Abstract
Under conventional heart failure therapy, inflammatory cardiomyopathy usually has a progressive course, merging for alternative interventional strategies. There is accumulating support for the application of cellular transplantation as a strategy to improve myocardial function. Mesenchymal stem cells (MSCs) have the advantage over other stem cells that they possess immunomodulatory features, making them attractive candidates for the treatment of inflammatory cardiomyopathy. Studies in experimental models of inflammatory cardiomyopathy have consistently demonstrated the potential of MSCs to reduce cardiac injury and to improve cardiac function. This paper gives an overview about how inflammation triggers the functionality of MSCs and how it induces cardiac homing. Finally, the potential of intravenous application of MSCs by inflammatory cardiomyopathy is discussed.
1. Introduction
Myocarditis, described as an inflammatory infiltration of the myocardium with necrosis and/or degeneration of cardiomyocytes, is likely caused by a wide variety of infectious organisms, autoimmune disorders, and exogenous agents [1]. The major long-term consequence of myocarditis is inflammatory dilated cardiomyopathy (DCMi) with chronic heart failure. Persistent DCMi usually has a progressive course under standard heart failure therapy. At present, specific treatment options are not yet available or have not yet been proofed in major trials. In virus-negative patients, immunosuppression [2] is an option whereas in patients with cardiac virus persistence, the role of immunoglobulin or immunomodulation with interferon (IFN) [3] is under investigation. Finally, immunoabsorption [4] could be an option in favour of the idea that also autoantibodies may play a role in a subset of DCMi populations. However, the search for alternative therapies is still open.
There is accumulating experimental [5, 6] and clinical support [7–9] for the application of cellular transplantation as a strategy to improve myocardial function. Mesenchymal stem cells (MSCs) have antiapoptotic [10], antifibrotic [11], and proangiogenic [12] features. They have the advantage over other stem cells that they have immunomodulatory properties [13], which make them an attractive cell source for the treatment of inflammatory cardiomyopathy, given the importance of the inflammatory component in this disorder. Application of MSCs in experimental models of Coxsackievirus- (CVB-3) induced myocarditis [14] and autoimmune myocarditis [5, 15], attenuated myocardial injury and dysfunction. Both intramyocardial and intravenous administration of MSCs were successful. However, the MSC-mediated reduction in cardiac injury in the acute model of CVB3-induced myocarditis was not paralleled with a decrease in cardiac viral load, disabling a view of the final outcome on the long term. Therefore, as long as evidence in models of chronic virus-induced myocarditis are lacking, the use of MSCs could be more important or restricted for the treatment of nonviral inflammatory cardiomyopathies.
Systemic delivery of MSCs requires efficient homing of MSCs to the place of injury. This review gives an overview about how inflammation triggers the functionality of MSCs and how it induces cardiac homing. The impact of inflammation/cytokine expression on the different aspects of homing, including chemokine-chemokine receptor interactions, adhesion on endothelial cells, transendothelial migration, and invasion through the extracellular matrix, is outlined. Finally, the potential of intravenous application of MSCs by inflammatory cardiomyopathy is discussed.
1.1. Mesenchymal Stem Cells
MSCs, which can be alternatively referred to as multipotent mesenchymal stromal cells or marrow stromal cells, are a heterogeneous population of cells which can proliferate in vitro as plastic-adherent cells, have fibroblast-like morphology, form colonies in vitro, and can differentiate into bone, cartilage, adipose, and stromal tissues [16, 17]. MSCs are positive for many characteristic markers including CD29, CD44, CD71, CD90, CD106, CD120a, CD124, SH2, SH3, and SH4, and negative for CD14, CD34, and CD45, which are specific markers of hematopoietic stem cells [18]. MSCs are rare in bone marrow, representing ~1 in 10,000 nucleated cells.
MSCs have the capacity to differentiate into cardiomyocytes, endothelial cells, and smooth muscle cells. Quevedo et al. [6] could demonstrate via using female swine and injecting male allogeneic MSCs 12 weeks after myocardial infarction that engrafted MSCs differentiated into cardiomyocytes as ascertained by colocalization with GATA-4, Nkx2.5, and α-sarcomeric actin. In addition, Ypos MSCs exhibited vascular smooth muscle and endothelial cell differentiation, contributing to large and small vessel formation. Besides their trilineage differentiation capacity [6], it is believed that the cardioprotective effects of MSC are predominantly due to facilitating endogenous repair processes via their antifibrotic, immunomodulatory, antiapoptotic and proangiogenic features.
In brief, MSCs have the capacity to reduce cardiac fibroblast proliferation [19] and collagen I and III expression [20], and they are able to promote matrix metalloproteinase secretion by cardiac fibroblasts [11], leading to reduced cardiac ventricular fibrosis. These effects may at least partially be mediated via the release of antifibrotic factors such as hepatocyte growth factor [21].
An emerging body of evidence indicates that MSCs possess immunomodulatory properties, affecting T cells [13], dendritic cells [22], B cells [23], and natural killer cells [24]. Immunosuppression occurs hereby most effectively under conditions in which MSCs make physical contact with allogeneic tissue and release soluble factors, including interleukin- (IL-)10, transforming growth factor- (TGF-)β, Indoleamine 2,3-dioxygenase [25], and prostaglandin E2. Moreover, the inflammatory environment/condition plays an important role in the ability of MSCs to exert their immunosuppressive effects. In fact, in the absence of T cell activation or exogenous inflammatory cytokines, MSCs actually prolong the survival of lymphocytes [26], supporting the immunomodulatory nature of MSCs.
Studies in models of diabetic cardiomyopathy [10], myocardial infarction [27], and myocarditis [14] demonstrated that MSC application leads to less cardiac apoptosis, which might be in part due to improved angiogenesis [27]. We demonstrated in vitro via coculture of MSCs with CVB3-infected HL-1 cardiomyocytes that MSCs have intrinsic antiapoptotic features, and that these effects are nitric oxide- (NO-)dependent [14]. Furthermore, Nagaya et al. [5] showed that cultured MSCs secreted large amounts of the angiogenic, antiapoptotic, and mitogenic factors vascular endothelial growth factor, hepatocyte growth factor, adrenomedullin, and insulin-like growth factor-1. Finally, MSCs also have proangiogenic effects: they can differentiate into endothelial cells [28], increase in vitro tube formation [12], and secrete proangiogenic factors including vascular endothelial growth factor [29, 30]. Their proangiogenic features have been demonstrated in models of peripheral hindlimb ischemia [31] and myocardial infarction [27, 32].
1.2. Inflammatory Cytokines Induce Mesenchymal Stem Cell Activation
Various reports have demonstrated the importance of IFN-γ for the activation of MSCs [13, 14, 33]. Sheng et al. [33] showed that IFN-γ, a well-known proinflammatory cytokine produced by activated T cells, plays an important role in priming the immunosuppressive property of MSCs. Mechanistically, IFN-γ acts directly on MSCs and leads to upregulation of B7-H1, an inhibitory surface molecule in these stem cells. MSCs primed by activated T cells derived from IFN-γ−/− mouse exhibited dramatically reduced ability to suppress T cell proliferation, a defect that could be rescued by supplying exogenous IFN-γ. Moreover, siRNA-mediated knockdown of B7-H1 in MSCs abolished immunosuppression by these cells. In agreement Ren et al. [13] found that the immunosuppressive function of MSCs is elicited by IFN-γ and the concomitant presence of any of three other proinflammatory cytokines, tumor necrosis factor-α (TNF-α), IL-1α, or IL-1β. These cytokine combinations provoke the expression of high levels of several chemokines and inducible nitric oxide synthase (iNOS) by MSCs. Chemokines drive T cell migration into the proximity of MSCs, where T cell responsiveness is suppressed by NO. This cytokine-induced immunosuppression is absent in MSCs derived from iNOS−/− or IFN-γR1−/− mice. Administration of wild-type MSCs, but not of IFN-γR1−/− MSCs, prevented graft-versus-host disease in mice, an effect reversed by anti-IFN-γ or iNOS inhibitors. Therefore, proinflammatory cytokines are required to induce immunosuppression by MSCs through the concerted action of chemokines and NO. Moreover, Ren et al. [34] found that intracellular adhesion molecule- (ICAM-)1 and vascular cell adhesion molecule- (VCAM-)1 were critical for MSC-mediated immunosuppression. When MSCs were cocultured with T cells in the presence of T cell antigen receptor activation, they significantly upregulated the adhesive capability of T cells due to the increased expression of ICAM-1 and VCAM-1. The greater the expression of ICAM-1 and VCAM-1 by MSCs was, the greater the immunosuppressive capacity that they exhibited. Furthermore, ICAM-1 and VCAM-1 were found to be inducible by the concomitant presence of IFN-γ and inflammatory cytokines (TNF-α, IL-1). Finally, MSC-mediated immunosuppression was significantly reversed in vitro and in vivo when the adhesion molecules were genetically deleted or functionally blocked, which corroborated the importance of cell-cell contact in immunosuppression by MSCs. These findings not only reveal a novel function of adhesion molecules in immunoregulation by MSCs, but also provide new insights for the clinical studies of antiadhesion therapies in various immune disorders.
Furthermore, we demonstrated that MSCs not only require IFN-γ to exert their immunomodulatory properties, but also to perform their antiapoptotic, antioxidative, and antiviral features [14]. Coculture of MSCs with Coxsackievirus B3-infected HL-1 cardiomyocytes reduced cardiomyocyte apoptosis, oxidative stress, and viral progeny release, an effect which was reduced in the presence of an IFN-γ antibody [14]. In line with Oh et al. [35], we could also demonstrate that MSCs request IFN-γ for the production of NO, which is known for its antiapoptotic [36] and antiviral properties [37]. Furthermore, Kemp et al. [38] demonstrated that the secretion of the antioxidative enzyme superoxide dismutase 3 by MSCs is regulated synergistically by TNF-α and IFN-γ, rather than through direct exposure to reactive oxygen species.
1.3. Cardiac Homing of Mesenchymal Stem Cells
Homing is a multistep process which involves chemokine-chemokine receptor interactions, adhesion to endothelial cells, transmigration through the endothelial vasculature, cytoskeleton rearrangement, and adhesive interactions with the extracellular matrix [39]. Numerous in vivo studies have shown that MSCs have the capability to migrate from the blood, across endothelial cells, into damaged tissues.
MSCs express a variety of chemokines [40–42], including CXCR4 and CCR2, the receptors for stromal cell-derived factor- (SDF-)1α and monocyte chemoattractant protein- (MCP-)1, respectively. SDF-1α is a CXC chemokine known to play a critical role in the trafficking of hematopoietic cells and stem cell progenitors and in maintaining hematopoietic stem cell niches in bone marrow [43]. SDF-1α expression has been shown to increase under hypoxic conditions [44] and thus may serve to attract stem cells to sites of tissue injury. Its expression is significantly upregulated in experimental rat and mouse models of myocardial infarction [45–47], in cardiac tissue of patients with myocardial ischemia [48], and in experimental CVB3-induced myocarditis (own unpublished data). Abbott et al. [47] could demonstrate that administration of AMD3100, which specifically blocks the binding of SDF-1α to its endogenous receptor CXCR4, diminished bone marrow cells recruitment after myocardial infarction, strongly suggesting a requirement for SDF-1α in bone marrow cell recruitment to the infarcted heart. Forced expression of SDF-1α in the heart by adenoviral gene delivery 48 hours after myocardial infarction doubled bone marrow cells recruitment over myocardial infarction. However, gene transfer of SDF-1α did not enhance recruitment in the absence of myocardial infarction, suggesting that SDF-1α can augment, but is not singularly sufficient for bone marrow cells recruitment to the heart. The importance of the SDF-CXCR4 axis in cardiac homing of MSCs has been demonstrated by CXCR-overexpressing MSCs showing improved targeted cardiac migration and engraftment of MSCs [49].
MCP-1 is upregulated upon myocardial infarction [50] and is induced in experimental CVB3-induced myocarditis [51], by which its cardiac expression correlates with the infiltration of mononuclear cells in the heart. Though, the importance of cardiac MCP-1 expression for the cardiac homing of MSCs follows from a study of Belema-Bedada et al. [52] which demonstrated that directed expression of MCP-1 in the myocardium led to specific homing of MSCs to the heart. In addition, they could show that MCP-1-mediated MSC migration depends on CCR2/FROUNT signaling in the MSC, by which the adapter molecule FROUNT is required for polarization, cytoskeletal reorganization, and clustering of CCR2 on the cell surface of MSCs. Besides the induction of cardiac chemokine expression, inflammation also triggers the expression of chemokine receptors on the MSCs. Only a small proportion of MSCs strongly expresses functionally active CXCR4 receptor [53]. Shi et al. [54] demonstrated that a cocktail of cytokines resulted in upregulation of both cell surface and intracellular CXCR4, increasing in vitro migration capacity to SDF-1α and homing to the bone marrow of irradiated NOD/SCID mice. Croitoru-Lamoury et al. [55] showed that TNF-α, IFN-γ, and IFN-β influence the expression of chemokines and their receptors in MSCs. This was further confirmed by Ponte et al. [40] demonstrating that TNF-α stimulation of MSCs increases chemotaxis towards chemokines but not towards growth factors. These data suggest that the mobilization of MSCs and their subsequent homing to injured tissues may depend on the systemic and local inflammatory state.
Numerous in vivo studies have shown that MSCs have the capacity to migrate from the blood, across endothelial cells into injured tissues. Many of the molecules, including integrins, selectins, and chemokine receptors, known to be involved in the tethering, rolling, adhesion, and transmigration of leukocytes from the bloodstream into tissues are also expressed on MSCs. MSCs express the integrins α1, α2, α3, α4, α5, αv, β1, β3, and β4, and approximately 50% of human MSCs are thought to express the integrin very late antigen- (VLA-)4 (α4β1, CD49d) [56]. The importance of VLA-4 in the adherence of human MSCs to endothelial cells, under shear flow conditions, follows from the observation that the use of a neutralizing antibody to this integrin decreased MSC binding to endothelial cells. On the other hand, it was observed that treating endothelial cells with a blocking antibody to its counterpart adhesion molecule, VCAM-1, induced a similar decrease in MSC adherence. Both findings indicate the importance of the VLA-4/VCAM-1 axis for firm MSC adherence to endothelial cells.
MSCs also express the adhesion molecules VCAM-1, ICAM-1, ICAM-3, ALCAM, and endoglin/CD105 [57]. Segers et al. [58] demonstrated that activation of both cardiac microvascular endothelial cells and MSCs with TNF-α or IL-1β before adhesion, increased the adhesion of MSCs to endothelial cells under shear stress concentration dependently. In agreement, in vivo, activation of MSCs with TNF-α before injection significantly enhanced cardiac homing of MSCs. TNF-α-induced adhesion could be completely blocked by pretreating either cardiac microvascular endothelial cells or MSCs with anti-VCAM-1 monoclonal antibodies but not by anti-ICAM-1 antibodies. Therefore, the adhesion of circulating MSCs in the heart appears to be an endothelium-dependent process and to be sensitive to modulation by activators of both MSCs and the endothelium. Inflammation and the expression of VCAM-1 but not of ICAM-1 on both MSCs and cardiac microvascular endothelial cells appear to have a regulatory effect on MSC homing in the heart. Furthermore, Rüster et al. [56] showed that MSCs display a coordinated rolling and adhesion behavior on endothelial cells similar to peripheral blood mononuclear cells for which they equally required P-selectin and VCAM-1/VLA-4. This involved rapid extension of podia, rolling, and subsequent firm adhesion was increased when endothelial cells were prestimulated with TNF-α.
A comparison between the cell adhesion molecule expression profile of the mobilized, circulating MSCs, and tissue-derived MSCs may provide further insight into the potential mechanisms of MSC homing.
The expression of chemokine receptors [49, 59] and integrins [60, 61] on MSCs is not only beneficial for cardiac migration/adhesion, but also for MSC survival and cardiac engraftment. However, the impact of inflammation on MSC survival and cardiac engraftment is beyond the scope of this review.
Upon adhesion, MSCs migrate through the endothelium. At present, little is known about the mechanism of MSC transendothelial migration and which adhesion molecules are involved. MSCs do not express PECAM-1/CD31, which is required in leukocyte transmigration across the endothelium. So, although the rolling and adhesion of MSCs on the endothelium are similar to those of leukocytes, it seems that MSCs use different adhesion molecules for transendothelial migration. Schmidt et al. [62] investigated the mechanism of transendothelial migration in vitro using a coculture of MSCs on an endothelial monolayer and analyzed direct interactions. An increasing flattened morphology of the MSCs, starting 30 minutes upon coculture was followed by total integration into the monolayer after 2 hours. In vivo, using isolated heart perfusions with gold-labelled MSCs and electron microscopy, they detected that MSCs exhibited direct cell-cell contacts. Tight junctions between the endothelial cells became abolished resulting in a distinct split between the cells. MSCs developed tight cell-cell contacts and became integrated into the endothelial wall of the capillary vessel. Finally, confocal laser scanning microscopy revealed that 30 minutes of MSC perfusion was sufficient to observe transmigration across the endothelium of approximately 30% of the cells. This percentage rose to 50% after 60 minutes and remained nearly unchanged thereafter. This finding is important in view of exposure times of MSCs in clinical settings. Steingen et al. [63] demonstrated that the time course of adhesion, integration, and transmigration depends on the endothelial phenotype and is most effective in venous vessels of the myocardium. Furthermore, they showed that transmigration not only requires the interaction of VCAM-1 and VLA-4, as verified by blocking experiments, but also triggers the clustering of beta1 integrins.
After transmigrating across the endothelium, MSCs have to traffic through the extracellular matrix (ECM) to first infiltrate sites of tissue damage. MSCs strongly express and synthesize matrix metalloproteinase 2 (MMP-2), membrane type 1 MMP (MT1-MMP), tissue inhibitor of metalloproteinase 1 (TIMP-1), and TIMP-2 [64]. In situ zymographies infer the activation of gelatinases at sites of MSC invasion into myocardial tissue [63]. The ability of MSCs to traverse reconstituted human basement membranes was effectively blocked in the presence of synthetic MMP inhibitors. Detailed studies by RNA interference revealed that gene knock-down of MMP-2, MT1-MMP, or TIMP-2 substantially impaired MSC invasion whereas silencing of TIMP-1 enhanced cell migration, indicating opposing roles of both TIMPs in this process. Moreover, the inflammatory cytokines TGF-β1, IL-1β, and TNF-α upregulated MMP-2, MT1-MMP, and/or MMP-9 production in these cells, resulting in a strong stimulation of chemotactic migration through ECM whereas the chemokine SDF-1α exhibited minor effects on MMP/TIMP expression and cell invasion [64].
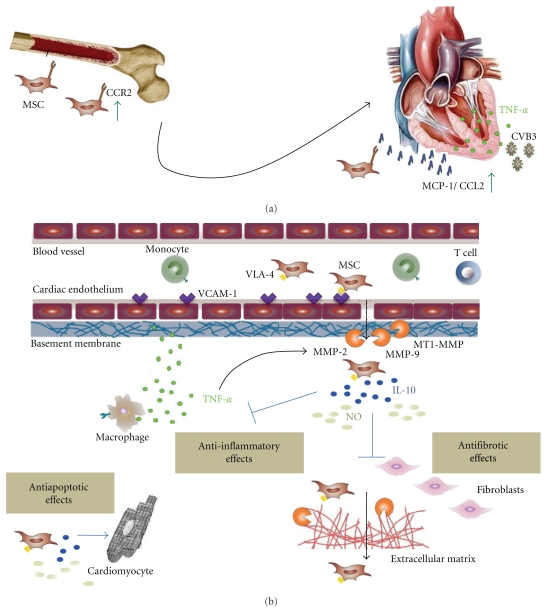
In summary, the homing of MSCs to the heart in inflammatory cardiomyopathy is triggered on different levels as outlined in Figure 1.
Figure 1.
Proposed pathways how inflammation induces cardiac homing of mesenchymal stem cells. (a) TNF-α and/or Coxsackievirus B3 (CVB3) trigger the induction of cardiac expression of monocyte chemoattractant protein-1 (MCP-1) also known as CCL2. At the same time, the expression of the corresponding chemokine receptor CCR2 is induced on mesenchymal stem cells (MSCs), stimulating cardiac migration. (b) TNF-α induces vascular cellular adhesion molecule- (VCAM-)1 expression on the cardiac endothelium. The VLA-4/VCAM-1 axis is important for firm MSC adherence to endothelial cells. Next, MSCs transmigrate through the endothelium which also requires the interaction of VCAM-1 and VLA-4. Finally, MSCs invade through the basement membrane and the extracellular matrix (ECM) via their secretion of matrix metalloproteinase- (MMP-)2, MMP-9, and membrane type 1 MMP (MT1-MMP), which are upregulated by TNF-α. In the heart, MSCs can exert cardioprotective effects, including their anti-inflammatory, antifibrotic, and antiapoptotic features, among others, via interleukin- (IL-)10 and nitric oxide (NO).
1.4. Route of Administration
Since MSCs have the capacity to home to injured tissue, intravenous application may be considered for inflammatory cardiomyopathy and might even have advantages over intracoronary or transendocardial application despite their reduced cardiac engraftment [65]. Indeed, via intravenous application, also the spleen could be reached. The spleen is the reservoir of monocytes which are recruited into the inflammatory heart via interaction of the chemokine MCP-1, released by the heart, with its cognate receptor CCR2, present on monocytes [51]. In case of myocardial infarction, 40% to 70% of monocytes acutely recruited to the infarct originate from a splenic reservoir [66]. Retrieved in the heart, cells then assume a central role in orchestrating the healing wound [67]. However, an excessive inflammatory response is deleterious. As shown for myocardial infarction where splenectomy or the arrested release of monocytes from the splenic reservoir via angotensin-converting enzyme inhibition reduced the recruitment into the healing infarct and improved the myocardial infarction outcome [68], an anti-inflammatory impact via MSCs on the splenic monocyte reservoir in the context of inflammatory cardiomyopathy could be further beneficial in addition to the direct MSC-mediated cardioprotective effects, favoring intravenous application.
In case of Coxsackievirus B3-induced inflammatory cardiomyopathy, the filtration of viral particles during acute infection takes place in the spleen [69] and pre-B, B cells, CD4+ helper T cells, and Mac-1+ macrophages are infected [70]. Homing of MSCs to the spleen might influence the condition of immune cells, including their activity [13], apoptosis [71], as well as their viral infection [14, 70] and potentially also their homing capacity [13]. The MSC-mediated dissemination of infected immune cells in the spleen might therefore reduce the infiltration of infected immune cells in the heart and consequently lead to a decrease in cardiac damage.
Finally, the ease of application, as well as the finding that MSCs transmigration is most effective in venous vessels of the myocardium [63], favor the intravenous route of administration.
2. Conclusion and Perspectives
In conclusion, consistent evidence from in vitro and in vivo experimental studies support a promoted cardiac homing of MSCs under inflammatory cardiomyopathy. This finding together with the activation of MSCs via cytokines, and the immunomodulatory properties of MSCs, make MSCs an attractive cell source for the treatment of inflammatory cardiomyopathy. The investigation of endogenous cardiac homing in patients with inflammatory cardiomyopathy versus control patients, currently ongoing in our working group, is needed to confirm these experimental findings in a patient setting and will underscore the potential use of intravenous application of MSCs for the treatment of inflammatory cardiomyopathy. Finally, the importance of adhesion molecules for cardiac homing of MSCs and the immunosuppressive effect of MSCs, position the use of antiadhesion therapies for inflammatory cardiomyopathy in perspective.
Acknowledgments
This paper was supported by the Berlin-Brandenburg Center for Regenerative Therapies—BCRT (Bundesministerium für Bildung und Forschung—0313911) to C. Tschöpe, and by the DFG Sonderforschungsbereich Transregio-19 B5 to C. Tschöpe.
Abbreviations
- CVB:
Coxsackievirus
- DCM:
Dilated cardiomyopathy
- DCMi:
Inflammatory dilated cardiomyopathy
- ECM:
Extracellular matrix
- IFN:
Interferon
- IL:
Interleukin
- iNOS:
Inducible nitric oxide synthase
- MMP:
Matrix metalloproteinase
- MT1-MMP:
Membrane type 1-MMP
- MSC:
Mesenchymal stem cell
- NO:
Nitric oxide
- SDF-1α:
Stromal derived factor-1 alpha
- TIMP:
Tissue inhibitor of metalloproteinase
- TNF-α:
Tumor necrosis factor-alpha
- TGF:
Transforming growth factor
- VCAM-1:
Vascular cell adhesion molecule-1
- VLA-4:
Very late antigen-4.
References
- 1.Aretz HT, Billingham ME, Edwards WD, et al. Myocarditis. A histopathologic definition and classification. The American Journal of Cardiovascular Pathology. 1987;1(1):3–14. [PubMed] [Google Scholar]
- 2.Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. European Heart Journal. 2009;30(16):1995–2002. doi: 10.1093/eurheartj/ehp249. [DOI] [PubMed] [Google Scholar]
- 3.Kühl U, Pauschinger M, Schwimmbeck PL, et al. Interferon-β treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation. 2003;107(22):2793–2798. doi: 10.1161/01.CIR.0000072766.67150.51. [DOI] [PubMed] [Google Scholar]
- 4.Staudt A, Herda LR, Trimpert C, et al. Fcgamma-receptor IIa polymorphism and the role of immunoadsorption in cardiac dysfunction in patients with dilated cardiomyopathy. Clinical Pharmacology and Therapeutics. 2010;87(4):452–458. doi: 10.1038/clpt.2009.246. [DOI] [PubMed] [Google Scholar]
- 5.Nagaya N, Kangawa K, Itoh T, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112(8):1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 6.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(33):14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yerebakan C, Kaminski A, Liebold A, Steinhoff G. Safety of intramyocardial stem cell therapy for the ischemic myocardium: results of the Rostock trial after 5-year follow-up. Cell Transplantation. 2007;16(9):935–940. doi: 10.3727/096368907783338280. [DOI] [PubMed] [Google Scholar]
- 8.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. Journal of the American College of Cardiology. 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Ramshorst J, Bax JJ, Beeres SLMA, et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. Journal of the American Medical Association. 2009;301(19):1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 10.Li JH, Zhang N, Wangi JA. Improved anti-apoptotic and anti-remodeling potency of bone marrow mesenchymal stem cells by anoxic pre-conditioning in diabetic cardiomyopathy. Journal of Endocrinological Investigation. 2008;31(2):103–110. doi: 10.1007/BF03345575. [DOI] [PubMed] [Google Scholar]
- 11.Mias C, Lairez O, Trouche E, et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27(11):2734–2743. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- 12.Sorrell JM, Baber MA, Caplan AI. Influence of adult mesenchymal stem cells on in vitro vascular formation. Tissue Engineering—Part A. 2009;15(7):1751–1761. doi: 10.1089/ten.tea.2008.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 14.van Linthout S, Savvatis K, Miteva K, et al. Mesenchymal stem cells improve murine acute Coxsackievurs B3-induced myocarditis. European Heart Journal. 2010 doi: 10.1093/eurheartj/ehq467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohnishi S, Yanagawa B, Tanaka K, et al. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. Journal of Molecular and Cellular Cardiology. 2007;42(1):88–97. doi: 10.1016/j.yjmcc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 17.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 18.Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13(1):69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- 19.Ohnishi S, Sumiyoshi H, Kitamura S, Nagaya N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Letters. 2007;581(21):3961–3966. doi: 10.1016/j.febslet.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Xu Z, Xu Y, Cui G. Selective down-regulation of extracellular matrix gene expression by bone marrow derived stem cell transplantation into infarcted myocardium. Circulation Journal. 2005;69(10):1275–1283. doi: 10.1253/circj.69.1275. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Zhang Y, Li Y, et al. Mesenchymal stem cell transplantation attenuates cardiac fibrosis associated with isoproterenol-induced global heart failure. Transplant International. 2008;21(12):1181–1189. doi: 10.1111/j.1432-2277.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- 22.Jiang XX, Zhang YI, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 23.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-γ in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 24.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 25.Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 26.Xu G, Zhang Y, Zhang L, Ren G, Shi Y. The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochemical and Biophysical Research Communications. 2007;361(3):745–750. doi: 10.1016/j.bbrc.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuleri KH, Amado LC, Boyle AJ, et al. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. American Journal of Physiology. 2008;294(5):H2002–H2011. doi: 10.1152/ajpheart.00762.2007. [DOI] [PubMed] [Google Scholar]
- 28.Oswald J, Boxberger S, Jørgensen B, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22(3):377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 29.Hung SC, Pochampally RR, Chen SYC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25(9):2363–2370. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- 30.Kinnaird T, Burnett ES, Shou M, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 31.Prather WR, Toren A, Meiron M, Ofir R, Tschope C, Horwitz E. The role of placental-derived adherent stromal cell (PLX-PAD) in the treatment of critical limb ischemi. Cytotherapy. 2009;11(4):427–434. doi: 10.1080/14653240902849762. [DOI] [PubMed] [Google Scholar]
- 32.Nagaya N, Fujii T, Iwase T, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. American Journal of Physiology. 2004;287(6):H2670–H2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 33.Sheng H, Wang Y, Jin Y, et al. A critical role of IFNγ in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Research. 2008;18(8):846–857. doi: 10.1038/cr.2008.80. [DOI] [PubMed] [Google Scholar]
- 34.Ren G, Zhao X, Zhang L, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. Journal of Immunology. 2010;184(5):2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh I, Ozaki K, Sato K, et al. Interferon-γ and NF-κB mediate nitric oxide production by mesenchymal stromal cells. Biochemical and Biophysical Research Communications. 2007;355(4):956–962. doi: 10.1016/j.bbrc.2007.02.054. [DOI] [PubMed] [Google Scholar]
- 36.Razavi HM, Hamilton JA, Feng Q. Modulation of apoptosis by nitric oxide: implications in myocardial ischemia and heart failure. Pharmacology and Therapeutics. 2005;106(2):147–162. doi: 10.1016/j.pharmthera.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Zell R, Markgraf R, Schmidtke M, et al. Nitric oxide donors inhibit the coxsackievirus B3 proteinases 2A and 3C in vitro, virus production in cells, and signs of myocarditis in virus-infected mice. Medical Microbiology and Immunology. 2004;193(2-3):91–100. doi: 10.1007/s00430-003-0198-6. [DOI] [PubMed] [Google Scholar]
- 38.Kemp K, Gray E, Mallam E, Scolding N, Wilkins A. Inflammatory cytokine induced regulation of superoxide dismutase 3 expression by human mesenchymal stem cells. Stem Cell Reviews and Reports. 2010;6(4):548–559. doi: 10.1007/s12015-010-9178-6. [DOI] [PubMed] [Google Scholar]
- 39.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 40.Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 41.Ringe J, Strassburg S, Neumann K, et al. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. Journal of Cellular Biochemistry. 2007;101(1):135–146. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- 42.Honczarenko M, Le YI, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24(4):1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 43.Kucia M, Jankowski K, Reca R, et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. Journal of Molecular Histology. 2004;35(3):233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 44.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature Medicine. 2004;10(8):858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 45.Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. The Lancet. 2003;362(9385):697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 46.Pillarisetti K, Gupta SK. Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1): SDF-1 α mRNA is selectively induced in rat model of myocardial infarction. Inflammation. 2001;25(5):293–300. doi: 10.1023/a:1012808525370. [DOI] [PubMed] [Google Scholar]
- 47.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1α plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110(21):3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 48.Yamani MH, Ratliff NB, Cook DJ, et al. Peritransplant ischemic injury is associated with up-regulation of stromal cell-derived factor-1. Journal of the American College of Cardiology. 2005;46(6):1029–1035. doi: 10.1016/j.jacc.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 49.Zhang D, Fan GC, Zhou X, et al. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. Journal of Molecular and Cellular Cardiology. 2008;44(2):281–292. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schenk S, Mal N, Finan A, et al. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25(1):245–251. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- 51.Shen Y, Xu W, Chu YIW, Wang Y, Liu QS, Xiong SID. Coxsackievirus group B type 3 infection upregulates expression of monocyte chemoattractant protein 1 in cardiac myocytes, which leads to enhanced migration of mononuclear cells in viral myocarditis. Journal of Virology. 2004;78(22):12548–12556. doi: 10.1128/JVI.78.22.12548-12556.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belema-Bedada F, Uchida S, Martire A, Kostin S, Braun T. Efficient homing of multipotent adult mesenchymal stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem Cell. 2008;2(6):566–575. doi: 10.1016/j.stem.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104(9):2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 54.Shi M, Li J, Liao L, et al. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92(7):897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- 55.Croitoru-Lamoury J, Lamoury FMJ, Zaunders JJ, Veas LA, Brew BJ. Human mesenchymal stem cells constitutively express chemokines and chemokine receptors that can be upregulated by cytokines, IFN-β, and copaxone. Journal of Interferon and Cytokine Research. 2007;27(1):53–64. doi: 10.1089/jir.2006.0037. [DOI] [PubMed] [Google Scholar]
- 56.Rüster B, Göttig S, Ludwig RJ, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108(12):3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 57.Krampera M, Pizzolo G, Aprili G, Franchini M. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone. 2006;39(4):678–683. doi: 10.1016/j.bone.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 58.Segers VFM, Van Riet I, Andries LJ, et al. Mesenchymal stem cell adhesion to cardiac microvascular endothelium: activators and mechanisms. American Journal of Physiology. 2006;290(4):H1370–H1377. doi: 10.1152/ajpheart.00523.2005. [DOI] [PubMed] [Google Scholar]
- 59.Huang J, Zhang Z, Guo J, et al. Genetic modification of mesenchymal stem cells overexpressing ccr1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circulation Research. 2010;106(11):1753–1762. doi: 10.1161/CIRCRESAHA.109.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song H, Chang W, Lim S, et al. Tissue transglutaminase is essential for integrin-mediated survival of bone marrow-derived mesenchymal stem cells. Stem Cells. 2007;25(6):1431–1438. doi: 10.1634/stemcells.2006-0467. [DOI] [PubMed] [Google Scholar]
- 61.Song SW, Chang W, Song BW, et al. Integrin-linked kinase is required in hypoxic mesenchymal stem cells for strengthening cell adhesion to ischemic myocardium. Stem Cells. 2009;27(6):1358–1365. doi: 10.1002/stem.47. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt A, Ladage D, Steingen C, et al. Mesenchymal stem cells transmigrate over the endothelial barrier. European Journal of Cell Biology. 2006;85(11):1179–1188. doi: 10.1016/j.ejcb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 63.Steingen C, Brenig F, Baumgartner L, Schmidt J, Schmidt A, Bloch W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. Journal of Molecular and Cellular Cardiology. 2008;44(6):1072–1084. doi: 10.1016/j.yjmcc.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 64.Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109(9):4055–4063. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- 65.Freyman T, Polin G, Osman H, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. European Heart Journal. 2006;27(9):1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 66.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325(5940):612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovascular Research. 2002;53(1):31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 68.Leuschner F, Panizzi P, Chico-Calero I, et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circulation Research. 2010;107(11):1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matteucci D, Toniolo A, Conaldi PG. Systemic lymphoid atrophy in coxsackievirus B3-infected mice: effects of virus and immunopotentiating agents. Journal of Infectious Diseases. 1985;151(6):1100–1108. doi: 10.1093/infdis/151.6.1100. [DOI] [PubMed] [Google Scholar]
- 70.Klingel K, Stephan S, Sauter M, et al. Pathogenesis of murine enterovirus myocarditis: virus dissemination and immune cell targets. Journal of Virology. 1996;70(12):8888–8895. doi: 10.1128/jvi.70.12.8888-8895.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19(9):1597–1604. doi: 10.1038/sj.leu.2403871. [DOI] [PubMed] [Google Scholar]