plant pathogens known to date, with sizes ranging from 250 to 400 nucleotides (Flores et al., 2005; Ding and Itaya, 2007; Tsagris et al., 2008; Ding, 2009). Lacking protein-coding capacity and helper viruses, viroid RNAs most likely interact directly with preexisting cellular machineries to move from cell to cell and from organ to organ to establish systemic infections. Therefore, viroids provide simple models to investigate the RNA structural elements critical for cell-to-cell trafficking (Ding and Wang, 2009; Wang and Ding, 2010). The 359-nucleotide genome of PSTVd folds into a rod-like secondary structure that has been supported by biophysical, structural and mutational studies (Flores et al., 2005; Owens, 2007; Ding, 2009). As shown in Figure 1, this secondary structure comprises 27 loops/bulges (collectively refered to as loops hereafter, for simplicity of description), numbered 1 to 27, starting from the left in Figure 1, that are flanked by short double-stranded helices. With the PSTVd model, our previous studies obtained genetic evidence for the role of RNA loops in mediating RNA trafficking between specific cells (Qi et al., 2004; Zhong et al., 2007).
Figure 1.
Secondary Structure of PSTVd.
The loops/bulges are numbered 1 to 27 from left to right. The inset shows the loop 6 sequence flanked by Watson-Crick base pairs.
Computational analyses, such as minimum free energy calculations (e.g., mfold) (Zuker, 2003), generally show RNA secondary structures as comprising short double helices punctuated by loops. In the absence of additional information, such loops are usually depicted as single-stranded regions lacking structure. However, many x-ray crystallographic and NMR studies in the last decade have provided evidence that nucleotides within most loops form distinct three-dimensional (3D) motifs via non-Watson-Crick (non-WC) base pairing and base stacking and that such motifs comprise the primary binding sites for RNA–RNA, RNA–protein, and RNA–small ligand interactions (Leontis et al., 2002a, 2002b, 2006; Noller, 2005; Steitz, 2008). RNA nucleobases interact with each other by hydrogen bonding at any one of three edges, the Watson-Crick (WC), Hoogsteen (H), and Sugar (S) edges (see Supplemental Figure 1 online). Non-WC base pairs are categorized into 12 geometric base pairing families according to the interacting edges and relative orientation (cis or trans) of glycosidic bonds of the paired bases (see Supplemental Figure 1 online) (Leontis and Westhof, 2001).
Many RNA 3D motifs recur in nonhomologous RNA molecules or in distinct sites of the same RNA molecule (Simons and Grunberg-Manago, 1998; Leontis et al., 2002a). Recurrent 3D motifs comprise sets of nucleotides with similar spatial arrangements, including the same non-WC base pairs. The 3D structures of recurrent motifs are more conserved than their sequences; in other words, different RNA sequences can fold into the same 3D structure by forming the same geometric non-WC base pairs (Leontis and Westhof, 1998a, 1998b; Leontis et al., 2002b; Schudoma et al., 2010). The set of base substitutions compatible with the 3D structure of a motif is its sequence signature. Thus, certain non-WC base pairs can substitute for each other in a motif without distorting the 3D structure of the motif; such base pairs are isosteric or nearly so (Michel and Westhof, 1990; Leontis and Westhof, 1998b; Leontis et al., 2002b). More specifically, isosteric pairs form hydrogen bonds using the same base edges and have the same glycosidic bond orientation and identical or similar C1’-C1’ (i.e., first carbon of ribose) distances. Isostericity matrices summarize the isosteric relationships for each geometric base pairing family and provide the basis for analyzing the sequence signatures of RNA motifs (Leontis et al., 2002b). Furthermore, isostericity matrices allow predictions of base substitutions in an RNA motif that will either disrupt or maintain the isostericity of a base pair and, consequently, the structural integrity of the motif. We used these principles to successfully predict, and then experimentally confirm by mutational and functional analyses, the 3D structures of PSTVd loop E, which is critical for replication (Zhong et al., 2006), and loop 7, which mediates trafficking from bundle sheath to phloem (Zhong et al., 2007).
We used mutational analysis to identify at least 11 loops in PSTVd critical for systemic trafficking in Nicotiana benthamiana plants (Zhong et al., 2007, 2008). This study investigates loop 6, which comprises the six nucleotides G36, A37, C38, C323, G324, and A325 in strain PSTVdInt (accession number NC002030; Figure 1). When we mutated three nucleotides (G36U/A37C/C38G) to form WC base pairs and thereby closed loop 6, as predicted by mfold (Zuker, 2003), systemic trafficking of the mutant PSTVd, but not its replication, was abolished (Zhong et al., 2008). To gain mechanistic insights into the function of loop 6, we investigated its 3D structure (i.e., specific non-WC base pairing) as well as the cellular boundary at which it functions to mediate trafficking. Here, we provide evidence that loop 6 plays a decisive role in PSTVd trafficking from palisade mesophyll to spongy mesophyll cells of N. benthamiana leaves. We also present a 3D structural model of loop 6 that specifies all non-WC base pair interactions. Our findings and approaches have broad implications for studying the 3D RNA structural motifs mediating trafficking of diverse RNA species across cellular boundaries and for studying the structure-function relationships of RNA motifs in other biological processes.
RESULTS AND DISCUSSION
A 3D Structural Model for PSTVd Loop 6
To infer the 3D structure of PSTVd loop 6, we used the Find RNA 3D (FR3D) program suite (Sarver et al., 2008) to search a nonredundant list of atomic-resolution Protein Database (PDB) files, updated on May 5, 2010. The goal was to find internal loop sequence matches of the PSTVd loop 6 sequence 5′-CGA-3′...5′-GAC-3′ flanked on both sides by WC (including near-isosteric G/U wobble) base pairs as templates for modeling the structure of loop 6. Although we did not find an exact sequence match in the 3D database, we did find two internal loops that each differ from PSTVd loop 6 at single base positions. One of these loops occurs in helix 89 (H89) of bacterial and archaeal 23S rRNAs. The sequence and 3D structure of this motif appear to be broadly conserved in bacterial and archaeal 23S rRNAs. The sequence of this internal loop is 5′-CAA-3′..5′-GAC-3′ in the 23S rRNAs of both the archaeon Haloarcula marismortui and the distantly related bacterium Escherichia coli, having the same crystal structure (Table 1). The boldface A in this bacterial and archaeal loop sequence differs from G at the equivalent position of PSTVd loop 6. The corresponding H89 motif in the 23S rRNAs of bacterium Deinococcus radiodurans has the sequence 5′-CGA-3′...5′-GGC-3′, with G being different from the A at position 37 of the PSTVd loop 6 (Table 1). The structure of this H89 motif is nearly identical to the E. coli and H. marismortui versions. In the following paragraphs, a boldface base in a loop sequence indicates that it is different from the base at the equivalent position in PSTVd loop 6. The other internal loop we found in the database has the sequence 5′-CAA-3′...5′-GAC-3′ in helix 41 (h41) of E. coli 16S rRNA (Table 1). It has the same 3D structure as the H89 internal loop but is not conserved in other 16S rRNAs.
Table 1.
The 3D Motifs in Crystal Structures of rRNAs Used to Model 3D Structure of PSTVd Loop 6
| Organism | Molecule/Location | Sequence | Nucleotide Nos. | PDB ID |
| PSTVd | Loop 6 | 5′-CGA-3′..5′-GAC-3′ | 323:325/36:38 | NA |
| E. coli | 23S rRNA, helix 89 | 5′-CAA-3′..5′-GAC-3′ | 2467:2469/2481:2483 | 2QBE |
| H. marismortui | 23S rRNA, helix 89 | 5′-CAA-3′..5′-GAC-3′ | 2502:2504/2516:2518 | 1S72 |
| D. radiodurans | 23S rRNA, helix 89 | 5′-CGA-3′..5′-GGC-3′ | 2446:2448/2516:2518 | 2ZJR |
| E. coli | 16S rRNA, helix 41 | 5′-CAA-3′..5′-GAC-3′ | 1273:1275/1260:1262 | 2QAN |
| Azoarcus sp | Group I intron | 5′-CAA-3′..5′-AAA-3′ | 85:87/57:59 | 1U6B |
The boldface nucleotide differs from that in the PSTVd loop 6.
To further explore the sequence variability that can produce the same geometry, we used the H89 internal loop structure to carry out geometric searches using FR3D to find geometrically similar 3D motifs. In addition to recovering the original motifs, we identified an internal loop in the 3D structure of the Azoarcus group I intron that adopts a 3D structure similar to that of the 23S H89 motif, even though its sequence, 5′-CAA-3′...5′-AAA-3′, is considerably different (three base changes) (Table 1). The results of this search, including 3D structure superpositions and detailed structure annotations, can be accessed at the interactive website http://rna.bgsu.edu/WebFR3D/Results/pstvd. A view of the web link is shown in Supplemental Figure 2 online.
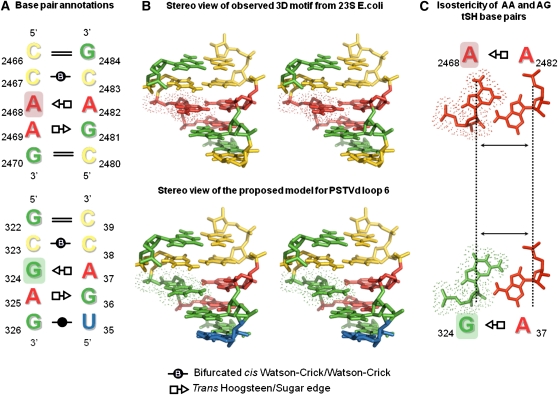
All of these motifs comprise a conserved set of three stacked non-WC base pairs flanked by WC base pairs, as shown in Figure 2. According to the Leontis-Westhof nomenclature of base pair classification (Leontis and Westhof, 2001), the first non-WC base pair is a cis Watson-Crick bifurcated pair, the second is a trans Sugar-Hoogsteen (tSH) pair, and the third is a trans Hoogsteen-Sugar (tHS) pair. Note that tSH GA and tHS AG indicate the same base pair geometry, with hydrogen bonding between the Hoogsteen edge of A and the Sugar edge of G.
Figure 2.
Proposed Non-WC Base Pairing for PSTVd Loop 6 Based on the Crystal Structure of a 23S rRNA Internal Loop as Template.
(A) and (B) Two-dimensional (2D) base pair annotations (A) and stereoview (B) of an observed RNA 3D motif from 23S E. coli rRNA (top) used as a template to manually build a structural model for PSTVd loop 6 (bottom). According to this loop 6 model, bases G322 and C39 make a cWC base pair, C323 and C38 make a bifurcated cWC base pair, bases G324 and A37 make a tSH pair, A325 and G36 make a tHS pair, and G326 and U35 make a cWC wobble pair. The only difference between the 23S rRNA loop and PSTVd loop 6 structures is the isosteric substitution of the AA tSH pair in the former loop by the GA tSH pair in the latter loop (the A and G bases are highlighted with the colored background in 2D annotations and with dots in the 3D structures). The 2D annotations are based on the Leontis-Westhof nomenclature (Leontis and Westhof, 2001). The 3D structure for this figure was taken from PDB 2QBE (23S rRNA of E. coli) (Borovinskaya et al., 2007).
(C) Isostericity of AA and GA tSH base pairs, with identical C1'-C1' distances in both pairs (dashed lines indicated by arrows) and the same orientation of the bases. The isodiscrepancy index calculated between exemplars for AA and AG is 1.56, typical for very similar base pairs (Stombaugh et al., 2009).
The sequence variability observed in the instances of this recurrent 3D motif recovered from the structure database is consistent with the base pair isostericity matrices defined for base pair families (Leontis et al., 2002b). GA, AA, and GG are mutually isosteric or near-isosteric tSH base pairs, and CC and AC are near-isosteric cWC bifurcated pairs. Most importantly, these same interactions can also be formed by the PSTVd sequence 5′-CGA-3′...5′-GAC-3′. Thus, we hypothesize that PSTVd loop 6 follows the same base pair pattern as seen in this 3D motif. Specifically, we predict that a GA tSH base pair forms between nucleotides 36 and 325, a tHS base pair between A37 and G324, and a bifurcated cWC pair between C38 and C323 (Figure 2). As can be seen in Figure 2, the only difference between the PSTVd sequence 5′-CGA-3′..5′-GAC-3′ and the 23S rRNA sequence 5′-CAA-3′..5′-GAC-3′ is the substitution of G for A in the second base pair (tSH). This implies the presence of a GA tSH pair in the viroid where an AA tSH pair is found in the H89 23S motif. As tSH AA and GA base pairs are isosteric (Stombaugh et al., 2009), which means that they occupy the same 3D space, this change does not significantly perturb the 3D structure of the motif. In fact, AA substitutions for GA tSH base pairs are very common in homologous RNA molecules (Stombaugh et al., 2009). Furthermore, the rRNA alignments show that GA is found at this position in some 23S rRNAs, as will be discussed below. Finally, we will show that a PSTVd loop 6 mutant with G-to-A substitution at this position, a sequence identical to the 23S rRNA motif, is indeed capable of systemic infection (see below). Figure 2C shows the structural similarity of the AA and GA tSH base pairs.
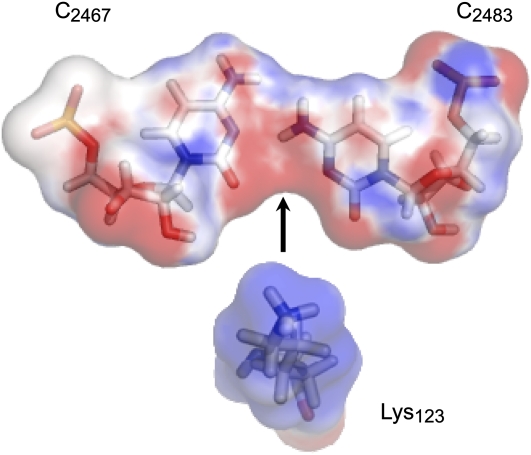
As mentioned above, the internal loop that we propose as a model for PSTVd loop 6 is highly conserved in H89 of archaeal and bacterial 23S rRNAs. The crystal structure shows that this motif provides a specific protein binding site for the conserved ribosomal protein L16 (called L10e in archaeal and eukaryal ribosomes). More specifically, Lys-123 from L16 protein in E. coli and the equivalent Lys-156 from L10e protein in H. marismortui interact with the conserved CC bifurcated pair, which provides an ideal docking site for the positively charged Lys amino group. Figure 3 shows the electrostatic complementarity between the terminal -NH3(+) group of Lys and the minor groove of the bifurcated CC pair. Examination of the L16/L10e alignment in the Pfam database (Finn et al., 2010) (Pfam id PF00252) showed this Lys to be almost universally conserved. Conservation of this docking interaction in bacterial and archaeal rRNA 3D structures as well as in sequence alignments indicates the biological significance of this docking interaction, which could also be relevant to other RNAs, including PSTVd.
Figure 3.
Docking Site for Lys-123 from L16 Ribosomal Protein Created by the Cytosine-Cytosine Bifurcated Pair.
The surface is colored by the electrostatic charge calculated using the PDB2PQR Web server (Dolinsky et al., 2004) and displayed with PyMOL (Schrodinger, 2010). Red color indicates negative charge, and blue color indicates positive charge. The 3D structure is based on 23S E. coli rRNA (PDB 2QBE).
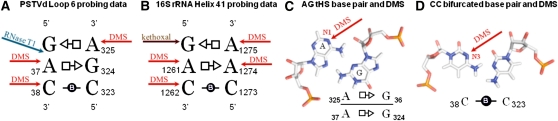
The Proposed PSTVd Loop 6 Structural Model Is Compatible with Chemical Probing Data
Our structural model for PSTVd loop 6 is also consistent with existing chemical probing data, which show that A37, A325, and C38 are highly modified by dimethylsulfide (DMS) (Gast et al., 1996). Figure 4A shows the proposed base-pairing model for loop 6 with the bases accessible to DMS marked by red arrows. DMS attacks the imino nitrogens of adenine (atom A-N1) and cytosine (atom C-N3) when the WC edges of these bases are exposed. If the WC edges were involved in base pairing, these imino nitrogens would not be reactive. In the proposed PSTVd loop 6 structural model, the As that form tHS base pairs using their Hoogsteen edges have exposed WC edges, as shown in Figure 4C, which are predicted to react with DMS, consistent with the chemical probing data. In fact, tHS pairs are among the most common non-WC base pairs, and As forming these base pairs are generally highly reactive to DMS probing unless such As use their WC edges to form another interaction, such as a base triple. Furthermore, the fact that C38 is DMS-reactive, whereas C323 is not, is also consistent with the proposed loop 6 model. As shown in Figure 4D, the bifurcated base pair between C38 and C323 is not symmetrical, as the WC edge of C323 forms bifurcated hydrogen bonds with the amino group of C38. This interaction protects the WC edge of C323 from DMS modification, while leaving the WC edge of C38 exposed and accessible to the probe.
Figure 4.
Chemical Probing Data in Support of the Proposed 3D Structure of PSTVd Loop 6.
(A) PSTVd loop 6 structure probing data (Gast et al., 1996) are consistent with the proposed model. Blue arrow denotes the RNase T1 cleavage site; red arrows show the DMS modification sites.
(B) Chemical probing data of a loop in the 16S rRNA helix 41 (Moazed et al., 1986) used as a model for the PSTVd loop 6.
(C) The N1 atoms of A325 and A37 of PSTVd loop 6 are accessible to DMS according to the proposed model because the Watson-Crick face of the adenines is not engaged in base pairing.
(D) The N3 atom of C38 in PSTVd loop 6 is also accessible for DMS in the proposed model.
Loop 6 is also susceptible to RNase T1 cleavage between G36 and A37 (Gast et al., 1996). RNase T1 cleaves on the 3′-side of guanines that are not engaged in WC base pairing. This is also consistent with the proposed base-pairing model of PSTVd loop 6, as G36 forms a non-WC tHS base pair with A325.
We also compared the chemical modification pattern of the PSTVd loop 6 with that observed in the h41 motif of 16S E. coli rRNA (Moazed et al., 1986) in the region corresponding to the proposed structural motif (nucleotides 1273:1275 and 1260:1262; Table 1). The pattern of reactivity in the h41 motif of 16S rRNA is essentially identical to that of PSTVd loop 6 (Figure 4B). All bases, except C1273, are accessible to chemical probes targeting atoms on the WC edges of the nucleotides. C1273 is the only base making an interaction that protects its N3 atom in this structure. These data support our PSTVd loop 6 model, especially for the bifurcated CC base pair. Unlike the h41 motif, PSTVd was not probed with kethoxal. Our model predicts that both G36 and G324 of PSTVd loop 6 should be accessible to kethoxal.
Pospiviroid and 23S rRNA Sequence Alignment Supports the PSTVd Loop 6 Structural Model
To further test the proposed PSTVd loop 6 structural model, we analyzed the sequence variability of this loop in all natural PSTVd variants as well as in different pospiviroid species. The question was whether the proposed model could accommodate the observed sequence variations. First, in the 138 unique sequences of PSTVd natural variants (among 156 isolates) registered in the Subviral RNA Database (Rocheleau and Pelchat, 2006) (http://subviral.med.uottawa.ca), PSTVd loop 6 sequence 5′-GAC-3′…5′-CGA-3′ is conserved in the equivalent genomic positions of all the variants. The alignment of PSTVd sequences was conducted by ClustalW program available at the Subviral RNA Database website. The result of this search is provided in Supplemental Figure 3 online and also can be accessed at the following website: http://subviral.med.uottawa.ca/cgi-bin/clustalw-results.cgi?jobID=clustalw-1283877994. Second, we downloaded sequences of eight species of the pospiviroid group from the NCBI Refseq collection (Pruitt et al., 2007) and aligned them using MAFFT (Katoh and Toh, 2008). Sequence variants identified in the pospiviroid alignment are summarized in Table 2. The details of sequence alignments are shown in Supplemental Figure 4 online. We excluded Pepper chat fruit viroid (Verhoeven et al., 2009) from the analysis because its sequence was poorly aligned with other pospiviroid sequences in the region of loop 6 due to low nucleotide sequence identity.
Table 2.
Sequence Variants of Loop 6 Based on Multiple Sequence Alignment of Eight Pospiviroid Species
| Sequence | Viroid |
| 5′-CGA-3′..5′-GAC-3′ | PSTVd |
| Tomato planta macho viroid | |
| Mexican papita viroid | |
| Iresine viroid 1 | |
| Columnea latent viroid | |
| Citrus exocortis viroid | |
| Tomato apical stunt viroid | |
| 5′-CCA-3′..5′-GAC-3′ | Chrysanthemum stunt viroid |
The boldface nucleotide differs from that in the PSTVd loop 6.
We analyzed the alignment using the Jalview program (Waterhouse et al., 2009). By looking at the columns corresponding to the location of PSTVd loop 6, we observed that the only difference in the pospiviroid sequences of loop 6 was a change from G to C in Chrysanthemum stunt viroid, at a position corresponding to G324 in PSTVd (Table 1). This substitution results in a change of an AG tHS pair to an isosteric AC tHS pair (Stombaugh et al., 2009), suggesting that the 3D structure of loop 6 may be conserved across all or most pospiviroid species. Intriguingly, the sequence of loop 6 equivalent from CSVd is the same as the sequence of an essential part of l-Trp binding motif (Majerfeld et al., 2010), again suggesting the possibility that PSTVd loop 6 and its equivalent in other pospiviroid species serve as protein binding sites.
We also examined rRNA alignments to gain additional insight into sequence variants capable of forming the proposed 3D motif. We used the Ribostral program (Mokdad and Leontis, 2006) to analyze nonredundant alignments of rRNA sequences (Stombaugh et al., 2009). We focused on the H89 motif from 23S rRNA because its structure is conserved in archaea and bacteria and because it binds a conserved protein. The h41 motif from 16S rRNA is not conserved, even within bacteria, and the sequence alignments show high variability, indicating variation in its 3D structure.
Table 3 summarizes the sequence variability observed for the H89 motif. Most species have the same sequence as that observed for E. coli, 5′-CAA-3′…5′-GAC-3′, which differs from PSTVd loop 6 at one position, as discussed above. However, five species have exactly the same sequence as PSTVd loop 6, providing additional support for the loop 6 structural model.
Table 3.
Comparison of Sequence Variability Observed for H89 Motif in 23S Bacterial rRNA Alignments with the Sequence of PSTVd Loop 6
| Sequence | Organism |
| Form same structure: | |
| 5′-CGA-3′..5′-GAC-3′ | PSTVd |
| 5′-CAA-3′..5′-GAC-3′ | 129 Species |
| 5′-CGA-3′..5′-GAC-3′ | Aquifex sp |
| Propionobacterium sp | |
| Wolbachia sp | |
| Thermotoga sp | |
| Chlorobium sp | |
| Form different structure: | |
| 5′-GCA-3′..5′-GGC-3′ | Pirellula sp |
| Thermus sp |
The boldface nucleotide differs from that in the PSTVd loop 6.
Mutational Analysis Provided Genetic Evidence in Support of the PSTVd Loop 6 Structural Model
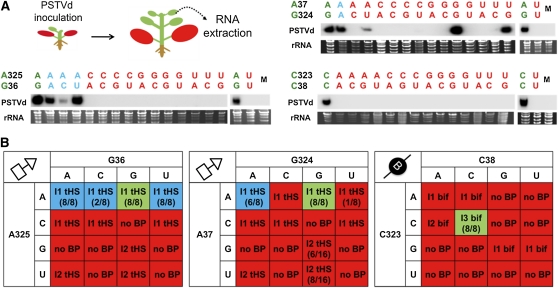
To experimentally test the 3D structural model of PSTVd loop 6, we generated all possible mutations for each base pair in the PSTVdInt (wild type) template and then assayed their systemic infection capabilities. The goal was to determine whether the functional consequence of a mutation could be accounted for by the structural model. For the systemic infection assay, in vitro transcripts were prepared for each mutant and used to inoculate the first two true leaves of N. benthamiana seedlings as described in Methods (Figure 5A). The systemic infection ability of each mutant was assayed by RNA gel blot analysis of RNA extracted from upper, noninoculated leaves (10th and 11th true leaves, collectively termed systemic leaves in this study) at 28 d after inoculation (DAI). The presence of (+)-circular PSTVd in the upper leaves, followed by sequencing to verify maintenance of the mutant sequence, demonstrates the ability of a mutant to mount a systemic infection. By contrast, absence of a mutant viroid in systemic leaves indicates a failed systemic infection. The experiments were repeated with at least eight biological replicates (i.e., individually inoculated plants) for each mutant. Inoculation with wild-type PSTVd and water served as positive and negative controls, respectively.
Figure 5.
Systemic Infection Analyses on PSTVd Loop 6 Mutants.
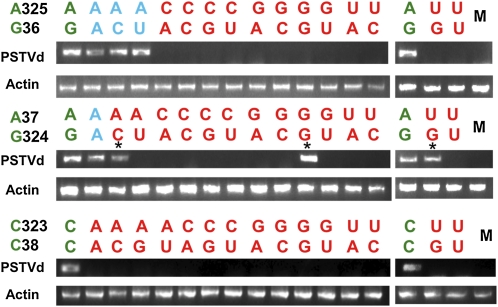
(A) The top left illustration shows the systemic infection assay system. The red-colored leaves were inoculated. Total RNA was collected from upper leaves and subjected to RNA gel blot analyses. Representative RNA gel blots are shown. Each lane indicates pooled sample from eight individual plants. Ethidium bromide staining of the corresponding samples is shown as a loading control. Representative RNA gel blots for individual plants are shown in Supplemental Figure 5 online.
(B) Summary of the systemic infection results of PSTVd loop 6 mutants presented in 4 × 4 matrices. Each matrix summarizes data for all mutants derived from one wild-type base pair of the proposed 3D model of loop 6. For each matrix, the wild-type sequence is indicated with a green background. All viable mutants capable of systemic infection are indicated with a blue background. Nonviable mutants (i.e., systemic infection defective) are indicated with a red background. The geometric type (tHS or bif) and isosteric group (I1 or I2) of a base pair formed by a sequence variant is indicated in each matrix. Base combinations that cannot form base pairs of the indicated geometric type are indicated with “no BP.” The number in parenthesis indicates the number of systemically infected plants out of the total number of inoculated plants.
A total of 45 mutants were analyzed. The systemic infection system and representative RNA gel blots are shown in Figure 5A. The results from multiple experiments are summarized in the three 4 × 4 matrices shown in Figure 5B, one for all mutants derived from each proposed base pair of the loop 6 3D model. The sequencing results of mutant progenies extracted from systemic leaves are presented in Supplemental Table 1 online. We categorized a mutant as viable only when the mutation(s) was maintained in systemic leaves. Additional representative RNA gel blots showing the systemic infection capacities of different mutants are presented in Supplemental Figure 5 online.
The first thing to note is that no viable variants (i.e., those capable of systemic infection) were observed for base combinations that cannot form a base pair of the indicated geometric type (no BP in the matrices in Figure 5). The second thing to note is that in each matrix, all viable sequence variants are able to form base pairs belonging to the same isosteric group as those predicted to occur in the wild-type PSTVd loop 6 sequence.
For the G36/A325 tSH base pair, only those mutants in which G36 was changed while retaining A at position 325 were capable of systemic infection. In all of these variants, the bases at positions 36 and 325 can form tSH base pairs, isosteric to the wild-type tSH GA base pair (Stombaugh et al., 2009). While tSH pairs in which C replaces A325 are also isosteric to GA, they are not viable. Evidently, A is required at position 325, perhaps for sequence-specific protein binding in the minor groove. Thus, besides the 3D structure of a motif, its primary nucleotide sequence can also be important for function.
Sequence variation is even more restricted for the A37/G324 tHS base pair, which only tolerates AA and AG. We observed reversion of three mutants (A37G, A37U, and G324U) to the wild type in infected plants in all the progeny clones that were sequenced (see Supplemental Table 1 online). This suggested that these mutants were incapable of systemic infection. In terms of the proposed model, we note that the tHS AG base pair is calculated to be the most energetically favorable pair of this type (Mládek et al., 2009). Interestingly, although the AA combination did not appear in the pospiviroid alignments, it was common among the bacterial 23S rRNA sequence alignments at the corresponding location. Here, it should be noted that G324U was reported as a viable sequence variant, and its reversion to the wild type also was observed in a previous study (Zhong et al., 2008).
The third pair, C38/C323, is required for PSTVd to traffic throughout the plant. All pospiviroid sequences analyzed also have two Cs at these positions, which, taken together with the fact that in the 23S rRNA the H89 motif interacts with a conserved protein, suggests that PSTVd loop 6 serves as a binding site for a protein responsible for trafficking.
All Systemic Infection-Defective Mutants of Loop 6 Fail to Exit the Inoculated Leaves
The experiments described above showed that most mutants of loop 6 were incapable of systemic infection. There could be two possible explanations: either the mutants failed to exit the inoculated leaves or they failed to traffic long distances after they left the inoculated leaves. To investigate this, we conducted RT-PCR (40 cycles) with RNA samples collected from the petioles of inoculated leaves for all mutants. The RNA samples were collected at 8 DAI, the same time point when RNA was collected from inoculated leaves for in planta replication assay as described below. Use of the same time point for these two types of assays ensured consistency of data interpretation. As shown in Figure 6, the progenies of these mutants were not detected in the petioles except for three mutants, G324C, A37G, and A37U. As described above, sequencing of the progenies of these three mutants from systemic leaves revealed reversion back to the wild-type PSTVd sequences. As expected, the four loop 6 mutants that showed systemic infection while maintaining their mutated sequences (blue color in Figures 5 and 6) were detected in the petioles.
Figure 6.
Detection of the Wild Type and Mutants of PSTVd Loop 6 in Petioles of Inoculated Leaves by RT-PCR.
The wild-type base pair is indicated by green color, mutant pairs capable of systemic infection by blue, and those incapable of systemic infection by red. M indicates mock inoculation. Asterisks indicate mutants that reverted to the wild type during infection. RT-PCR amplified actin RNA serves as loading control.
The Failure of Some Loop 6 Mutants to Establish Systemic Infection Is Not Due to Deficiency in Replication
Given that the systemic infection-defective loop 6 mutants did not exit the inoculated leaves, the next question was whether these mutants failed to replicate in the inoculated leaves. To address this question, in vitro transcripts of each loop 6 mutant were inoculated onto young N. benthamiana leaves as for the systemic infection assays described above. Wild-type PSTVd and water inoculations served as positive and negative controls, respectively. The inoculated leaves were harvested at 8 DAI and chemically fixed for whole-mount in situ hybridization using digoxigenin-labeled PSTVd-specific riboprobes. Here, whole-mount refers to the fact that pieces of a leaf, rather than thin sections, were used for in situ hybridization. We established that wild-type PSTVd could be detected in the petioles of inoculated leaves at 5 DAI; therefore, 8 DAI was a sufficiently long time interval to analyze the replication patterns. The assay was repeated with seven to eight biological replicates (i.e., individual plants inoculated) for each mutant.
Figure 7 shows typical images of PSTVd localization patterns from such analyses. The dark purple spots represent PSTVd hybridization signals in the nuclei. Because of overlap of cells and compression of the tissues incurred during the fixation/in situ hybridization processes, it was not feasible to identify clearly the types of cells containing the PSTVd signals. This issue will be addressed below by in situ hybridization on thin sections. However, our objective here was to detect signs of viroid replication/accumulation, regardless of cell type. Most mutants showed hybridization signals, suggesting that they were capable of replication. However, there were generally fewer cells exhibiting hybridization signals for the mutants than for the wild-type PSTVd. This could be due to low infection efficiency (i.e., low capacity to initiate replication in all inoculated cells), low levels of replication in some cells, or limited trafficking between cells.
Figure 7.
Representative Images of Whole-Mount in Situ Hybridization to Assay Replication of Loop 6 Mutants.
The purple dots, some indicated by arrows, represent viroid hybridization signals in the nuclei. Bars = 100 μm.
(A) Wild-type PSTVd.
(B) G36A (systemic infection-competent mutant).
(C) A325G (systemic infection-defective mutant).
(D) Mock inoculation.
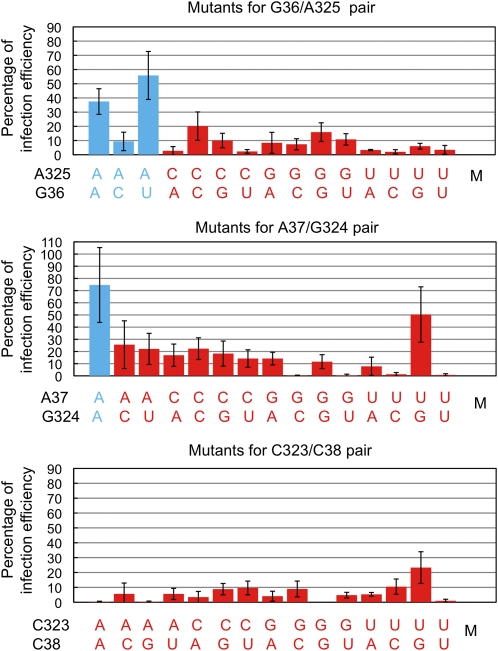
The next question was whether the failed systemic infection of a mutant was due to fewer cells supporting its replication/accumulation. To address this, we determined the percentages of cells showing visible accumulation of all mutants as well as the wild type. We counted the number of PSTVd-accumulating cells per 6.25 mm2 (2.5 × 2.5 mm) area of inoculated leaves. The number of cells accumulating a mutant was calculated as a percentage of that accumulating the wild type, to represent a measure of relative replication efficiency. The data are shown in Figure 8. A key observation is that mutant G36C, which is capable of systemic infection (Figure 5), was detected in <10% of the cells relative to the wild type. We therefore postulated that a 10% infection efficiency was sufficient to enable a mutant to achieve systemic trafficking. Then, for any mutants showing 10% or higher infection efficiency in the inoculated leaves, their absence from systemic leaves could be interpreted as chiefly the consequence of failed cell-to-cell trafficking. Fifteen mutants fit this criterion, including G36C/A325C, A325C, A325G, G36U/A325G, G324C, G324U, A37C/G324A, A37C/G324C, A37C, A37C/G324U, A37G/G324A, A37G, A37U, C323U, and C38G/C323U. For mutants that exhibited <10% of the wild-type infection efficiency, it is unclear whether their failure to develop systemic infection is due to impaired intracellular trafficking such as nuclear import/export, replication, in vivo stability, or cell-to-cell trafficking.
Figure 8.
Infection Efficiencies of PSTVd Loop 6 Mutants as Percentages of the Wild Type (Set to 100%) as Determined by Whole-Mount in Situ Hybridization.
Mutant pairs capable of systemic infection are indicated by blue and those incapable of systemic infection by red. M indicates mock inoculation. Each error bar indicates sd.
We recognize that using 10% of infection efficiency as a threshold level sufficient for systemic infection is arbitrary. However, it is a useful and practical approach in the context of this investigation. What is important is that a relatively low infection efficiency compared with the wild type, in terms of the percentage of cells infected, could still enable a viroid mutant to achieve successful systemic infection if trafficking is not impaired.
Loop 6 Is Required for Trafficking from Palisade to Spongy Mesophyll
As described above, the whole-mount in situ hybridization images do not allow assessment of infection efficiencies of loop 6 mutants in distinct cell types and therefore do not reveal the cellular boundary at which loop 6 functions to mediate trafficking. However, the data allowed us to choose particular mutants for further in situ hybridization on thin sections of inoculated leaves to identify this cellular boundary. Mutant A325G was first chosen because its 15% of the wild-type infection efficiency represents nearly the median level among all mutants. Furthermore, we did not observe any reversion of this mutant to the wild type in our extensive infection experiments, which ensures consistency of observations in multiple samples.
The A325G-inoculated N. benthamiana leaves were chemically fixed at 8 DAI to obtain paraffin sections for in situ hybridization. Figure 9A illustrates the leaf cell types in a transverse view and shows that viroid inocula were applied to the upper epidermis. Figure 9B shows typical in situ hybridization images of transverse sections of N. benthamiana leaves inoculated with wild-type PSTVd (positive control), mutant A325G, and water (mock negative control). The wild-type PSTVd hybridization signal was detected in the nuclei in upper epidermal, palisade mesophyll, and spongy mesophyll cells. It was also detected in the phloem cells (see Supplemental Figure 6 online). Mutant A325G was localized in the nuclei of upper epidermal and palisade mesophyll cells but very rarely in spongy mesophyll cells. Furthermore, the mutant was not observed in any bundle sheath or phloem cells (see Supplemental Figure 6 online). Hybridization signal was completely absent from any cells in mock-inoculated leaves (Figure 9B). These observations suggest preliminarily that loop 6 plays a critical role in PSTVd trafficking from the palisade mesophyll to spongy mesophyll.
Figure 9.
In Situ Hybridization Localization of the Wild Type and A325G Mutant PSTVd in Inoculated Leaves of N. benthamiana.
(A) Schematic representation of a transverse section of a young N. benthamiana leaf.
(B) In situ hybridization on 14-μm thin sections of N. benthamiana leaves inoculated by wild-type PSTVd, A325G mutant, and water as a mock. Purple dots indicate viroid hybridization signals in the nuclei. Bars = 100 μm.
(C) Quantitative analysis of the cellular distribution of mutant A325G (n = 17) as well as wild-type PSTVd (n = 14). The “n” number represents the number of individual leaves (and therefore individual plants) sampled. See Methods for details on sampling and analysis. The data in the two columns indicated by an asterisk are significantly different (t test, P < 0.05). The numbers in the two columns indicated by double asterisks also are significantly different (P < 0.05). The nonhighlighted data in the two columns are not significantly different (P > 0.05). Each error bar indicates sd.
To further test this possibility, we analyzed the number of epidermal, palisade and spongy mesophyll cells exhibiting mutant A325G signal compared with the wild-type control. We analyzed 88 sections from 17 leaves individually inoculated with mutant A325G and showing viroid hybridization signals, and 103 sections from 14 leaves individually inoculated with wild-type PSTVd and showing viroid hybridization signals. As demonstrated in Figure 9C, the numbers of viroid-accumulating epidermal cells per section (~1 × 1 mm for each section) are not significantly different between A325G- and wild type–inoculated leaves (t test, P > 0.05). However, the numbers of A325G-infected cells are significantly lower for palisade (P < 0.05) and spongy mesophyll (P < 0.05) compared with the wild-type infected cells. These quantitative data demonstrate, importantly, that mutant A325G has similar replication/accumulation capacity as the wild-type PSTVd, as shown in epidermal cells. The lower number of palisade mesophyll cells showing A325G accumulation may then be due to impaired trafficking from the epidermis into these cells. It is possible that comprised lateral trafficking between palisade mesophyll cells also contributed to the lower number of infected cells. Most significantly, however, the rare presence of mutant A325G in spongy mesophyll cells is best explained by the requirement of loop 6 for PSTVd trafficking from the palisade to spongy mesophyll.
In addition to A325G, we analyzed A37U, which had a relatively high infection efficiency (50% of the wild-type level) as shown by whole-mount in situ hybridization. In some leaves, A37U was localized only in the upper epidermal and palisade mesophyll cells, similar to mutant A325G (see Supplemental Figure 7 online). In other leaves, it was detected in a few spongy mesophyll as well as upper epidermal and palisade mesophyll cells (see Supplemental Figure 7 online). Because mutant A37U often reverted to the wild type (see Supplemental Table 1 online), we postulate that this instability may well account for the localization of A37U in the spongy mesophyll in some cases, although direct evidence was difficult to obtain from the fixed leaf samples. This reversion may also lead to an arbitrarily high infection efficiency of A37U among all mutants. Overall, exclusive localization of A37U in epidermal and palisade mesophyll cells in many cases is consistent with a decisive role of loop 6 in trafficking from palisade to spongy mesophyll as revealed by the A325G mutant.
Although our current data support a critical role of loop 6 in PSTVd trafficking from palisade to spongy mesophyll cells in young N. benthamiana leaves, we do not know whether this motif is also required for trafficking in the reverse direction (i.e., from spongy to palisade) or whether leaf development impacts loop 6 function at a specific cellular boundary. This is an important question to be addressed in future studies, given our previous finding that a bipartitie PSTVd motif, whose 3D structure remains to be determined, is required for unidirectional trafficking from mesophyll to bundle sheath in a leaf development-dependent manner in tobacco (Nicotiana tabacum) (Qi et al., 2004).
These thin-section in situ hybridization data may provide an explanation for the low infection efficiencies for many mutants as shown from whole-mount in situ hybridization. Specifically, some mutants, like A325G, may have similar infection efficiency as the wild type in the epidermis. Their impaired trafficking into mesophyll cells, especially the spongy mesophyll, would then lead to the overall lower infection efficiency when infected cells are counted from whole-mount in situ hybridization samples. In support of this interpretation, our counting of epidermal cells infected by A37U, on in situ–hybridized thin sections of leaf samples in which this mutant was absent from the spongy mesophyll, led to a number not significantly different from those for A325G- and wild type–inoculated leaves (see Supplemental Figure 8 online). For other mutants, other possibilities may also be considered. First, their low infection efficiency may suggest an additional role of loop 6 in replication. However, it is possible that any mutations in a loop, while disrupting the major biological function of the loop, leads to disturbance to some degree the overall viroid structure so as to indirectly impact other biological functions of the RNA as a whole. This would not be surprising if we consider any one motif to function as an integral part of the whole RNA, rather than an isolated entity. Second, for those mutations that caused reduction in infection efficiency to barely detectable levels (e.g., A37G/G324C, A37G/G324U, A37U/G324C, A37U/G324U, C323A/C38A, C323A/C38G, C323G/C38G, and C323U/C38U), we cannot exclude the possibility that some of these mutations produce a dominant-negative effect by interacting with some cellular factors to interfere with intracellular trafficking or replication. Finally, whether any mutations cause or enhance RNA instability in vivo is an open question. These issues may provide opportunities for new learning about RNA structures in relation to function and for developing more comprehensive research tools to advance studies on RNA structure-function relationships.
In summary, our work provides evidence that the PSTVd loop 6 plays a decisive role in mediating RNA trafficking from palisade mesophyll to spongy mesophyll in N. benthamiana leaves. Our structural and bioinformatic analyses, together with the experimental data including structure probing data of PSTVd and the functional data on the loop 6 mutants, support the proposed 3D structural model of PSTVd loop 6. While it is formally possible that other 3D models could also be consistent with these data, at present we are not aware of any such models. Whether the primary sequence and proposed 3D structure of this loop also function similarly in other host plant species remains to be investigated. Together with previous identification of PSTVd U43/C318 (loop 7) 3D motif mediating trafficking from the bundle-sheath to phloem in N. benthamiana (Zhong et al., 2007), our findings provide compelling evidence to support the hypothesis that distinct 3D RNA motifs mediate trafficking across specific cellular boundaries. Further studies will determine whether one or more motifs are involved in trafficking across a particular boundary. This hypothesis and our experimental approaches may be useful for identifying the 3D motifs in other RNAs that mediate trafficking between specific cells. A cis-element in the 5′-untranslated region (UTR) of potexviral RNA, which plays a role in replication (Miller et al., 1998), was found to mediate cell-to-cell transport of a fused green fluorescent protein reporter RNA (Lough et al., 2006). A green fluorescent protein reporter system also was used to show the existence of RNA trafficking signals in nucleotides 1 to 102 of the Arabidopsis FLOWERING LOCUS T mRNA (Li et al., 2009) and in the 3′-UTR of GIBBERELLIC ACID-INSENSITIVE mRNA (Huang and Yu, 2009). The UTRs of potato BEL5 mRNA are important for long-distance movement (Banerjee et al., 2006, 2009). Systemic spread of Brome mosaic virus RNAs in the absence of replication suggests that they have sequence or structural elements recognized by cellular factors (Gopinath and Kao, 2007). The identification of 3D structural motifs in these RNAs mediating trafficking between specific cells should significantly advance our mechanistic knowledge of RNA trafficking.
How might PSTVd loop 6 or other motifs mediate trafficking between specific cells? A simple working model predicts that these motifs are recognized by distinct cellular factors that are components of the cell-specific RNA trafficking machinery. There is evidence for cellular proteins involved in trafficking cellular RNAs. Xoconostle-Cázares et al. (1999) isolated CmPP16-1 from pumpkin (Cucurbita maxima) phloem exudates that binds and traffics endogenous RNAs. Yoo et al. (2004) identified pumpkin Phloem SMALL RNA BINDING PROTEIN1, which binds synthetic single-stranded siRNAs and potentiates their trafficking in N. benthamiana mesophyll as shown by microinjection. Recent studies identified a mobile 50-kD RNA binding protein (RBP50) from the pumpkin phloem exudates that binds polypyrimidine tract in mRNAs, providing a platform for additional protein interactions to form a ribonucleoprotein complex in the phloem translocation stream (Ham et al., 2009). While the putative cellular factors recognizing any particular PSTVd motifs are yet to be identified, the observations that rRNA 3D motifs similar or identical to PSTVd loop 6 and loop 7 represent protein binding sites support the proposition that these motifs are recognized by palisade- and bundle sheath-specific factors, respectively, to initiate trafficking.
METHODS
cDNA Construction of PSTVd Loop 6 Mutants
Plasmid pRZ6-2 containing the cDNA of PSTVdInt (pRZ6-2-Int) was constructed by Hu et al. (1997) and was a gift from Robert Owens. All PSTVd mutants described in this article were generated by site-directed mutagenesis based on this template, as previously described (Zhong et al., 2008). All introduced mutations were verified by DNA sequencing with a 3730 DNA Analyzer (Applied Biosystems) at the Plant-Microbe Genomics Facility of Ohio State University. Plasmid pInter (−) was constructed as previously described (Qi and Ding, 2002).
Plant Growth and Infection
Nicotiana benthamiana plants were grown in a growth chamber maintained at 16-h-light/8-h-dark (28°C) cycles. The in vitro transcripts of PSTVd mutants were mechanically inoculated onto the first two true leaves of 2-week-old N. benthamiana plants (200 ng/leaf) dusted with carborundum powder. Diethylpyrocarbonate-treated water was used for mock inoculation. All inocula were applied exclusively to the upper surfaces of leaves.
In Vitro Transcription
To prepare in vitro transcripts of wild-type and mutant PSTVd for systemic infection and replication experiments, HindIII-linearized pRZ6-2-Int and all mutant derivatives were transcribed using the T7 Megascript kit according to the instructions provided by the manufacturer (Ambion). Antisense PSTVd riboprobes for in situ hybridization and RNA gel blots were prepared by in vitro transcription of SpeI-linearized pInter (−) plasmid using T7 Maxiscript kit (Ambion). [α-32P]UTP-labeled riboprobes were used for RNA gel blots, and digoxigenin (DIG)-UTP labeled riboprobes were used for in situ hybridization. All RNA transcripts were purified by the MEGAClear kit (Ambion) after DNase I digestion.
RNA Extraction and RNA Gel Blotting
Total RNA from leaves of PSTVd- or mock-inoculated N. benthamiana plants was extracted by Ribozol reagent (Amresco) by following the manufacturer’s instructions. RNA gel blotting was performed essentially as described in Zhong et al. (2006).
RT-PCR
Total RNA from petioles of PSTVd- or mock-inoculated N. benthamiana leaves was extracted using Ribozol reagent. The extracted RNA was purified by RNeasy mini (Qiagen) followed by RNA cleanup using the manufacturer’s protocol. A total of 500 ng of pooled RNA was reverse transcribed using Superscript III (Invitrogen). The synthesized cDNAs were amplified with Taq polymerase using PSTVd-specific forward primer (5′-CGGAACTAAACTCGTGGTTCCT-3′) and reverse primer (5′-AGGAACCAACTGCGGTTCCA-3). As an internal control, actin mRNA was RT-PCR amplified using actin-specific forward primer (5′-CACCATGGCAGATGGAGAGGATATTCAGCC-3′) and reverse primer (5′-AGGAACCAACTGCGGTTCCA-3′). The PCR products were run on 1.2% agarose gel and visualized with ethidium bromide staining.
Sequencing of PSTVd Progenies
The PSTVd progenies were sequenced in a manner similar to that described by Qi and Ding (2002). Briefly, after the PSTVd signal detection either by RNA gel blotting or RT-PCR, the PSTVd RNA was RT-PCR amplified as described above except using Pfu Turbo DNA polymerase (Agilent Technologies) instead of Taq polymerase. Next, the Pfu-amplified product was incubated with Taq for 20 min at 72°C and ligated into pGEM-T vector (Promega). The inserted DNA was sequenced as described above.
Tissue Processing and in Situ Hybridization
The tissue fixation and processing followed the protocols previously described (Zhu et al., 2001; Qi et al., 2004) with slight modifications. Briefly, 8 DAI leaf samples were fixed in a solution of 50% ethanol/5% formaldehyde/5% acetic acid for 30 min and then dehydrated by a step-wise gradient of ethanol solutions. The samples for whole-mount in situ hybridization were washed by PBS and treated with 10 μg/mL of proteinase K for 20 min at 37°C. Then, the samples were hybridized with DIG-labeled PSTVd antisense riboprobes at 55°C overnight. The samples were washed and incubated with alkaline phosphatase–conjugated DIG antibodies (Roche) followed by visualization with NBT/BCIP (Roche) color reaction.
To obtain thin sections, fixed and dehydrated samples were infiltrated with paraffin and embedded in paraffin blocks. Sections of 14 μm in thickness were obtained with a HM 340 E rotary microtome (Microm International). PSTVd accumulation was detected by in situ hybridization followed by the color reaction as describe above.
In situ hybridized samples were examined and photographed under a Nikon Eclipse E600 light microscope. Images were captured and processed with a SPOT RT KE Slider camera and the associated software (SPOT Advanced; Diagnostic Instruments). The size bar in each picture was stamped on each image after magnification calibration using SPOT Advanced and cut and pasted to the intended location of the same picture, if needed, by Adobe Photoshop.
Quantitative Analysis of PSTVd-Accumulating Cells
PSTVd-accumulating cells were counted by visual inspection under the light microscope. For whole-mount in situ hybridization samples, the counting was conducted in a 2.5 × 2.5-mm area per leaf sample. For thin-section in situ hybridization samples, the number of PSTVd-accumulating cells was determined separately for different cell types: epidermis, palisade mesophyll, and spongy mesophyll. The number was counted per section, which was ~1 × 1 mm. For the wild type, 103 sections from 14 leaves (each from an individual plant) were used. For mutant A325G, 88 sections from 17 leaves were used. For each leaf, at least four different sections were analyzed, and the average of numbers from these sections was used to represent the number from each leaf for statistical analysis, so that comparisons were made among biological replicates, rather than among individual sections. The data were analyzed by t test using Microsoft Excel (two-tailed distribution, two-sample unequal variance). P value < 0.05 is treated as a significantly different value and is indicated in the text.
Accession Number
The PSTVdInt sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under accession number NC002030.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. RNA Nucleobase Interactions.
Supplemental Figure 2. RNA 3D Motifs Used for Modeling of PSTVd Loop 6.
Supplemental Figure 3. The Alignment of PSTVd Natural Variants Showing Conservation of Loop 6 Sequence.
Supplemental Figure 4. Pospiviroid Sequence Alignments Provide Support to the PSTVd Loop 6 Structural Model.
Supplemental Figure 5. Representative RNA Gel Blots Showing Systemic Infection of PSTVd Loop 6 Mutants.
Supplemental Figure 6. In Situ Hybridization on 14-μm Paraffin Sections of N. benthamiana Leaves Inoculated with PSTVd or Water (Mock Control).
Supplemental Figure 7. In Situ Hybridization 14-μm Paraffin Sections of N. benthamiana Leaves Inoculated with A37U Mutant.
Supplemental Figure 8. Comparison of Infection Efficiencies of the Wild Type, A325G, and A37U in Leaf Epidermis of Inoculated N. benthamiana.
Supplemental Table 1. Sequencing of PSTVd Loop 6 Mutant Progenies in Systemically Infected Plants.
Supplementary Material
Acknowledgments
We thank Jesse Stombaugh and Xuehua Zhong for their initial insights into the analysis of loop 6 structure and function, Mark Forster for helpful discussions, and Woong June Park, Ying Wang, Xiaorui Yang, Yuan Tian, Carter Mason, and Farah Khan for critical reading of this manuscript. This work was supported by grants from the U.S. National Science Foundation (IOB-0840906 to B.D. and MCB-0443508 to N.B.L.) and from the National Institutes of Health (2 R15 GM055898-05 to N.B.L.).
References
- Banerjee A.K., Chatterjee M., Yu Y., Suh S.G., Miller W.A., Hannapel D.J. (2006). Dynamics of a mobile RNA of potato involved in a long-distance signaling pathway. Plant Cell 18: 3443–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A.K., Lin T., Hannapel D.J. (2009). Untranslated regions of a mobile transcript mediate RNA metabolism. Plant Physiol. 151: 1831–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovinskaya M.A., Pai R.D., Zhang W., Schuwirth B.S., Holton J.M., Hirokawa G., Kaji H., Kaji A., Cate J.H. (2007). Structural basis for aminoglycoside inhibition of bacterial ribosome recycling. Nat. Struct. Mol. Biol. 14: 727–732 [DOI] [PubMed] [Google Scholar]
- Buhtz A., Pieritz J., Springer F., Kehr J. (2010). Phloem small RNAs, nutrient stress responses, and systemic mobility. BMC Plant Biol. 10: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhtz A., Springer F., Chappell L., Baulcombe D.C., Kehr J. (2008). Identification and characterization of small RNAs from the phloem of Brassica napus. Plant J. 53: 739–749 [DOI] [PubMed] [Google Scholar]
- Carlsbecker A., et al. (2010). Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 465: 316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood D.H., Nogueira F.T., Howell M.D., Montgomery T.A., Carrington J.C., Timmermans M.C. (2009). Pattern formation via small RNA mobility. Genes Dev. 23: 549–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood D.H., Timmermans M.C. (2010). Small RNAs are on the move. Nature 467: 415–419 [DOI] [PubMed] [Google Scholar]
- Chuck G., O’Connor D. (2010). Small RNAs going the distance during plant development. Curr. Opin. Plant Biol. 13: 40–45 [DOI] [PubMed] [Google Scholar]
- David-Schwartz R., Runo S., Townsley B., Machuka J., Sinha N. (2008). Long-distance transport of mRNA via parenchyma cells and phloem across the host-parasite junction in Cuscuta. New Phytol. 179: 1133–1141 [DOI] [PubMed] [Google Scholar]
- Deeken R., Ache P., Kajahn I., Klinkenberg J., Bringmann G., Hedrich R. (2008). Identification of Arabidopsis thaliana phloem RNAs provides a search criterion for phloem-based transcripts hidden in complex datasets of microarray experiments. Plant J. 55: 746–759 [DOI] [PubMed] [Google Scholar]
- Ding B. (2009). The biology of viroid-host interactions. Annu. Rev. Phytopathol. 47: 105–131 [DOI] [PubMed] [Google Scholar]
- Ding B., Itaya A. (2007). Viroid: a useful model for studying the basic principles of infection and RNA biology. Mol. Plant Microbe Interact. 20: 7–20 [DOI] [PubMed] [Google Scholar]
- Ding B., Wang Y. (2009). Viroids: uniquely simple and tractable models to elucidate regulation of cell-to-cell trafficking of RNA. DNA Cell Biol. 28: 51–56 [DOI] [PubMed] [Google Scholar]
- Ding S.W., Voinnet O. (2007). Antiviral immunity directed by small RNAs. Cell 130: 413–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinger M.E., Mercer T.R., Mattick J.S. (2008). RNAs as extracellular signaling molecules. J. Mol. Endocrinol. 40: 151–159 [DOI] [PubMed] [Google Scholar]
- Doering-Saad C., Newbury H.J., Couldridge C.E., Bale J.S., Pritchard J. (2006). A phloem-enriched cDNA library from Ricinus: Insights into phloem function. J. Exp. Bot. 57: 3183–3193 [DOI] [PubMed] [Google Scholar]
- Dolinsky T.J., Nielsen J.E., McCammon J.A., Baker N.A. (2004). PDB2PQR: An automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 32: W665–W667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunoyer P., Brosnan C.A., Schott G., Wang Y., Jay F., Alioua A., Himber C., Voinnet O. (2010b). An endogenous, systemic RNAi pathway in plants. EMBO J. 29: 1699–1712 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dunoyer P., Schott G., Himber C., Meyer D., Takeda A., Carrington J.C., Voinnet O. (2010a). Small RNA duplexes function as mobile silencing signals between plant cells. Science 328: 912–916 [DOI] [PubMed] [Google Scholar]
- Finn R.D., et al. (2010). The Pfam protein families database. Nucleic Acids Res. 38(Database issue): D211–D222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischhacker M., Schmidt B. (2007). Circulating nucleic acids (CNAs) and cancer—A survey. Biochim. Biophys. Acta 1775: 181–232 [DOI] [PubMed] [Google Scholar]
- Flores R., Hernández C., Martínez de Alba A.E., Daròs J.A., Di Serio F. (2005). Viroids and viroid-host interactions. Annu. Rev. Phytopathol. 43: 117–139 [DOI] [PubMed] [Google Scholar]
- Gast F.U., Kempe D., Spieker R.L., Sänger H.L. (1996). Secondary structure probing of potato spindle tuber viroid (PSTVd) and sequence comparison with other small pathogenic RNA replicons provides evidence for central non-canonical base-pairs, large A-rich loops, and a terminal branch. J. Mol. Biol. 262: 652–670 [DOI] [PubMed] [Google Scholar]
- Giakountis A., Coupland G. (2008). Phloem transport of flowering signals. Curr. Opin. Plant Biol. 11: 687–694 [DOI] [PubMed] [Google Scholar]
- Gopinath K., Kao C.C. (2007). Replication-independent long-distance trafficking by viral RNAs in Nicotiana benthamiana. Plant Cell 19: 1179–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham B.K., Brandom J.L., Xoconostle-Cázares B., Ringgold V., Lough T.J., Lucas W.J. (2009). A polypyrimidine tract binding protein, pumpkin RBP50, forms the basis of a phloem-mobile ribonucleoprotein complex. Plant Cell 21: 197–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannapel D.J. (2010). A model system of development regulated by the long-distance transport of mRNA. J. Integr. Plant Biol. 52: 40–52 [DOI] [PubMed] [Google Scholar]
- Haywood V., Yu T.S., Huang N.C., Lucas W.J. (2005). Phloem long-distance trafficking of GIBBERELLIC ACID-INSENSITIVE RNA regulates leaf development. Plant J. 42: 49–68 [DOI] [PubMed] [Google Scholar]
- Hu Y., Feldstein P.A., Hammond J., Hammond R.W., Bottino P.J., Owens R.A. (1997). Destabilization of potato spindle tuber viroid by mutations in the left terminal loop. J. Gen. Virol. 78: 1199–1206 [DOI] [PubMed] [Google Scholar]
- Huang N.C., Yu T.S. (2009). The sequences of Arabidopsis GA-INSENSITIVE RNA constitute the motifs that are necessary and sufficient for RNA long-distance trafficking. Plant J. 59: 921–929 [DOI] [PubMed] [Google Scholar]
- Jose A.M., Hunter C.P. (2007). Transport of sequence-specific RNA interference information between cells. Annu. Rev. Genet. 41: 305–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantidis K., Schumacher H.T., Alexiadis T., Helm J.M. (2008). RNA silencing movement in plants. Biol. Cell 100: 13–26 [DOI] [PubMed] [Google Scholar]
- Katoh K., Toh H. (2008). Improved accuracy of multiple ncRNA alignment by incorporating structural information into a MAFFT-based framework. BMC Bioinformatics 9: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J., Buhtz A. (2008). Long distance transport and movement of RNA through the phloem. J. Exp. Bot. 59: 85–92 [DOI] [PubMed] [Google Scholar]
- Kim M., Canio W., Kessler S., Sinha N. (2001). Developmental changes due to long-distance movement of a homeobox fusion transcript in tomato. Science 293: 287–289 [DOI] [PubMed] [Google Scholar]
- Kosaka N., Iguchi H., Yoshioka Y., Takeshita F., Matsuki Y., Ochiya T. (2010). Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 285: 17442–17452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehesranta S.J., Lichtenberger R., Helariutta Y. (2010). Cell-to-cell communication in vascular morphogenesis. Curr. Opin. Plant Biol. 13: 59–65 [DOI] [PubMed] [Google Scholar]
- Leontis N.B., Lescoute A., Westhof E. (2006). The building blocks and motifs of RNA architecture. Curr. Opin. Struct. Biol. 16: 279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontis N.B., Stombaugh J., Westhof E. (2002a). Motif prediction in ribosomal RNAs Lessons and prospects for automated motif prediction in homologous RNA molecules. Biochimie 84: 961–973 [DOI] [PubMed] [Google Scholar]
- Leontis N.B., Westhof E. (1998a). A common motif organizes the structure of multi-helix loops in 16 S and 23 S ribosomal RNAs. J. Mol. Biol. 283: 571–583 [DOI] [PubMed] [Google Scholar]
- Leontis N.B., Westhof E. (1998b). Conserved geometrical base-pairing patterns in RNA. Q. Rev. Biophys. 31: 399–455 [DOI] [PubMed] [Google Scholar]
- Leontis N.B., Westhof E. (2001). Geometric nomenclature and classification of RNA base pairs. RNA 7: 499–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontis N.B., Stombaugh J., Westhof E. (2002b). The non-Watson-Crick base pairs and their associated isostericity matrices. Nucleic Acids Res. 30: 3497–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Zhang K., Zeng X., Jackson S., Zhou Y., Hong Y. (2009). A cis element within flowering locus T mRNA determines its mobility and facilitates trafficking of heterologous viral RNA. J. Virol. 83: 3540–3548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.I., Chiang S.F., Lin W.Y., Chen J.W., Tseng C.Y., Wu P.C., Chiou T.J. (2008). Regulatory network of microRNA399 and PHO2 by systemic signaling. Plant Physiol. 147: 732–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough T.J., Lee R.H., Emerson S.J., Forster R.L., Lucas W.J. (2006). Functional analysis of the 5′ untranslated region of potexvirus RNA reveals a role in viral replication and cell-to-cell movement. Virology 351: 455–465 [DOI] [PubMed] [Google Scholar]
- Lucas W.J. (2006). Plant viral movement proteins: Agents for cell-to-cell trafficking of viral genomes. Virology 344: 169–184 [DOI] [PubMed] [Google Scholar]
- Lucas W.J., Ham B.K., Kim J.Y. (2009). Plasmodesmata - Bridging the gap between neighboring plant cells. Trends Cell Biol. 19: 495–503 [DOI] [PubMed] [Google Scholar]
- Majerfeld I., Chocholousova J., Malaiya V., Widmann J., McDonald D., Reeder J., Iyer M., Illangasekare M., Yarus M., Knight R. (2010). Nucleotides that are essential but not conserved; a sufficient L-tryptophan site in RNA. RNA 16: 1915–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Westhof E. (1990). Modelling of the three-dimensional architecture of group I catalytic introns based on comparative sequence analysis. J. Mol. Biol. 216: 585–610 [DOI] [PubMed] [Google Scholar]
- Miller E.D., Plante C.A., Kim K.H., Brown J.W., Hemenway C. (1998). Stem-loop structure in the 5′ region of potato virus X genome required for plus-strand RNA accumulation. J. Mol. Biol. 284: 591–608 [DOI] [PubMed] [Google Scholar]
- Mládek A., Sharma P., Mitra A., Bhattacharyya D., Sponer J., Sponer J.E. (2009). Trans Hoogsteen/sugar edge base pairing in RNA. Structures, energies, and stabilities from quantum chemical calculations. J. Phys. Chem. B 113: 1743–1755 [DOI] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H.F. (1986). Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J. Mol. Biol. 187: 399–416 [DOI] [PubMed] [Google Scholar]
- Mokdad A., Leontis N.B. (2006). Ribostral: An RNA 3D alignment analyzer and viewer based on basepair isostericities. Bioinformatics 22: 2168–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A., Melnyk C.W., Bassett A., Hardcastle T.J., Dunn R., Baulcombe D.C. (2010). Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science 328: 872–875 [DOI] [PubMed] [Google Scholar]
- Noller H.F. (2005). RNA structure: Reading the ribosome. Science 309: 1508–1514 [DOI] [PubMed] [Google Scholar]
- Omid A., Keilin T., Glass A., Leshkowitz D., Wolf S. (2007). Characterization of phloem-sap transcription profile in melon plants. J. Exp. Bot. 58: 3645–3656 [DOI] [PubMed] [Google Scholar]
- Owens R.A. (2007). Potato spindle tuber viroid: The simplicity paradox resolved? Mol. Plant Pathol. 8: 549–560 [DOI] [PubMed] [Google Scholar]
- Pant B.D., Buhtz A., Kehr J., Scheible W.R. (2008). MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J. 53: 731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel D.M., Cosmopoulos K., Thorley-Lawson D.A., van Eijndhoven M.A., Hopmans E.S., Lindenberg J.L., de Gruijl T.D., Würdinger T., Middeldorp J.M. (2010). Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. USA 107: 6328–6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt K.D., Tatusova T., Maglott D.R. (2007). NCBI reference sequences (RefSeq): A curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 35: D61–D65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y., Ding B. (2002). Replication of Potato spindle tuber viroid in cultured cells of tobacco and Nicotiana benthamiana: The role of specific nucleotides in determining replication levels for host adaptation. Virology 302: 445–456 [DOI] [PubMed] [Google Scholar]
- Qi Y., Pélissier T., Itaya A., Hunt E., Wassenegger M., Ding B. (2004). Direct role of a viroid RNA motif in mediating directional RNA trafficking across a specific cellular boundary. Plant Cell 16: 1741–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechavi O., Erlich Y., Amram H., Flomenblit L., Karginov F.V., Goldstein I., Hannon G.J., Kloog Y. (2009). Cell contact-dependent acquisition of cellular and viral nonautonomously encoded small RNAs. Genes Dev. 23: 1971–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheleau L., Pelchat M. (2006). The Subviral RNA Database: A toolbox for viroids, the hepatitis delta virus and satellite RNAs research. BMC Microbiol. 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roney J.K., Khatibi P.A., Westwood J.H. (2007). Cross-species translocation of mRNA from host plants into the parasitic plant dodder. Plant Physiol. 143: 1037–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Medrano R., Xoconostle-Cázares B., Lucas W.J. (1999). Phloem long-distance transport of CmNACP mRNA: Implications for supracellular regulation in plants. Development 126: 4405–4419 [DOI] [PubMed] [Google Scholar]
- Sarver M., Zirbel C.L., Stombaugh J., Mokdad A., Leontis N.B. (2008). FR3D: Finding local and composite recurrent structural motifs in RNA 3D structures. J. Math. Biol. 56: 215–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Chino M., Hayashi H., Fujiwara T. (1998). Detection of several mRNA species in rice phloem sap. Plant Cell Physiol. 39: 895–897 [DOI] [PubMed] [Google Scholar]
- Scholthof H.B. (2005). Plant virus transport: motions of functional equivalence. Trends Plant Sci. 10: 376–382 [DOI] [PubMed] [Google Scholar]
- Schrodinger L. (September 21, 2010) The PyMOL Molecular Graphics System. Version 1.3r1. http://www.pymol.org/
- Schudoma C., May P., Nikiforova V., Walther D. (2010). Sequence-structure relationships in RNA loops: establishing the basis for loop homology modeling. Nucleic Acids Res. 38: 970–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab R., Maizel A., Ruiz-Ferrer V., Garcia D., Bayer M., Crespi M., Voinnet O., Martienssen R.A. (2009). Endogenous TasiRNAs mediate non-cell autonomous effects on gene regulation in Arabidopsis thaliana. PLoS ONE 4: e5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M., Raposo G. (2009). Exosomes—Vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21: 575–581 [DOI] [PubMed] [Google Scholar]
- Simons R.W., Grunberg-Manago M. (1998). RNA Structure and Function. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; ). [Google Scholar]
- Steitz T.A. (2008). A structural understanding of the dynamic ribosome machine. Nat. Rev. Mol. Cell Biol. 9: 242–253 [DOI] [PubMed] [Google Scholar]
- Stombaugh J., Zirbel C.L., Westhof E., Leontis N.B. (2009). Frequency and isostericity of RNA base pairs. Nucleic Acids Res. 37: 2294–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsagris E.M., Martínez de Alba A.E., Gozmanova M., Kalantidis K. (2008). Viroids. Cell. Microbiol. 10: 2168–2179 [DOI] [PubMed] [Google Scholar]
- Turgeon R., Wolf S. (2009). Phloem transport: Cellular pathways and molecular trafficking. Annu. Rev. Plant Biol. 60: 207–221 [DOI] [PubMed] [Google Scholar]
- Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9: 654–659 [DOI] [PubMed] [Google Scholar]
- Varkonyi-Gasic E., Gould N., Sandanayaka M., Sutherland P., MacDiarmid R.M. (2010). Characterisation of microRNAs from apple (Malus domestica ‘Royal Gala’) vascular tissue and phloem sap. BMC Plant Biol. 10: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven J.T., Jansen C.C., Roenhorst J.W., Flores R., de la Peña M. (2009). Pepper chat fruit viroid: biological and molecular properties of a proposed new species of the genus Pospiviroid. Virus Res. 144: 209–214 [DOI] [PubMed] [Google Scholar]
- Vlassov V.V., Laktionov P.P., Rykova E.Y. (2007). Extracellular nucleic acids. Bioessays 29: 654–667 [DOI] [PubMed] [Google Scholar]
- Wang Y., Ding B. (2010). Viroids: Small probes for exploring the vast universe of RNA trafficking in plants. J. Integr. Plant Biol. 52: 28–39 [DOI] [PubMed] [Google Scholar]
- Waterhouse A.M., Procter J.B., Martin D.M., Clamp M., Barton G.J. (2009). Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 25: 1189–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xoconostle-Cázares B., Xiang Y., Ruiz-Medrano R., Wang H.L., Monzer J., Yoo B.C., McFarland K.C., Franceschi V.R., Lucas W.J. (1999). Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science 283: 94–98 [DOI] [PubMed] [Google Scholar]
- Yoo B.C., Kragler F., Varkonyi-Gasic E., Haywood V., Archer-Evans S., Lee Y.M., Lough T.J., Lucas W.J. (2004). A systemic small RNA signaling system in plants. Plant Cell 16: 1979–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Sun L., Kragler F. (2009). The phloem-delivered RNA pool contains small noncoding RNAs and interferes with translation. Plant Physiol. 150: 378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Archual A.J., Amin A.A., Ding B. (2008). A genomic map of viroid RNA motifs critical for replication and systemic trafficking. Plant Cell 20: 35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Leontis N.B., Qian S., Itaya A., Qi Y., Boris-Lawrie K., Ding B. (2006). Tertiary structural and functional analyses of Loop E motif in viroid RNA reveal its essential role in RNA-templated RNA replication by the nuclear transcription machinery. J. Virol. 80: 8566–8581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Tao X., Stombaugh J., Leontis N., Ding B. (2007). Tertiary structure and function of an RNA motif required for plant vascular entry to initiate systemic trafficking. EMBO J. 26: 3836–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Green L., Woo Y.M., Owens R., Ding B. (2001). Cellular basis of potato spindle tuber viroid systemic movement. Virology 279: 69–77 [DOI] [PubMed] [Google Scholar]
- Zuker M. (2003). Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31: 3406–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.