Abstract
Biallelic inactivating germline mutations in the base excision repair MUTYH (MYH) gene have been shown to predispose to MUTYH-associated polyposis (MAP), which is characterized by multiple colorectal adenomas and carcinomas. In this study, we successfully prepared highly homogeneous human MUTYH type 2 recombinant proteins and compared the DNA glycosylase activity of the wild-type protein and fourteen variant-type proteins on adenine mispaired with 8-hydroxyguanine, an oxidized form of guanine. The adenine DNA glycosylase activity of the p.I195V protein, p.G368D protein, p.M255V protein, and p.Y151C protein was 66.9%, 15.2%, 10.7%, and 4.5%, respectively, of that of the wild-type protein, and the glycosylase activity of the p.R154H, p.L360P, p.P377L, p.452delE, p.R69X, and p.Q310X proteins as well as of the p.D208N negative control form was extremely severely impaired. The glycosylase activity of the p.V47E, p.R281C, p.A345V, and p.S487F proteins, on the other hand, was almost the same as that of the wild-type protein. These results should be of great value in accurately diagnosing MAP and in fully understanding the mechanism by which MUTYH repairs DNA in which adenine is mispaired with 8-hydroxyguanine. © 2010 Wiley-Liss, Inc.
Keywords: base excision repair, 8-hydroxyguanine, MUTYH, MUTYH-associated polyposis, MAP, DNA glycosylase, colorectal cancer
INTRODUCTION
8-Hydroxyguanine (8-OHG) is an oxidized form of guanine (Kasai and Nishimura, 1991), and because 8-OHG can pair with adenine as well as cytosine, formation of 8-OHG in DNA causes a G:C to T:A transversion mutation (Shibutani et al., 1991). MUTYH protein (MIM# 604933), also known as MYH protein, is a DNA glycosylase that catalyzes the removal of adenine mispaired with 8-OHG in double-stranded DNA (Slupska et al., 1999; Shinmura et al., 2000; Tao et al., 2008). Two major MUTYH proteins, i.e., type 1 and type 2, are expressed in human cells as a result of the presence of multiple transcription initiation sites and alternative splicing of mRNA transcripts (Takao et al., 1999; Ohtsubo et al., 2000). Type 1 is composed of 535 amino acids, and because it contains a mitochondrial targeting signal (MTS) in its N-terminal, it is localized in the mitochondria. Type 2 is composed of only 521 amino acid, because it lacks the N-terminal 14 amino acids of type 1, which contain the MTS, and as a result type 2 is localized in the nucleus (Takao et al., 1999; Ohtsubo et al., 2000). The excisional repair activity of the type 2 protein is greater than that of the type 1 protein under certain conditions (Shinmura et al., 2000).
Biallelic inactivating germline mutations in the MUTYH gene predispose to MUTYH-associated polyposis (MAP; MIM# 608456), an autosomal recessive disorder characterized by multiple colorectal adenomas and carcinomas (Al-Tassan et al., 2002; Jones et al., 2002; Sampson et al., 2003; Sieber et al., 2003). Since the diagnosis of MAP depends on the level of repair activity of the MUTYH variants encoded in the two MUTYH alleles of the patient and the presence of the clinical phenotype characteristic of MAP, even when MUTYH gene variations are present in a patient, information on the level of repair activity of the MUTYH variants is indispensable to making the diagnosis of MAP. However, even though more than 80 MUTYH variants have been described in the MUTYH gene in colorectal polyposis and colorectal cancer patients (reviewed in Cheadle and Sampson, 2007; Vogt et al., 2009), the effect of only a small number of variations on human MUTYH protein activity has been investigated (Wooden et al., 2004; Bai et al., 2005; Bai et al., 2007; Ali et al., 2008; Kundu et al., 2009; Forsbring et al., 2009; Molatore et al., 2010). One of the reasons for not investigating the effect of more variations is that human MUTYH recombinant proteins cannot be efficiently overexpressed and purified in Escherichia coli (E. coli) and baculovirus cultures or in a cell-free system. Thus, even in previous investigations of variant MUTYH proteins, the purified protein fraction also contained multiple other proteins, judging from the photographs of the SDS-PAGE gels. The authors of one paper (Bai et al., 2005) estimated that the purity of the GST-MUTYH fusion proteins used in the analysis was approximately 15%. Too much amount of contamination by other proteins can interfere with accurate determination of the repair activity of variant MUTYH proteins. Thus, improvement of the production and purification system is needed to enable accurate evaluation of the repair activity of variant MUTYH proteins. Moreover, since somatic APC (MIM# 611731) mutations occur in the nuclear DNA of a high proportion of MAP tumors (Al-Tassan et al., 2002), it is preferable to evaluate the repair activity of the type 2 protein localized in the nucleus, not the type 1 mitochondrial protein. However, except for the study by Molatole et al. (2010), the repair activity of variants of the type 1 mitochondrial MUTYH form, not the type 2 nuclear form, has been studied in previous studies. Therefore, in the present study we established an experimental system for the purification of MUTYH type 2 recombinant proteins and evaluated 14 type 2 variants, i.e., p.V47E, p.Y151C, p.R154H, p.I195V, p.M255V, p.R281C, p.A345V, p.L360P, p.G368D, p.P377L, p.452delE, p.S487F, p.R69X, and p.Q310X, which correspond to type 1 proteins p.V61E, p.Y165C, p.R168H, p.I201V, p.M269V, p.R295C, p.A359V, p.L374P, p.G382D, p.P391L, p.466delE, p.S501F, p.R83X, and p.Q324X, respectively. All of the above are MUTYH variants that have been identified in patients with colorectal polyposis and/or with colorectal cancer (Halford et al., 2003; Sieber et al., 2003; Aceto et al., 2005; Aretz et al., 2006; Kanter-Smoler et al., 2006; Lejeune et al., 2006; Peterlongo et al., 2006; Russell et al., 2006; Yanaru-Fujisawa et al., 2008). This study assessed the adenine excisional activity of a larger number of MUTYH variants than in previous studies, and the repair activity of the type 2 protein of 11 of the 14 MUTYH variants (p.V47E, p.R154H, p.I195V, p.M255V, p.R281C, p.A345V, p.L360P, p.P377L, p.S487F, p.R69X, and p.Q310X) was examined for the first in this study.
MATERIALS AND METHODS
Plasmid construction
The human MUTYH type 2 cDNA sequence was inserted into a pET25b(+) expression vector (Novagen, Darmstadt, Germany). The expression vector for 13 missense-type variants was generated by site-directed mutagenesis with a QuikChange Site-directed Mutagenesis kit (Stratagene, La Jolla, CA). The expression vector for p.R69X and p.Q310X types were constructed by inserting MUTYH cDNA sequence (nucleotides 1-204 and 1-927, respectively) into the pET25b(+) expression vector. All vectors were confirmed by DNA sequencing with a BigDye Terminator Cycle Sequencing Reaction Kit (Applied Biosystems, Tokyo, Japan) and an ABI 3100 Genetic Analyzer (Applied Biosystems).
Preparation of the recombinant MUTYH proteins
E. coli BL21-CodonPlus (DE3)-RP competent cells (Stratagene) were transformed with the MUTYH-pET25b vector and cultured at 37°C until an A600 of 0.6. After incubation with 0.1 mM IPTG at 15°C for 12 h, MUTYH-His6 protein was purified with TALON metal affinity resins (Clontech, Palo Alto, CA) and a TALON 2-ml disposable gravity column (Clontech). The protein was then dialyzed against buffer containing 10 mM sodium phosphate (pH 7.6), 50 mM NaCl, 0.5 mM DTT, 0.1 mM EDTA, 0.5 mM PMSF, 2 μg/ml pepstatin, 2 μg/ml leupeptin, 50 μM chymostatin, and 10% glycerol. The quality and concentration of MUTYH proteins were determined by using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA) and Image J software (National Institutes of Health, Bethesda, MD).
Western blot analysis
Purified recombinant protein was mixed with an equal volume of 2x SDS sample buffer and boiled. A 2 ug protein was subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and electrophoretically transferred to a polyvinylidene difluoride membrane (GE Healthcare Bio-Science Corp., Piscataway, NJ). The membrane was blocked with non-fat milk and incubated with an anti-MUTYH polyclonal antibody (Ohtsubo et al., 2000). After washing, the membrane was incubated with an anti-rabbit HRP-conjugated secondary antibody (GE Healthcare Bio-Science Corp.). The membrane was then washed, and immunoreactivity was visualized with an ECL Plus chemiluminescence system (GE Healthcare Bio-Science Corp.).
DNA cleavage activity assay
30-mer oligonucleotides containing and not containing a single 8-OHG (5′-CTG GTG GCC TGA C[8-OHG or T]C ATT CCC CAA CTA GTG-3′) were chemically synthesized and purified by PAGE (Japan Bio Services, Saitama, Japan). Complementary oligonucleotides containing an adenine opposite the 8-OHG or T were 32P-labeled at the 5′ terminus with a MEGALABEL kit (Takara, Osaka, Japan) and a [γ-32P]ATP (PerkinElmer, Tokyo, Japan), and then annealed to oligonucleotides containing a single 8-OHG or T. The reaction mixture containing 20 mM sodium phosphate (pH 7.6), 100 mM NaCl, 0.5 mM DTT, 0.5 mM EDTA, 5 μM ZnCl2, 1.5% glycerol, 2.5 nM labeled oligonucleotide, 50 μg/ml BSA, and purified MUTYH protein was incubated at 37°C, and the mixture was treated with 0.1 M NaOH at 95°C for 4 min. After adding denaturing formamide dye to the mixture, it was heated at 95°C for 3 min, and subjected to 20% PAGE. A 32P-labeled marker oligonucleotide was used as a size marker for the cleavage products. The radioactivity of intact and cleaved oligonucleotides was quantified by using an FLA-3000 fluoroimage analyzer (Fuji Film, Tokyo, Japan) and ImageGauge software (Fuji Film) (Goto et al., 2009).
Active site titration and evaluation of the rate constant kf
The active site titration and evaluation of the rate constant were performed as described previously (Fersht, 1985; Kundu et al., 2009). A 100 ng amount of total proteins was incubated at 37°C for 0 - 30 min with 5 nM 8-OHG containing substrate and the cleavage products were monitored. To determine the amplitude of the burst (A0), which is proportional to the active protein fraction concentration, the data were fitted to equation (1):
| (1) |
where [P]t is the cleavage product concentration at time t and kb and kl are the rate constants of the burst phase and the linear phase, respectively.
Rate constant, kf, was evaluated under single-turnover conditions. A 10 nM concentration of active MUTYH enzymes was incubated at 37°C for 0-15 min with 2.5 nM 8-OHG containing substrate and the cleavage products were monitored. To estimate the kf the data were fitted to equation (2):
| (2) |
Mutation nomenclature and reference sequence
Mutation nomenclature is according to den Dunnen and Antonarakis (2000) and den Dunnen and Paalman (2003). The reference sequence for the MUTYH gene encoding type 2 protein is accession number NM_001048174.1. The nucleotide numbering system uses the A of the ATG translation initiation start site as nucleotide +1.
RESULTS
To overcome the difficulty of preparing highly purified recombinant MUTYH proteins, in this study, we used a pET25b(+) expression vector and BL21-CodonPlus (DE3)-RP E. coli host cells for induction of MUTYH expression and a TALON metal affinity resin and gravity column for purification of the MUTYH proteins. Wild-type human MUTYH type 2 protein tagged with His6 at its C-terminus was successfully overexpressed in E. coli and purified to approximately 90% homogeneity (Figure 1A). The specificity of the purified MUTYH protein was confirmed by Western blotting with anti-MUTYH polyclonal antibody (Figure 1B). Their molecular size of approximately 61 kDa was determined by SDS-PAGE / Coomassie Brilliant Blue (CBB) staining and Western blotting, and it corresponded to their size calculated from the cDNA sequence. The DNA glycosylase activity of the wild-type MUTYH protein was tested by determining its capacity to cleave a double-stranded oligonucleotide containing an adenine mispaired with 8-OHG. The cleavage products were analyzed on a denaturing polyacrylamide gel, and their mobility was compared with that of a marker oligonucleotide. No clear cleavage products were detected when oligonucleotide containing an unmodified A:T base pair was exposed to the MUTYH protein, but cleavage products having the same mobility as the marker oligonucleotide were detected when MUTYH proteins were allowed to react with oligonucleotide containing an A:8-OHG base pair (Figure 1C). No cleavage was detected when allowed to react after heat-inactivation of the MUTYH protein (Figure 1C). The amount of cleavage products was calculated as percent of total oligonucleotides and expressed as % incision, and the % incision of protein substrate containing an A:8-OHG mispair increased in a protein-concentration-dependent manner (Figure 1D). The time-course assay of the cleavage activity of MUTYH protein on substrate containing an A:8-OHG mispair indicated that the amount of cleavage products peaked within 5 min and was almost constant from 5 min to 60 min (Figure 1E). The results showed that highly purified wild-type MUTYH type 2 protein had been obtained with our expression and purification system, and the adenine DNA glycosylase activity of the protein on an A: 8-OHG mispair was satisfactorily detected by our assay. We therefore decided to apply our experimental systems to assessment of the adenine DNA glycosylase activity of various MUTYH variant proteins.
Figure 1.
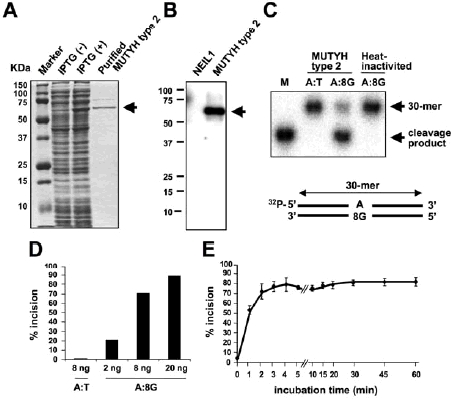
Measurement of the adenine DNA glycosylase activity of wild-type MUTYH type 2 protein. (A) Purification of wild-type MUTYH type 2 protein resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and stained with Coomassie Brilliant Blue. Lysates of E. coli culture without or with IPTG induction and purified MUTYH type 2 protein are shown. The arrow points to the MUTYH-His6 protein band. (B) Western blot of purified wild-type MUTYH type 2 protein tagged with His6. MUTYH-His6 protein is indicated by the arrow. Purified recombinant NEIL1 (MIM# 608844)-His6 protein, which was prepared by using pET25b(+) vector (Novagen) and E. coli BL21-CodonPlus (DE3)-RP cells (Stratagene) previously (Shinmura et al., 2004), was included as a negative control. (C) The DNA glycosylase activity of MUTYH type 2 protein on double-stranded DNA containing an A:8-hydroxyguanine (8-OHG). The MUTYH type 2 protein and a 32P-labeled double-stranded oligonucleotides containing or not containing a single 8-OHG mispair were incubated and subjected to 20% PAGE. The intact 30-mer oligonucleotides and cleavage products are indicated by the arrows. Heat-inactivation of the MUTYH protein was accomplished by heating the protein at 100°C for 5 min. 8G means 8-hydroxyguanine. (D) Protein concentration dependency of cleavage of DNA containing an A: 8-OHG by MUTYH type 2 protein. The MUTYH protein (2, 8, and 20 ng) was incubated at 37°C for 15 min with a 30-mer oligonucleotide containing an A: 8-OHG or A:T (50 fmole). The amount of cleavage products as a proportion of total oligonucleotides was calculated as % incision. (E) Time-course assay of cleavage of DNA containing an A: 8-OHG by MUTYH type 2 protein. The 8 ng amount of MUTYH type 2 protein was incubated at 37°C for 0-60 min with double-stranded oligonucleotide containing an A: 8-OHG (50 fmole). The % incision valves are means ± standard errors of data from three independent experiments.
Fourteen MUTYH variants that had previously been identified in patients with colorectal polyposis and/or colorectal cancer were selected, and their expression vectors were prepared by site-directed mutagenesis. Since the Asp222 in MUTYH type 1 is the active site, and the p.D222N mutant is known not to have DNA glycosylase activity (Wooden et al., 2004), we prepared a type 2-p.D208N construct corresponding to type 1-p.D222N as a negative control. A total of 15 MUTYH type 2 proteins were successfully expressed and purified to a high level of homogeneity (Figure 2). Their molecular sizes determined by SDS-PAGE / CBB staining corresponded with their sizes calculated from their cDNA sequences. Each MUTYH variant protein was almost always reproducibly obtained in the three independent protein preparation (Table 1). Next, we compared the DNA glycosylase activity of each variant protein on oligonucleotide containing an A:8-OHG mispair with that of the wild-type MUTYH type 2 protein (Figure 3 and Table 1). As expected, no clear cleavage products were detected when any of the variant proteins were allowed to act on oligonucleotide containing an unmodified A:T base pair (Figure 3). The adenine DNA glycosylase activity of the MUTYH variant proteins on oligonucleotide containing an A:8-OHG mispair varied (Figure 3 and Table 1). The adenine DNA glycosylase activity of the p.V47E, p.R281C, p.A345V, and p.S487F proteins on the A:8-OHG substrate under conditions of 37°C for 15 min were similar to that of the wild-type protein or only slightly different (102.8% - 128.1%, with activity of the wild-type protein set equal to 100%), whereas p.I195V protein exhibited slightly lower glycosylase activity (66.9%). The p.Y151C protein, p.M255V protein and p.G368D protein exhibited only 4.5%, 10.7%, and 15.2%, respectively, of the glycosylase activity of the wild-type protein, and the adenine DNA glycosylase activity of the p.R154H, p.L360P, p.P377L, p.452delE, p.R69X, and p.Q310X proteins as well as of the p.D208N negative control protein was almost at the background level. We also attempted to determine whether the recombinant type 1 variant proteins had a level of repair activity that was similar to that of the corresponding type 2 proteins. We randomly chose type 1-p.R168H and type 1-p.S501F, which correspond to type 2-p.R154H and type 2-p.S487F, respectively, and found that the activity level of the type 1 and type 2 proteins of at least these two MUTYH variants in comparison with the wild-type protein is similar (Supp. Figure S1A-E). We also estimated the rate constant kf of some type 2 proteins on the A:8-OHG substrate after correction for the active enzyme fraction as described previously (Fersht, 1985; Kundu et al., 2009). The rate constant for adenine excision by wild-type protein was 0.524, and similar to the constant for p.R281C (kf = 0.489) (Table 2). However, the kf value of p.M255V was 0.024 and more than 20-fold lower than that of the wild-type protein, indicating that the glycosylase activity of p.M255V was severely reduced. The above findings indicate that the adenine DNA glycosylase activity of nine of the 14 MUTYH type 2 variant proteins tested is severely impaired.
Figure 2.
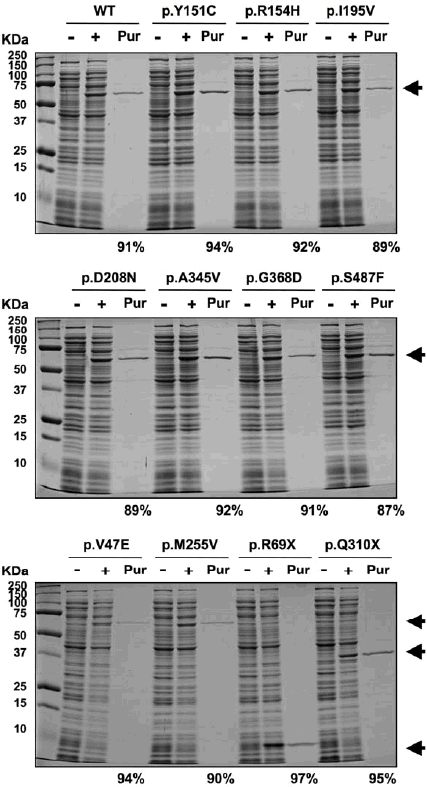
Purification of variant-type MUTYH type 2 recombinant proteins. Each protein was overexpressed and purified under conditions essentially the same as used for the wild-type (WT) protein. Representative results of expression and purification of MUTYH proteins resolved by SDS-PAGE and stained with Coomassie Brilliant Blue are shown. ‘−’ and ‘+’ mean absence and presence, respectively, of IPTG induction, and ‘Pur’ means purified MUTYH type 2 proteins. The arrow points to the MUTYH-His6 protein band. The purification level is indicated below the SDS-PAGE panels.
Table 1.
DNA glycosylase activity of 14 variants of MUTYH type 2 protein on DNA containing adenine mispaired with 8-hydroxyguanine
| MUTYH type 2 protein | Type of mutationa | Purified protein yield (ng) per 10 ml cultureb | Relative % incisionc |
|---|---|---|---|
| WT | 4365, 3562, 3454 | 100 | |
| p.D208N | negative control | 2073, 1826, 1808 | extremely severely defective |
| p.V47E | c.140T>A, missense | 1018, 1893, 1916 | 128.1±5.20 |
| p.Y151C | c.452A>G, missense | 5485, 4779, 2835 | 4.5±0.21 |
| p.R154H | c.461G>A, missense | 894, 1501, 2109 | extremely severely defective |
| p.I195V | c.583A>G, missense | 1733, 1102, 671 | 66.9±0.35 |
| p.M255V | c.763A>G, missense | 2446, 1726, 2488 | 10.7±0.47 |
| p.R281C | c.841C>T, missense | 912, 934, 447 | 103.0±1.39 |
| p.A345V | c.1034C>T, missense | 2564, 2435, 3864 | 103.5±5.43 |
| p.L360P | c.1079T>C, missense | 152, 303, 120 | extremely severely defective |
| p.G368D | c.1103G>A, missense | 2486, 1246, 2270 | 15.2±0.71 |
| p.P377L | c.1130C>T, missense | 885, 626, 430 | extremely severely defective |
| p.452delE | c.1353_1355delGGA, inframe deletion | 822, 614, 364 | extremely severely defective |
| p.S487F | c.1460C>T, missense | 1099, 984, 706 | 102.8±2.31 |
| p.R69X | c.205C>T, nonsense | 6289, 5245, 6289 | extremely severely defective |
| p.Q310X | c.928C>T, nonsense | 918, 5726, 2771 | extremely severely defective |
The reference sequence for the MUTYH gene encoding type 2 protein is accession number NM_001048174.1.
Amount of MUTYH proteins purified from 10 ml of E. coli culture expressing MUTYH type 2 protein. Each protein was purified three times and has been listed in this table.
The DNA cleavage activity of MUTYH protein was measured under conditions of 37°C for 15 min. The amount of cleavage products as a proportion of total oligonucleotides was calculated as % incision, and the % incision of each variant-type MUTYH protein is shown relative to that of wild-type (WT) MUTYH protein, which has been set equal to 100. Values are means ± standard errors of data obtained from three independent experiments in which three independently prepared MUTYH proteins were used.
Figure 3.
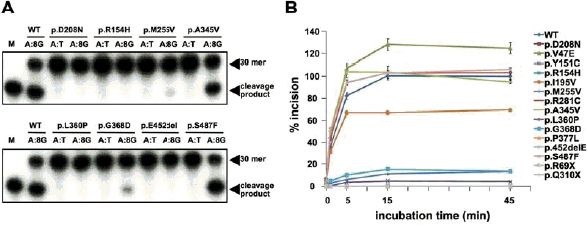
Measurement of the adenine DNA glycosylase activity of variant MUTYH type 2 proteins. (A) Representative results of DNA cleavage assays of MUTYH variant proteins are shown. MUTYH proteins (130 fmole) were allowed to act on double-stranded oligonucleotide containing a single A: 8-OHG (8G) mispair at 37°C for 15 min. The reaction mixture was analyzed by 20% PAGE. A 32P-labeled marker oligonucleotide was used as a size marker for the cleavage products. The intact 30-mer oligonucleotides and cleavage products are indicated by the arrows. (B) Time-course assay of cleavage of DNA containing an A:8-OHG by MUTYH type 2 protein. MUTYH type 2 proteins (130 fmole) were incubated at 37°C for 0 - 45 min with double-stranded oligonucleotide containing an A: 8-OHG (50 fmole). The amount of cleavage products as a proportion of total oligonucleotides was calculated as % incision, and the % incision of each variant-type MUTYH protein is shown relative to that of wild-type (WT) MUTYH protein, which has been set equal to 100. The % incision valves are means ± standard errors of data obtained from three independent experiments in which three independently prepared MUTYH proteins were used.
Table 2.
Active yield and rate constant kf evaluated for MUTYH type 2 protein on DNA containing adenine mispaired with 8-hydroxyguanine
| MUTYH type 2 protein | Active protein yield (μg) per 1L culturea | kf(min−1)b |
|---|---|---|
| WT | 2706, 1248,2045 | 0.524±0.034 |
| p.M255V | 58,72,75 | 0.024±0.004 |
| p.R281C | 944, 1128,751 | 0.489±0.044 |
Values were obtained by incubating 100 ng of total proteins with 5 nM of substrate. Results obtained with three separate protein preparations are shown.
Values were obtained by incubating 10 nM of active MUTYH enzyme and 2.5 nM of substrate. Values are means ± standard errors of data obtained from three independent experiments using independently prepared proteins.
DISCUSSION
The cumulative results of recent screenings of colorectal polyposis patients for MUTYH mutations have revealed many MUTYH gene variants (reviewed in Cheadle and Sampson, 2007; Vogt et al., 2009), but the repair activity of the type 2 protein of most of the variants has never been tested. In this study we improved the method of expressing and purifying the recombinant MUTYH type 2 proteins and assessed the adenine DNA glycosylase activity of various type 2 proteins. The results revealed that the p.V47E, p.R281C, p.A345V, and p.S487F proteins largely retain adenine removing activity, but that the adenine removing activity of the p.I195V protein is mildly impaired and the activity of the p.Y151C, p.R154H, p.M255V, p.L360P, p.G368D, p.P377L, p.452delE, p.R69X, and p.Q310X proteins is severely impaired. This information should be of great help in accurately diagnosing MAP and managing MAP patients.
The adenine DNA glycosylase activity of the type 1 or type 2 MUTYH variants p.Y151C, p.G368D, p.P377L, p.452delE, and p.S487F has been assessed previously (Wooden et al., 2004; Ali et al., 2008; Kundu et al., 2009; Forsbring et al., 2009; Molatore et al., 2010). The results showed that the repair activity of p.Y151C, p.G368D, p.P377L, and p.452delE was impaired while that of p.S487F was retained, findings that were consistent with our own, and the consistency confirms the reliability of the results of our study. Interestingly, a slight difference (approximately 5% vs. approximately 15%) in repair activity was observed between p.Y151C and p.G368D in our study, and the same difference was reported in several previous papers. Nielsen et al. (2009) recently reported finding that MAP patients homozygous for a p.G368D allele have a milder clinical phenotype than MAP patients homozygous for a p.Y151C allele. The difference in adenine removing activity between the p.Y151C protein and p.G368D protein may be related to the difference in clinical phenotype.
Retention of DNA glycosylase activity by the human MUTYH proteins p.V47E, p.R281C, and p.A345V as well as mild impairment of the activity of p.I195V and severe impairment of the activity of p.R154H, p.M255V, p.L360P, p.R69X, and p.Q310X were documented for the first time in this study. Although no analyses of crystal structure of the MUTYH has been reported, based on the cumulative results of previous studies of the biochemistry of MUTYH (reviewed in Cheadle and Sampson, 2007; Ali et al., 2008), p.R154, p.M255, and p.L360 are located in regions suspected of being important to catalytic activity or substrate recognition. Moreover, the p.R154, p.M255, and p.L360 in MUTYH protein are conserved among E. coli, Mus musculus, Rattus norvegicus, Pan troglodytes, Canis familiaris, and Homo sapiens. Substitution of any of these amino acids appears to result in a functional abnormality, resulting in severe reduction of adenine DNA glycosylase activity on DNA containing an A:8-OHG mispair. The loss of large parts of the MUTYH protein in the frameshift-type variant proteins p.R69X and p.Q310X may be responsible for the severe impairment of their adenine removing activity. An amino acid substitution in p.V47E, p.R281C, p.A345V, and p.S487F did not greatly affect their adenine DNA glycosylase activity. These results are consistent with the prediction of a possible impact of an amino acid substitution on the structure and function of MUTYH type 2 protein by the PolyPhen-2 program (http://genetics.bwh.harvard.edu/pph2/index.shtml) (Adzhubei et al., 2010). Since p.V47E, p.R281C, p.A345V, and p.S487F are not located in the region suspected of being important to catalytic activity or in a well-conserved position of the substrate recognition region, the amino acid localization may be one of the reasons for the retention of enzymatic activity. In the future, a crystal structure of the MUTYH protein alone and covalently complexed with DNA, in conjunction with the present findings on MUTYH variants, should contribute to establishing further correlations between the structure and repair function of the MUTYH protein.
Highly homogeneous MUTYH type 2 recombinant protein was prepared by using the method described in this study. As far as we have been able to determine in a review of the literature, the level of purification of the proteins in our study was higher than level of purification estimated from the SDS-PAGE images of MUTYH type 1 or type 2 proteins in previous papers and purification levels described in the other papers. Use of E. coli BL21-CodonPlus (DE3)-RP cells, which contain extra copies of the genes that encode the tRNAs of rare E. coli codons, is thought to be responsible for the increase in level of MUTYH protein expression, because the MUTYH gene contains many rare codons. The expression conditions (temperature and IPTG concentration), combination of expression vector and competent cells, and metal-affinity purification conditions are also thought to possibly have contributed to the improvement in the level of purification in this study. Since genetic screening of colorectal polyposis patients for MUTYH mutations will continue to be performed worldwide, the same as genetic screening for APC mutations (reviewed in Lynch et al., 2008), our method of expression and purification of human MUTYH protein should be useful for assessing MUTYH variants newly identified by genetic screening as well as MUTYH variants that have not been examined.
As shown in Supp. Figure S1E, the activity level of the type 1 and type 2 proteins of two MUTYH variants in comparison with the wild-type protein was similar. Although comparisons were not made for the other variants, it may not always be necessarily to study the type 2 form when analyzing MUTYH protein. However, since type 2, and not type 1, is a nuclear form (Takao et al., 1999; Ohtsubo et al., 2000), and somatic APC mutations occur in the nuclear DNA of MAP tumors (Al-Tassan et al., 2002), we think that evaluation of type 2 variants is likely to be more preferable when we investigate the possible pathogenic role of MUTYH in MAP. Assessment of the glycosylase activity level of the wild-type type 1 and type 2 proteins showed that the activity of type 2 was greater than that of type 1 (Figure 1 and Supp. Figure S1), a finding that is consistent with a previous report (Shinmura et al., 2000). The reason for the difference in the catalysis of adenine excision between the type 1 and type 2 proteins is unclear. One point that requires caution is that there is evidence that the type 1 protein is processed during mitochondrial transport in cells and the mature form of the protein never been identified, and thus the repair activity of the full-length type 1 may not necessarily reflect the activity in vivo. With regard to the method of evaluating MUTYH variant proteins, since MUTYH possesses suppressive activity against G:C to T:A mutations caused by 8-OHG (Yamane et al., 2003) and biallelic MUTYH inactivation leads to somatic APC mutation in MAP tumors (Al-Tassan et al., 2002), analysis of the mutation rate of the APC gene in MUTYH variant-expressing cells may be an alternative way of evaluating the level of repair activity of a MUTYH variant. A combination of mutation rate analysis and DNA glycosylase analysis would provide more definitive proof of the pathogenicity of a MUTYH variant.
Acknowledgments
Contract grant sponsor: The Ministry of Health, Labour and Welfare for the Comprehensive 10-Year Strategy for Cancer Control (19-19) and the Third Term Comprehensive Control Research for Cancer, the Japan Society for the Promotion of Science for Scientific Research (19790286, 22590356, 22790378), the Ministry of Education, Culture, Sports, Science and Technology for priority area (18014009, 20014007, 221S0001), and the Smoking Research Foundation.
SUPPORTING INFORMATION
Supp. Figure S1.
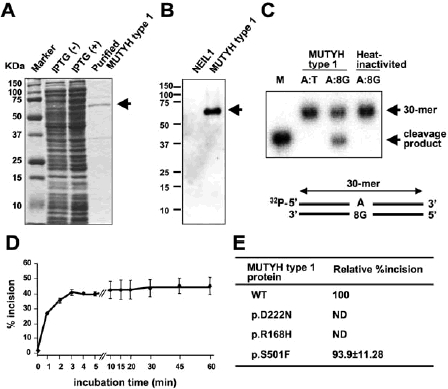
Measurement of DNA glycosylase activity of MUTYH type 1 protein. (A) Purification of wild-type MUTYH type 1 protein resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and stained with Coomassie Brilliant Blue (CBB). The human MUTYH type 1 cDNA sequence was inserted into a pET25b(+) expression vector (Novagen, Darmstadt, Germany). E. coli BL21-CodonPlus (DE3)-RP competent cells (Stratagene, La Jolla, CA) were transformed with the MUTYH-pET25b vector and cultured at 37°C until an A600 of 0.6. After incubation with 0.1 mM IPTG at 20°C for 12 h, MUTYH-His6 protein was purified with TALON metal affinity resins (Clontech, Palo Alto, CA) and a TALON 2-ml disposable gravity column (Clontech). The protein was then dialyzed against buffer containing 10 mM sodium phosphate (pH 7.6), 50 mM NaCl, 0.5 mM DTT, 0.1 mM EDTA, 0.5 mM PMSF, 2 μg/ml pepstatin, 2 μg/ml leupeptin, 50 μM chymostatin, and 10% glycerol. Lysates of E. coli culture without or with IPTG induction and purified MUTYH type 1 protein are shown. The arrow points to the MUTYH-His6 protein band. (B) Western blot of purified wild-type MUTYH type 1 protein tagged with His6. Purified recombinant NEIL1-His6 protein, which was prepared by using pET25b(+) vector (Novagen) and E. coli BL21-CodonPlus (DE3)-RP cells (Stratagene) previously (Shinmura et al., 2004), was included as a negative control. Purified recombinant protein was mixed with an equal volume of 2x SDS sample buffer and boiled. A 2 ug protein was subjected to SDS-PAGE and electrophoretically transferred to a polyvinylidene difluoride membrane (GE Healthcare Bio-Science Corp., Piscataway,NJ). The membrane was blocked with nonfat milk and incubated with an anti-MUTYH polyclonal antibody (Ohtsubo et al., 2000). After washing, the membrane was incubated with an anti-rabbit HRP-conjugated secondary antibody (GE Healthcare Bio-Science Corp.). The membrane was then washed, and immunoreactivity was visualized with an ECL Plus chemiluminescence system (GE Healthcare Bio-Science Corp.). MUTYH-His6 protein is indicated by the arrow. (C) The DNA glycosylase activity of wild-type MUTYH type 1 protein on double-stranded DNA containing an A:8-hydroxyguanine (8-OHG). 30-mer oligonucleotides containing and not containing a single 8-OHG (5′-CTG GTG GCC TGA C[8-OHG or T]C ATT CCC CAA CTA GTG-3′) were chemically synthesized and purified by PAGE (Japan Bio Services, Saitama, Japan). Complementary oligonucleotides containing an adenine opposite the 8-OHG or T were 32P-labeled at the 5′ terminus with a MEGALABEL kit (Takara, Osaka, Japan) and a [γ-32P]ATP (PerkinElmer, Tokyo, Japan), and then annealed to oligonucleotides containing a single 8-OHG or T. The reaction mixture containing 20 mM sodium phosphate (pH 7.6), 100 mM NaCl, 0.5 mM DTT, 0.5 mM EDTA, 5 μM ZnCl2, 1.5% glycerol, 2.5 nM labeled oligonucleotide, 50 μg/ml BSA, and purified MUTYH protein was incubated at 37°C, and the mixture was treated with 0.1 M NaOH at 95°C for 4 min. After adding denaturing formamide dye to the mixture, it was heated at 95°C for 3 min, and subjected to 20% PAGE. A 32P-labeled marker oligonucleotide was used as a size marker for the cleavage products. The radioactivity of intact and cleaved oligonucleotides was quantified by using an FLA-3000 fluoroimage analyzer (Fuji Film, Tokyo, Japan) and ImageGauge software (Fuji Film) (Goto et al., 2009). The intact 30-mer oligonucleotides and cleavage products are indicated by the arrows. Heat-inactivation of the MUTYH protein was accomplished by heating the protein at 100°C for 5 min. 8G means 8-hydroxyguanine. (D) Time-course assay of cleavage of DNA containing an A:8-OHG by wild-type MUTYH type 1 protein. The MUTYH type 1 protein (260 fmole) was incubated at 37°C for 0 - 60 min with double-stranded oligonucleotide containing an A:8-OHG (50 fmole). The amount of cleavage products as a proportion of total oligonucleotides was calculated as % incision. The % incision values are shown as means ± standard errors of data from three independent experiments. (E) DNA glycosylase activities of wild-type MUTYH type 1 protein and their variant proteins on an A:8-OHG substrate. DNA cleavage activities of MUTYH type 1 proteins were measured at 37°C for 15 min. The amount of cleavage products as a proportion of total oligonucleotides was calculated as % incision, and the % incision of each variant-type MUTYH protein is shown relative to that of wild-type (WT) MUTYH protein, which has been set equal to 100. Values are means ± standard errors of data from three independent experiments. ND, not detected.
REFERENCES
- Aceto G, Cristina Curia M, Veschi S, De Lellis L, Mammarella S, Catalano T, Stuppia L, Palka G, Valanzano R, Tonelli F, Casale V, Stigliano V, Cetta F, Battista P, Mariani-Costantini R, Cama A. Mutations of APC and MYH in unrelated Italian patients with adenomatous polyposis coli. Hum Mutat. 2005;26:394. doi: 10.1002/humu.9370. [DOI] [PubMed] [Google Scholar]
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tassan N, Chmiel NH, Maynard J, Fleming N, Livingston AL, Williams GT, Hodges AK, Davies DR, David SS, Sampson JR, Cheadle JP. Inherited variants of MYH associated with somatic G:C–->T:A mutations in colorectal tumors. Nat Genet. 2002;30:227–32. doi: 10.1038/ng828. [DOI] [PubMed] [Google Scholar]
- Ali M, Kim H, Cleary S, Cupples C, Gallinger S, Bristow R. Characterization of mutant MUTYH proteins associated with familial colorectal cancer. Gastroenterology. 2008;135:499–507. doi: 10.1053/j.gastro.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aretz S, Uhlhaas S, Goergens H, Siberg K, Vogel M, Pagenstecher C, Mangold E, Caspari R, Propping P, Friedl W. MUTYH-associated polyposis: 70 of 71 patients with biallelic mutations present with an attenuated or atypical phenotype. Int J Cancer. 2006;119:807–14. doi: 10.1002/ijc.21905. [DOI] [PubMed] [Google Scholar]
- Bai H, Jones S, Guan X, Wilson TM, Sampson JR, Cheadle JP, Lu AL. Functional characterization of two human MutY homolog (hMYH) missense mutations (R227W and V232F) that lie within the putative hMSH6 binding domain and are associated with hMYH polyposis. Nucleic Acids Res. 2005;33:597–604. doi: 10.1093/nar/gki209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Grist S, Gardner J, Suthers G, Wilson TM, Lu AL. Functional characterization of human MutY homolog (hMYH) missense mutation (R231L) that is linked with hMYH-associated polyposis. Cancer Lett. 2007;250:74–81. doi: 10.1016/j.canlet.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheadle JP, Sampson JR. MUTYH-associated polyposis—from defect in base excision repair to clinical genetic testing. DNA Repair (Amst) 2007;6:274–9. doi: 10.1016/j.dnarep.2006.11.001. [DOI] [PubMed] [Google Scholar]
- den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat. 2000;15:7–12. doi: 10.1002/(SICI)1098-1004(200001)15:1<7::AID-HUMU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- den Dunnen JT, Paalman MH. Standardizing mutation nomenclature: why bother? Hum Mutat. 2003;22(3):181–182. doi: 10.1002/humu.10262. [DOI] [PubMed] [Google Scholar]
- Fersht A. Enzyme Structure and Mechanism. 2nd edn. New York: Freeman WH; 1985. Measurement and magnitude of enzymatic rate constants; pp. 121–154. [Google Scholar]
- Forsbring M, Vik ES, Dalhus B, Karlsen TH, Bergquist A, Schrumpf E, Bjørås M, Boberg KM, Alseth I. Catalytically impaired hMYH and NEIL1 mutant proteins identified in patients with primary sclerosing cholangitis and cholangiocarcinoma. Carcinogenesis. 2009;30:1147–54. doi: 10.1093/carcin/bgp118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Shinmura K, Igarashi H, Kobayashi M, Konno H, Yamada H, Iwaizumi M, Kageyama S, Tsuneyoshi T, Tsugane S, Sugimura H. Altered expression of the human base excision repair gene NTH1 in gastric cancer. Carcinogenesis. 2009;30:1345–52. doi: 10.1093/carcin/bgp108. [DOI] [PubMed] [Google Scholar]
- Halford SE, Rowan AJ, Lipton L, Sieber OM, Pack K, Thomas HJ, Hodgson SV, Bodmer WF, Tomlinson IP. Germline mutations but not somatic changes at the MYH locus contribute to the pathogenesis of unselected colorectal cancers. Am J Pathol. 2003;162:1545–8. doi: 10.1016/S0002-9440(10)64288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Emmerson P, Maynard J, Best JM, Jordan S, Williams GT, Sampson JR, Cheadle JP. Biallelic germline mutations in MYH predispose to multiple colorectal adenoma and somatic G:C—>T:A mutations. Hum Mol Genet. 2002;11:2961–7. doi: 10.1093/hmg/11.23.2961. [DOI] [PubMed] [Google Scholar]
- Kanter-Smoler G, Björk J, Fritzell K, Engwall Y, Hallberg B, Karlsson G, Grönberg H, Karlsson P, Wallgren A, Wahlström J, Hultcrantz R, Nordling M. Novel findings in Swedish patients with MYH-associated polyposis: mutation detection and clinical characterization. Clin Gastroenterol Hepatol. 2006;4:499–506. doi: 10.1016/j.cgh.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Kasai H, Nishimura S. Formation of 8-hydroxyguanosine in DNA by oxygen radicals and its biological significance. In: Sies H, editor. Oxidative Stress: Oxidants and Antioxidants. London, UK: Academic Press; 1991. pp. 99–116. [Google Scholar]
- Kundu S, Brinkmeyer MK, Livingston AL, David SS. Adenine removal activity and bacterial complementation with the human MutY homologue (MUTYH) and Y165C, G382D, P391L and Q324R variants associated with colorectal cancer. DNA Repair (Amst) 2009;8:1400–10. doi: 10.1016/j.dnarep.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune S, Guillemot F, Triboulet JP, Cattan S, Mouton C, PAFNORD Group. Porchet N, Manouvrier S, Buisine MP. Low frequency of AXIN2 mutations and high frequency of MUTYH mutations in patients with multiple polyposis. Hum Mutat. 2006;27:1064. doi: 10.1002/humu.9460. [DOI] [PubMed] [Google Scholar]
- Lynch HT, Lynch JF, Lynch PM, Attard T. Hereditary colorectal cancer syndromes: molecular genetics, genetic counseling, diagnosis and management. Fam Cancer. 2008;7:27–39. doi: 10.1007/s10689-007-9165-5. [DOI] [PubMed] [Google Scholar]
- Molatore S, Russo MT, D'Agostino VG, Barone F, Matsumoto Y, Albertini AM, Minoprio A, Degan P, Mazzei F, Bignami M, Ranzani GN. MUTYH mutations associated with familial adenomatous polyposis: functional characterization by a mammalian cell-based assay. Hum Mutat. 2010;31:159–66. doi: 10.1002/humu.21158. [DOI] [PubMed] [Google Scholar]
- Nielsen M, Joerink-van de Beld MC, Jones N, Vogt S, Tops CM, Vasen HF, Sampson JR, Aretz S, Hes FJ. Analysis of MUTYH genotypes and colorectal phenotypes in patients With MUTYH-associated polyposis. Gastroenterology. 2009;136:471–6. doi: 10.1053/j.gastro.2008.10.056. [DOI] [PubMed] [Google Scholar]
- Ohtsubo T, Nishioka K, Imaiso Y, Iwai S, Shimokawa H, Oda H, Fujiwara T, Nakabeppu Y. Identification of human MutY homolog (hMYH) as a repair enzyme for 2-hydroxyadenine in DNA and detection of multiple forms of hMYH located in nuclei and mitochondria. Nucleic Acids Res. 2000;28:1355–64. doi: 10.1093/nar/28.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlongo P, Mitra N, Sanchez de Abajo A, de la Hoya M, Bassi C, Bertario L, Radice P, Glogowski E, Nafa K, Caldes T, Offit K, Ellis NA. Increased frequency of disease-causing MYH mutations in colon cancer families. Carcinogenesis. 2006;27:2243–9. doi: 10.1093/carcin/bgl093. [DOI] [PubMed] [Google Scholar]
- Russell AM, Zhang J, Luz J, Hutter P, Chappuis PO, Berthod CR, Maillet P, Mueller H, Heinimann K. Prevalence of MYH germline mutations in Swiss APC mutation-negative polyposis patients. Int J Cancer. 2006;118:1937–40. doi: 10.1002/ijc.21470. [DOI] [PubMed] [Google Scholar]
- Sampson JR, Dolwani S, Jones S, Eccles D, Ellis A, Evans DG, Frayling I, Jordan S, Maher ER, Mak T, Maynard J, Pigatto F, Shaw J, Cheadle JP. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39–41. doi: 10.1016/S0140-6736(03)13805-6. [DOI] [PubMed] [Google Scholar]
- Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991;349:431–4. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Tao H, Goto M, Igarashi H, Taniguchi T, Maekawa M, Takezaki T, Sugimura H. Inactivating mutations of the human base excision repair gene NEIL1 in gastric cancer. Carcinogenesis. 2004;25:2311–7. doi: 10.1093/carcin/bgh267. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Yamaguchi S, Saitoh T, Takeuchi-Sasaki M, Kim SR, Nohmi T, Yokota J. Adenine excisional repair function of MYH protein on the adenine: 8-hydroxyguanine base pair in double-stranded DNA. Nucleic Acids Res. 2000;28:4912–8. doi: 10.1093/nar/28.24.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber OM, Lipton L, Crabtree M, Heinimann K, Fidalgo P, Phillips RK, Bisgaard ML, Orntoft TF, Aaltonen LA, Hodgson SV, Thomas HJ, Tomlinson IP. Multiple colorectal adenomas, classic adenomatous polyposis, and germ-line mutations in MYH. N Engl J Med. 2003;348:791–9. doi: 10.1056/NEJMoa025283. [DOI] [PubMed] [Google Scholar]
- Slupska MM, Luther WM, Chiang JH, Yang H, Miller JH. Functional expression of hMYH, a human homolog of the Escherichia coli MutY protein. J Bacteriol. 1999;181:6210–3. doi: 10.1128/jb.181.19.6210-6213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao M, Zhang QM, Yonei S, Yasui A. Differential subcellular localization of human MutY homolog (hMYH) and the functional activity of adenine: 8-oxoguanine DNA glycosylase. Nucleic Acids Res. 1999;27:3638–44. doi: 10.1093/nar/27.18.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Shinmura K, Suzuki M, Kono S, Mibu R, Tanaka M, Kakeji Y, Maehara Y, Okamura T, Ikejiri K, Futami K, Yasunami Y, Maekawa T, Takenaka K, Ichimiya H, Imaizumi N, Sugimura H. Association between genetic polymorphisms of the base excision repair gene MUTYH and increased colorectal cancer risk in a Japanese population. Cancer Sci. 2008;99:355–60. doi: 10.1111/j.1349-7006.2007.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt S, Jones N, Christian D, Engel C, Nielsen M, Kaufmann A, Steinke V, Vasen HF, Propping P, Sampson JR, Hes FJ, Aretz S. Expanded extracolonic tumor spectrum in MUTYH-associated polyposis. Gastroenterology. 2009;137:1976–85. doi: 10.1053/j.gastro.2009.08.052. [DOI] [PubMed] [Google Scholar]
- Wooden SH, Bassett HM, Wood TG, McCullough AK. Identification of critical residues required for the mutation avoidance function of human MutY (hMYH) and implications in colorectal cancer. Cancer Lett. 2004;205:89–95. doi: 10.1016/j.canlet.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Yamane A, Shinmura K, Sunaga N, Saitoh T, Yamaguchi S, Shinmura Y, Yoshimura K, Murakami H, Nojima Y, Kohno T, Yokota J. Suppressive activities of OGG1 and MYH proteins against G:C to T:A mutations caused by 8-hydroxyguanine but not by benzo[a]pyrene diol epoxide in human cells in vivo. Carcinogenesis. 2003;24:1031–7. doi: 10.1093/carcin/bgg056. [DOI] [PubMed] [Google Scholar]
- Yanaru-Fujisawa R, Matsumoto T, Ushijima Y, Esaki M, Hirahashi M, Gushima M, Yao T, Nakabeppu Y, Iida M. Genomic and functional analyses of MUTYH in Japanese patients with adenomatous polyposis. Clin Genet. 2008;73:545–53. doi: 10.1111/j.1399-0004.2008.00998.x. [DOI] [PubMed] [Google Scholar]


