Abstract
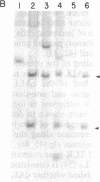
To examine the distribution of rearrangements of the gamma- and beta-chain T-cell receptor (TCR) genes in T- and non-T acute lymphoblastic leukemias (ALLs), and potentially to determine which genes rearrange first in ontogeny, we analyzed high molecular weight DNA from 102 patients with acute leukemia. Rearranged gamma- and beta-chain genes were found in all T-cell ALLs (22/22) examined. Overall, 27% (18/66) of B-lineage ALLs had beta-chain gene rearrangements, and 41% (24/58) had gamma-chain gene rearrangements, but the distribution of rearranged genes varied according to the stage of B-cell differentiation. The gamma-chain genes were rearranged in 11% (1/9) of the B-lineage patients negative for the common acute lymphoblastic leukemia antigen (cALLA) and 50% (23/46) of cALLA+ ALL patients, while the beta-chain genes were not rearranged in any of the 7 cALLA- ALL patients examined but were rearranged in 32% (18/56) of the cALLA+ patients. Neither TCR gene was found to be rearranged in acute nonlymphoid leukemia patients (0/12) or in patients with B-cell (surface immunoglobulin-positive) leukemia (0/3). Of the 44 cALLA+ patients in which a direct comparison of gamma- and beta-chain gene rearrangements could be made, 34% had both genes rearranged, 16% had only gamma-chain gene rearrangements, and the remaining 50% had both genes in the germ-line configuration. beta-Chain rearrangements have not been found in the absence of gamma-chain rearrangements, thus supporting a proposed hierarchy of TCR gene rearrangements. A provocative finding was that only a small percentage (11%) of the patients with cALLA- B precursor cell ALLs had rearranged TCR genes, while 50% of the cALLA+ leukemia patients had at least gamma-chain rearrangement, raising a question as to whether indeed cALLA- cells are precursors to cALLA+ cells. Interestingly, 18% (2/11) of the cytoplasmic immunoglobulin (cIg)-positive cALLA+ (pre-B) ALLs involved TCR gene rearrangements, compared to 60% (21/35) of the cIg-negative cases, suggesting the possibility that the majority of functional B cells are derived from the cALLA+ pool that contains immunoglobulin but not TCR gene rearrangements.
Full text
PDF
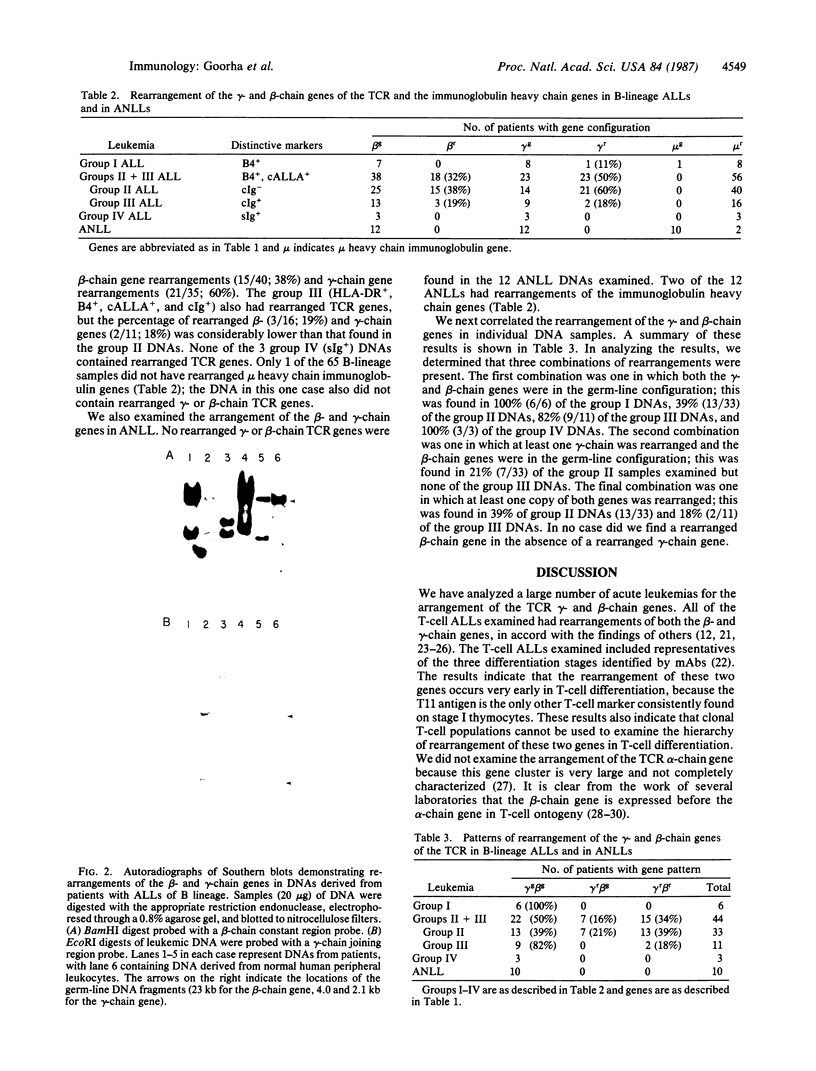


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuto O., Hussey R. E., Fitzgerald K. A., Protentis J. P., Meuer S. C., Schlossman S. F., Reinherz E. L. The human T cell receptor: appearance in ontogeny and biochemical relationship of alpha and beta subunits on IL-2 dependent clones and T cell tumors. Cell. 1983 Oct;34(3):717–726. doi: 10.1016/0092-8674(83)90528-7. [DOI] [PubMed] [Google Scholar]
- Arnold A., Cossman J., Bakhshi A., Jaffe E. S., Waldmann T. A., Korsmeyer S. J. Immunoglobulin-gene rearrangements as unique clonal markers in human lymphoid neoplasms. N Engl J Med. 1983 Dec 29;309(26):1593–1599. doi: 10.1056/NEJM198312293092601. [DOI] [PubMed] [Google Scholar]
- Bank I., DePinho R. A., Brenner M. B., Cassimeris J., Alt F. W., Chess L. A functional T3 molecule associated with a novel heterodimer on the surface of immature human thymocytes. Nature. 1986 Jul 10;322(6075):179–181. doi: 10.1038/322179a0. [DOI] [PubMed] [Google Scholar]
- Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976 Aug;33(4):451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Dialynas D. P., Strominger J. L., Smith J. A., Owen F. L., Seidman J. G., Ip S., Rosen F., Krangel M. S. Identification of a putative second T-cell receptor. Nature. 1986 Jul 10;322(6075):145–149. doi: 10.1038/322145a0. [DOI] [PubMed] [Google Scholar]
- Cheng G. Y., Minden M. D., Toyonaga B., Mak T. W., McCulloch E. A. T cell receptor and immunoglobulin gene rearrangements in acute myeloblastic leukemia. J Exp Med. 1986 Feb 1;163(2):414–424. doi: 10.1084/jem.163.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. K., Kissonerghis A. M., Dunne M. J., Watson C. J., Rigby P. W., Owen M. J. Transcripts from an aberrantly re-arranged human T-cell receptor beta-chain gene. EMBO J. 1985 May;4(5):1211–1215. doi: 10.1002/j.1460-2075.1985.tb03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M. P., Bongiovanni K. F., Kaulfersch W., Quertermous T., Seidman J. G., Hershfield M. S., Kurtzberg J., Haynes B. F., Davis M. M., Waldmann T. A. Immunoglobulin and T-cell receptor gene rearrangement and expression in human lymphoid leukemia cells at different stages of maturation. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8759–8763. doi: 10.1073/pnas.83.22.8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Foa R., Migone N., Saitta M., Fierro M. T., Giubellino M. C., Lusso P., Cordero di Montezemolo L., Miniero R., Lauria F. Different stages of B cell differentiation in non-T acute lymphoblastic leukemia. J Clin Invest. 1984 Nov;74(5):1756–1763. doi: 10.1172/JCI111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furley A. J., Mizutani S., Weilbaecher K., Dhaliwal H. S., Ford A. M., Chan L. C., Molgaard H. V., Toyonaga B., Mak T., van den Elsen P. Developmentally regulated rearrangement and expression of genes encoding the T cell receptor-T3 complex. Cell. 1986 Jul 4;46(1):75–87. doi: 10.1016/0092-8674(86)90861-5. [DOI] [PubMed] [Google Scholar]
- Hayday A. C., Saito H., Gillies S. D., Kranz D. M., Tanigawa G., Eisen H. N., Tonegawa S. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell. 1985 Feb;40(2):259–269. doi: 10.1016/0092-8674(85)90140-0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Kappler J., Kubo R., Haskins K., White J., Marrack P. The mouse T cell receptor: comparison of MHC-restricted receptors on two T cell hybridomas. Cell. 1983 Oct;34(3):727–737. doi: 10.1016/0092-8674(83)90529-9. [DOI] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Kitchingman G. R., Rovigatti U., Mauer A. M., Melvin S., Murphy S. B., Stass S. Rearrangement of immunoglobulin heavy chain genes in T cell acute lymphoblastic leukemia. Blood. 1985 Mar;65(3):725–729. [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Ravetch J. V., Poplack D. G., Waldmann T. A., Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFranc M. P., Forster A., Baer R., Stinson M. A., Rabbitts T. H. Diversity and rearrangement of the human T cell rearranging gamma genes: nine germ-line variable genes belonging to two subgroups. Cell. 1986 Apr 25;45(2):237–246. doi: 10.1016/0092-8674(86)90388-0. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- McIntyre B. W., Allison J. P. The mouse T cell receptor: structural heterogeneity of molecules of normal T cells defined by xenoantiserum. Cell. 1983 Oct;34(3):739–746. doi: 10.1016/0092-8674(83)90530-5. [DOI] [PubMed] [Google Scholar]
- Melvin S. L. Immunological definition of leukemic cell surface phenotypes. Cancer Res. 1981 Nov;41(11 Pt 2):4790–4793. [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden M. D., Toyonaga B., Ha K., Yanagi Y., Chin B., Gelfand E., Mak T. Somatic rearrangement of T-cell antigen receptor gene in human T-cell malignancies. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1224–1227. doi: 10.1073/pnas.82.4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirro J., Zipf T. F., Pui C. H., Kitchingman G., Williams D., Melvin S., Murphy S. B., Stass S. Acute mixed lineage leukemia: clinicopathologic correlations and prognostic significance. Blood. 1985 Nov;66(5):1115–1123. [PubMed] [Google Scholar]
- Nadler L. M., Ritz J., Bates M. P., Park E. K., Anderson K. C., Sallan S. E., Schlossman S. F. Induction of human B cell antigens in non-T cell acute lymphoblastic leukemia. J Clin Invest. 1982 Aug;70(2):433–442. doi: 10.1172/JCI110633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor N. T., Wainscoat J. S., Weatherall D. J., Gatter K. C., Feller A. C., Isaacson P., Jones D., Lennert K., Pallesen G., Ramsey A. Rearrangement of the T-cell-receptor beta-chain gene in the diagnosis of lymphoproliferative disorders. Lancet. 1985 Jun 8;1(8441):1295–1297. doi: 10.1016/s0140-6736(85)92791-6. [DOI] [PubMed] [Google Scholar]
- Pelicci P. G., Knowles D. M., 2nd, Dalla Favera R. Lymphoid tumors displaying rearrangements of both immunoglobulin and T cell receptor genes. J Exp Med. 1985 Sep 1;162(3):1015–1024. doi: 10.1084/jem.162.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessano S., Palumbo A., Ferrero D., Pagliardi G. L., Bottero L., Lai S. K., Meo P., Carter C., Hubbell H., Lange B. Subpopulation heterogeneity in human acute myeloid leukemia determined by monoclonal antibodies. Blood. 1984 Jul;64(1):275–281. [PubMed] [Google Scholar]
- Quertermous T., Murre C., Dialynas D., Duby A. D., Strominger J. L., Waldman T. A., Seidman J. G. Human T-cell gamma chain genes: organization, diversity, and rearrangement. Science. 1986 Jan 17;231(4735):252–255. doi: 10.1126/science.3079918. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Acuto O., Fabbi M., Bensussan A., Milanese C., Royer H. D., Meuer S. C., Schlossman S. F. Clonotypic surface structure on human T lymphocytes: functional and biochemical analysis of the antigen receptor complex. Immunol Rev. 1984 Oct;81:95–129. doi: 10.1111/j.1600-065x.1984.tb01106.x. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovigatti U., Mirro J., Kitchingman G., Dahl G., Ochs J., Murphy S., Stass S. Heavy chain immunoglobulin gene rearrangement in acute nonlymphocytic leukemia. Blood. 1984 May;63(5):1023–1027. [PubMed] [Google Scholar]
- Sabbath K. D., Ball E. D., Larcom P., Davis R. B., Griffin J. D. Heterogeneity of clonogenic cells in acute myeloblastic leukemia. J Clin Invest. 1985 Feb;75(2):746–753. doi: 10.1172/JCI111756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster R. N., Minowada J., Suciu-Foca N., Minden M., Mak T. W. Rearrangement and expression of the alpha, beta, and gamma chain T cell receptor genes in human thymic leukemia cells and functional T cells. J Exp Med. 1986 Jun 1;163(6):1491–1508. doi: 10.1084/jem.163.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu G., Clark S. P., Yoshikai Y., Malissen M., Yanagi Y., Strauss E., Mak T. W., Hood L. The human T cell antigen receptor is encoded by variable, diversity, and joining gene segments that rearrange to generate a complete V gene. Cell. 1984 Jun;37(2):393–401. doi: 10.1016/0092-8674(84)90369-6. [DOI] [PubMed] [Google Scholar]
- Snodgrass H. R., Dembić Z., Steinmetz M., von Boehmer H. Expression of T-cell antigen receptor genes during fetal development in the thymus. Nature. 1985 May 16;315(6016):232–233. doi: 10.1038/315232a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I., Koga M., Srivastava P. Phorbol ester-induced differentiation of a non-T/non-B human leukemic cell line (REH) to macrophage-like cells. Cancer Res. 1984 Jul;44(7):3017–3021. [PubMed] [Google Scholar]
- Stass S. A., Schumacher H. R., Keneklis T. P., Bollum F. J. Terminal deoxynucleotidyl transferase immunofluorescence of bone marrow smears: experience in 156 cases. Am J Clin Pathol. 1979 Dec;72(6):898–903. doi: 10.1093/ajcp/72.6.898. [DOI] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Single-cell origin of mouse hemopoietic colonies expressing multiple lineages in variable combinations. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6689–6693. doi: 10.1073/pnas.80.21.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawa A., Hozumi N., Minden M., Mak T. W., Gelfand E. W. Rearrangement of the T-cell receptor beta-chain gene in non-T-cell, non-B-cell acute lymphoblastic leukemia of childhood. N Engl J Med. 1985 Oct 24;313(17):1033–1037. doi: 10.1056/NEJM198510243131701. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Clark S. P., Taylor S., Sohn U., Wilson B. I., Minden M. D., Mak T. W. Organization and sequences of the variable, joining and constant region genes of the human T-cell receptor alpha-chain. 1985 Aug 29-Sep 4Nature. 316(6031):837–840. doi: 10.1038/316837a0. [DOI] [PubMed] [Google Scholar]
- Zauderer M., Iwamoto A., Mak T. W. Gamma gene rearrangement and expression in autoreactive helper T cells. J Exp Med. 1986 May 1;163(5):1314–1318. doi: 10.1084/jem.163.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]