Abstract
Objectives
Antipsychotics are routinely administered to traumatic brain injured (TBI) patients even though the benefits vs. risks of this approach on behavioral recovery are unclear. To clarify the issue, the present study evaluated the effect of single and multiple administrations of haloperidol and risperidone on functional outcome after TBI.
Design
Prospective and randomized study in rodents.
Setting
Experimental research laboratory at the University of Pittsburgh.
Subjects
Sixty adult male Sprague-Dawley rats weighing 300–325 g.
Interventions
Anesthetized rats received either a cortical impact or sham injury and then were randomly assigned to five TBI groups (risperidone 0.045 mg/kg, 0.45 mg/kg, 4.5 mg/kg, haloperidol 0.5 mg/kg, or vehicle 1 mL/kg) or three sham groups (risperidone 4.5 mg/kg, haloperidol 0.5 mg/kg, or vehicle 1 mL/kg). The experiment consisted of three phases. In the first phase, a single treatment was provided (i.p.) 24 hr after surgery and motor and cognitive function was assessed on post-operative days 1–5 and 14–18, respectively. During the second phase, after completion of the initial behavioral tasks, the same rats were treated once daily for 5 days and behavior was reevaluated. During the third phase, treatments were discontinued and 3 days later the rats were assessed one final time.
Measurements and Main Results
Time (sec) to maintain beam balance, traverse an elevated beam, and to locate a submerged platform in a Morris water maze. Neither motor nor cognitive performance was affected after a single treatment, regardless of group assignment (p > 0.05). In contrast, both behavioral deficits reoccurred after daily treatments of risperidone (4.5 mg/kg) and haloperidol (p < 0.05). The cognitive deficits persisted even after a 3-day washout period during the third phase.
Conclusions
These data suggest that while single or multiple low doses of risperidone and haloperidol may be innocuous to subsequent recovery after TBI, chronic high-dose treatments are detrimental.
Keywords: beam balance, catecholamines, cognition, controlled cortical impact, functional recovery, Morris water maze, traumatic brain injury
Introduction
Traumatic brain injury (TBI) induces a plethora of secondary sequelae, which include, but is not limited to, alterations in neurotransmitter systems (1–5) and neurobehavioral dysfunction (6–11). Regarding the former, regional increases in dopamine (DA) levels have been detected as early as 5 min to 1 hr after acceleration-deceleration brain injury (3). Cortical DA levels have been reported to decrease by 1 hr after fluid percussion-induced brain injury and remain depressed for up to 2 weeks (5). DA tissue levels and metabolism increase acutely, within 1 hr after controlled cortical impact (CCI), and then subsequently normalize over a 2-week period (4). Furthermore, both tyrosine hydroxylase, the rate-limiting enzyme in the synthesis of catecholamines, and the DA transporter, which plays a critical role in maintaining DA homeostasis in the CNS, are temporally altered after CCI injury (12–15). Moreover, the motor and cognitive deficits produced, in part, by these DA alterations are attenuated by treatment with dopamine D2 receptor agonists in both experimental (9–11,16,17) and clinical (18–21) studies. Taken together, these data suggest an important role for the DA neurotransmitter system in enhancing and maintaining functional recovery after TBI (22).
In addition to the motor and cognitive deficits, other behavioral manifestations of clinical TBI include agitation and aggression. Managing these symptoms is one of the more frustrating aspects in caring for TBI patients because they may resist treatment, become disruptive, and pose a physical risk to themselves and/or hospital staff, which ultimately impedes rehabilitation efforts (23–25). The administration of antipsychotics is routine for managing TBI-induced agitation albeit the benefits vs. risks of this approach on behavioral recovery are unclear. Because many of the commonly used antipsychotics exert much of their effect by acting as high affinity dopamine D2 receptor antagonists, this approach may have deleterious consequences after TBI, especially in light of the beneficial actions of D2 receptor agonists. Several experimental studies lend support for this assertion. For example, haloperidol, the prototype for the typical antipsychotics, hinders motor recovery when given singly (2,26,27) or chronically (28) after cortical damage. Haloperidol has also been reported to impair cognitive performance when provided chronically after fluid percussion injury (29). In contrast, neither a single low dose of clozapine nor multiple doses of olanzapine, which have a low affinity for dopamine D2 receptors, have been reported to negatively affect motor and cognitive performance, respectively (27,29). Thus, despite several investigative studies the rehabilitative consequences of administering antipsychotics, particularly those with a high affinity for dopamine D2 receptors, after TBI remain ambiguous. It is likely that the dose, timing, and duration of drug treatment may be compounding the issue. The goal of this study is to help clarify this ambiguity by empirically evaluating the effects of single and multiple administrations of the typical and atypical antipsychotics, haloperidol and risperidone, respectively, on motor and cognitive outcome after experimental TBI.
Materials and methods
Subjects and surgery
Sixty adult male Sprague-Dawley rats (Harlan Inc., Indianapolis, IN) were housed in standard steel-wire mesh cages and maintained in a temperature (21 ± 1°C) and light (on 7:00 a.m. to 7:00 p.m.) controlled environment with food and water available ad libitum. After one week of acclimatization the rats (300–325 g) were randomly assigned to either controlled cortical impact (CCI) or sham injury groups. In addition to the contusion, CCI injury produces many other morphological and cerebrovascular responses that are seen after human TBI, such as subarachnoid hemorrhage, hematoma, hippocampal cell loss, axonal injury, edema, altered cerebral blood flow & metabolism, and ischemia. CCI injury also produces neurobehavioral deficits (e.g., motor skills such as locomotion) and cognitive impairments (spatial learning, retention, and working memory) that are reminiscent of those seen clinically (30). Surgical procedures have been reported in detail elsewhere (8,10). Briefly, isoflurane (4% in 2:1 N2O/O2) anesthetized rats were intubated, mechanically ventilated, and then subjected to either a right hemisphere CCI (2.7 mm tissue deformation at 4 m/sec) or craniectomy alone (i.e., sham). Core temperature was maintained at 37 ± 0.5°C during surgery. All experimental procedures were approved by the Animal Care and Use Committee at the University of Pittsburgh and were conducted in accordance with the recommendations provided in the Guide for the Care and Use of Laboratory Animals (National Academy Press, 1996). Every attempt was made to limit the number of subjects used and to minimize suffering.
Acute neurological evaluation
To assess hind limb reflexive ability the rats were given brief paw pinches every 10 sec following the cessation of anesthesia and the time to elicit a withdrawal was recorded. To evaluate the return of righting ability the rats were placed on their back and the time required to turn onto their stomach for three consecutive times was recorded.
Motor training
Beam balance and beam walk tasks were utilized to assess gross and fine motor function, respectively. The beam balance task consists of placing the rat on an elevated (90 cm) narrow wooden beam (1.5 cm wide) and recording the duration it remains on for a maximum of 60 sec. The beam walk task, originally devised by Feeney et al (26), allows for the assessment of locomotion. Briefly, the task consists of training/assessing rats using a negative-reinforcement paradigm to escape ambient light and high decibel white noise by traversing an elevated (90 cm) narrow wooden beam (2.5 × 100 cm) and entering a darkened goal box at the opposite end. The termination of the aversive stimuli (light and noise) serves as reinforcement (reward). Performance is assessed by the time required to traverse the beam. Rats were tested for both beam balance and beam walk ability on post-operative days 1–5. Three trials (60 sec allotted time) per day were provided on each task and the average daily scores for each subject were used in the statistical analyses.
Cognitive training (spatial learning acquisition and memory)
A Morris water maze task that is sensitive to cognitive function/dysfunction following TBI (7,8,10) was used to compare acquisition rates among groups. The maze consisted of a plastic pool (180 cm diameter; 60 cm high) filled with water (26 ± 1°C) to a depth of 28 cm and was situated in a room with salient visual cues that remained constant throughout the study. The platform consisted of a clear Plexiglas stand (10 cm diameter, 26 cm high) that was positioned 26 cm from the maze wall in the SW quadrant and held constant throughout the experiment. Spatial learning acquisition and memory began on post-operative day 14 and consisted of providing a block of four daily trials for five consecutive days (days 14–18) to locate the platform when it was submerged 2 cm below the water surface (i.e., invisible to the rat), followed by an additional day (day 19) to locate the platform when it stood 2 cm above the water surface (visible to the rat). For each daily block of trials the rats were placed in the pool facing the wall at each of the four possible start locations (north, east, south, and west) in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. Rats that failed to locate the goal within the allotted time were manually guided to it. All rats remained on the platform for 30 sec before being placed in a heated incubator between trials (4-min inter-trial interval). The average time of the 4 trials for each rat were used in the statistical analyses. Swim speed was recorded and assessed with a video analysis system (Chromatrack, San Diego Instruments, San Diego, CA).
Drug administration
Risperidone (Research Diagnostics Inc., Flanders NJ) and haloperidol (Sigma, St. Louis, MO) were prepared daily by dissolving in 1:1 DMSO/saline v/v, which also served as the vehicle. TBI animals were randomly assigned to receive intraperitoneal injections of risperidone (0.045 mg/kg, 0.45 mg/kg, or 4.5 mg/kg, n=9 each group), haloperidol (0.5 mg/kg, n=9), or vehicle (1 mL/kg, n=9). Sham animals received risperidone (4.5 mg/kg, n=5), haloperidol (0.5 mg/kg, n=5), or vehicle (1 mL/kg, n=5). During phase 1 of the study, all rats received a single intraperitoneal injection 24 hr after surgery. During phase 2 (post-operative days 19–23), all groups, which consisted of the same rats from phase 1, received daily injections 1 hr prior to testing. Treatments were discontinued during phase 3 (i.e., drug washout period) and the rats were reevaluated (see Fig. 1 for an outline of the experimental phases). The 0.45 mg/kg dose of risperidone and the 0.5 mg/kg dose of haloperidol were chosen because these concentrations are equivalent to those used clinically to control psychosis (31). Risperidone concentrations 10-fold lower and 10-fold higher were also evaluated in an effort to establish a behavioral response profile.
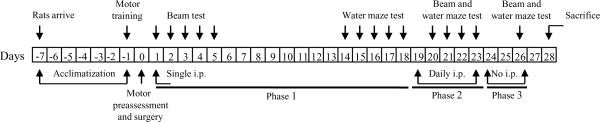
Fig. 1.
Flow chart of the experimental paradigm. The thick black lines refer to the time points (days) corresponding with the three phases of the experiment. Phase 1 included post-operative days 1–18, in which rats received a single administration of one of the treatments (risperidone, 0.045 mg/kg, 0.45 mg/kg, 4.5 mg/kg; 0.5 mg/kg haloperidol, or 1 mL/kg vehicle) and tested on the beam on post-operative days 1–5 and in a hidden platform version of the water maze on days 14–18. Phase 2 consisted of days 19–23 in which a single visible platform test was provided on day 19, just prior to commencement of daily treatments (same rats and drug doses as in phase 1) that continued for 5 days. Motor and water maze performance were reevaluated beginning one day after the induction of daily treatments. Phase 3 consisted of days 24–26, which was considered a drug washout period. All rats underwent final behavioral assessments on the beam and water maze on day 26 (three days after discontinuation of treatments) and then sacrificed on post-operative day 28.
Statistical analyses
Statistical analyses were performed on data collected by observers blinded to treatment conditions by repeated measures analysis of variance (ANOVA) using Statview 4.51 software (Abacus Concepts, Inc., Berkeley, CA). When the ANOVA revealed a significant effect, the Bonferonni post-hoc test was utilized to determine specific group differences. The data are expressed as the mean ± standard error of the mean (SE) and are considered significant when corresponding p values are ≤ 0.05 or as dictated by Bonferroni corrections for multiple comparisons.
Results
Exclusions and data pooling
Two rats from the TBI+vehicle group were unsuccessful in locating the visible platform during the allotted two min period for each location, which is suggestive of impaired visual acuity, and thus were excluded from the cognitive analyses. Furthermore, post-hoc analyses did not reveal significant differences among the sham groups regardless of treatment in any of the tasks and thus the data were pooled and analyzed as one group (designated “SHAM”).
Acute neurological evaluation
No significant differences were observed among the TBI groups in hind limb reflex withdrawal latency [range 171.9 ± 3.9 sec to 188.1 ± 10.4, p > 0.05] or return of righting ability [range 351.2 ± 24.4 sec to 398.8 ± 24.5, p > 0.05]. The lack of significant differences with these acute neurological indices suggests that all TBI groups experienced equivalent injury severities.
Motor performance (beam balance)
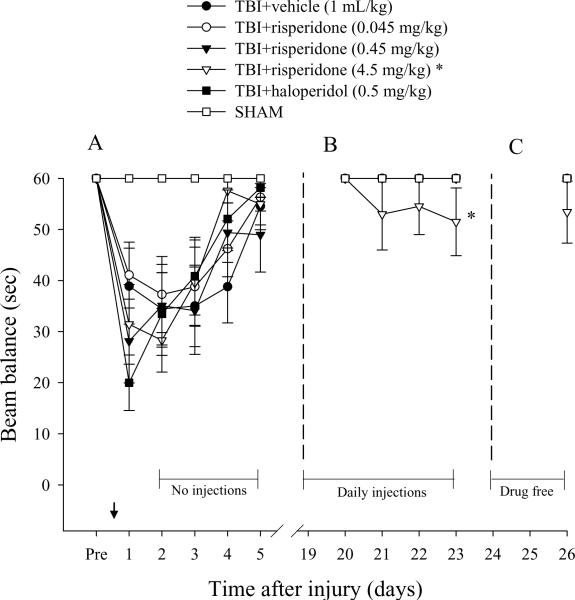
All groups were able to balance on the beam for the allotted 60 sec on each of three trials prior to surgery. In contrast, all injured groups displayed significant beam balance impairments on post-operative days 1–3, regardless of treatment. Moreover, all injured groups exhibited a similar rate of spontaneous improvement over time (Fig. 2A) and as such no significant differences were revealed among any of the TBI groups vs. SHAM on post-operative days 4–5 [all p's > 0.05, Bonferroni]. However, during the daily treatment paradigm (second phase of the study) beam balance deficits reoccurred in the TBI+risperidone (4.5 mg/kg) group. The impairment was different [p < 0.05] from the other groups, which did not exhibit a reoccurrence of deficits (Fig. 2B). Lastly, all groups were reevaluated after a 3-day drug washout period and although the TBI+risperidone (4.5 mg/kg) group did not return to baseline performance, no statistically significant difference was revealed (Fig. 2C).
Fig. 2.
Mean (± SE) balance ability as measured by time (sec) spent on an elevated wooden beam prior to, and after, TBI or SHAM injury. During the first phase of the experiment a single drug injection was provided 1 hr prior to testing on post-operative day 1 (arrow), which was followed by daily administrations on days 19–23 during the second phase, and no treatments during the third phase, which consisted of days 24–26. (A) No significant differences were revealed among TBI groups, albeit all were markedly impaired relative to SHAM controls. (B) * p < 0.05 vs. all other groups during daily administration. (C) Assessment at three days after the discontinuation of daily treatment revealed no statistically significant differences among groups (p > 0.05, Bonferroni post-hoc).
Motor performance (beam walk)
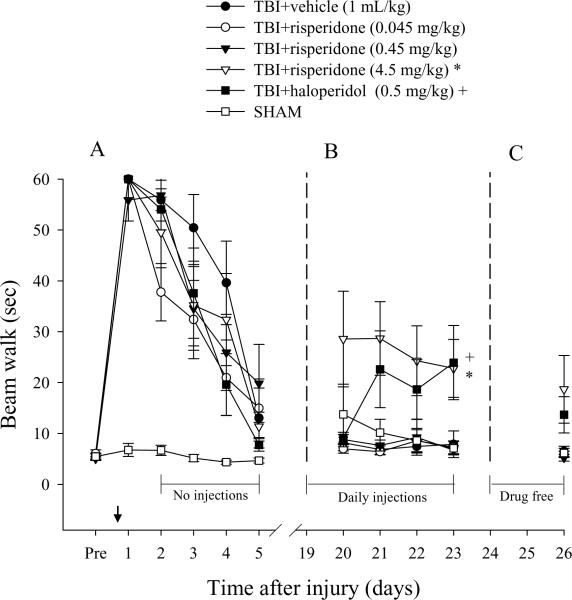
Similar to the beam balance results, there were no significant differences in time to traverse the beam among groups prior to surgery [p > 0.05]. However, for the first 4 days after TBI there was a significant increase in beam-walking time for all injured groups vs. SHAM controls. Time to traverse the beam did not differ significantly between the injured groups on any testing day, regardless of treatment [p > 0.05] as all groups recovered at the same rate and exhibited near baseline performance by the end of the testing period (Fig. 3A). During the second phase of the study, both the TBI+risperidone (4.5 mg/kg) and TBI+haloperidol groups exhibited (Fig. 3B) significant increases in beam walk latencies vs. all other groups [p < 0.05]. While the TBI+risperidone (4.5 mg/kg) and TBI+haloperidol groups still appeared deficient in beam walk ability after 3 drug free days (Fig. 3C), the post-hoc analysis was not significant [p > 0.05].
Fig. 3.
Mean (± SE) walking ability as measured by time (sec) to traverse an elevated wooden beam prior to, and after, TBI or SHAM injury. During the first phase of the experiment a single drug injection was provided 1 hr prior to testing on post-operative day 1 (arrow), which was followed by daily administrations on days 19–23 during the second phase, and no treatments during the third phase, which consisted of days 24–26. (A) No significant differences were revealed among TBI groups, albeit all were markedly impaired relative to SHAM controls. (B) +*p < 0.05 vs. all other groups, but not each other, during daily administration. (C) Assessment at three days after the discontinuation of daily treatment revealed no statistically significant differences among groups (p > 0.05, Bonferroni post-hoc).
Cognitive performance (spatial learning acquisition)
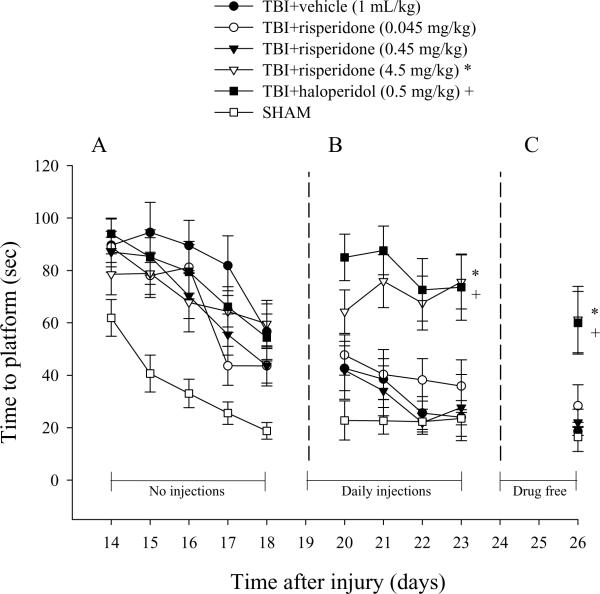
Analysis of spatial acquisition revealed significant TBI-induced water maze performance deficits. Despite the initial water maze impairment, all injured groups became progressively better at locating the submerged platform over time, but were still significantly impaired relative to the SHAM group, which became quite proficient in performing the task over the testing period (Fig. 4A). Reevaluation of cognitive performance during the second phase of the study when all groups were receiving daily treatments showed that both the TBI+risperidone (4.5 mg/kg) and TBI+haloperidol groups required significantly more time to locate the platform vs. all other groups [p < 0.05], suggesting a reinstatement of deficits (Fig. 4B). The deficits were still present 3 days after discontinuation of drug treatments (Fig. 4C), as evidenced by both groups still differing significantly from the TBI+vehicle group [p = 0.0019 and 0.0025, respectively]. No significant difference in swim speed (range = 27.2 ± 3.3 to 31.3 ± 1.3 cm/sec [p > 0.05]) or visible platform acquisition [p > 0.05] was observed among the injured groups, indicating that neither motor impairments nor visual disparities precluded the accurate assessment of place learning during the first phase of the study. However, during the second phase when treatments were provided daily, both the TBI+risperidone (4.5 mg/kg) and TBI+haloperidol groups displayed slower, albeit not statistically significant [p > 0.05], swim speeds (range = 21.4 ± 4.1 to 26.3 ± 2.4 cm/sec) relative to the other TBI groups (range = 29.3 ± 2.2 to 33.4 ± 1.7 cm/sec).
Fig. 4.
Mean (± SE) time (sec) to locate a hidden platform in a water maze. During the first phase of the experiment a single drug injection was provided 1 hr prior to testing on post-operative day 1 and thus rats were not being treated during testing days 14–18. However, daily administrations were provided during the second phase (post-operative days 19–23). No treatments were provided during the third phase (post-operative days 24–26). (A) No significant differences were revealed among TBI groups, albeit all are markedly impaired relative to SHAM controls. (B) *+p < 0.05 vs. all other groups, but not each other, during daily administrations. (C) *+p < 0.05 vs. all other groups, but not each other, three days after discontinuation of treatments.
Discussion
The present study sought to evaluate the effect of typical (haloperidol) and atypical (risperidone) antipsychotics on both motor and cognitive performance after TBI induced by a clinically relevant model (30). The experiment was conducted in three phases (Fig. 1) and was designed to determine the effects of a single and early treatment (24 hr post-TBI or sham surgery), delayed and chronic treatments (18 to 23 days post-TBI), and persistence of neurobehavioral effects (after a 3-day drug washout period). The results from the first phase of the study revealed that neither haloperidol nor risperidone, regardless of dose, exerted a deleterious effect on functional outcome as evidenced by a similar rate of motor recovery and cognitive performance for all antipsychotic-treated groups relative to vehicle-treated controls. In marked contrast, the findings from the second phase showed that daily administration of both haloperidol and the high dose of risperidone (4.5 mg/kg) led to a reoccurrence of beam-walking and water maze impairments. Moreover, as revealed in the third phase, the water maze spatial learning deficits were persistent as they were still present in the high dose risperidone-treated group three days after discontinuation of treatment.
The lack of significant motor impairment after one treatment is in contrast to that of previously published studies reporting that a single administration of haloperidol 24 hr after sensorimotor cortex ablation impairs motor recovery for several weeks (2,26,27). The results from the second phase of the study showing a significant deleterious effect of daily administrations of haloperidol on motor function is comparable to a previously published report where brain trauma was induced via cortical weight drop (28), but not fluid percussion (29). However, water maze performance was impaired in the latter study (29). The discrepant findings may be attributed to differences in location and size of the lesions, as well as selective cell loss produced by the different brain injury models (i.e., focal cortical impact vs. cortical ablation and fluid percussion). Another explanation for the differences may stem from inherent characteristics of the motor tasks, such as sensitivity and scoring criteria. For instance, motor performance was evaluated in the current study by recording the overall time required to traverse a beam, which is not as sensitive as evaluating fine motor coordination, such as determining the number of foot slips during beam walking as was done in the studies by Feeney (2,26) and Goldstein (27).
Because time to traverse the beam or locate the submerged platform was the criteria used for beam-walking and water maze performance, an intuitive explanation for the reoccurrence of deficits is that slower speed, perhaps produced by catalepsy, accounted for the impairments. However, the impaired rats (i.e., high dose risperidone and haloperidol treated) were generally not slower overall, as demonstrated by traversal times that were similar to the other groups after gentle prodding (that elicits locomotion) and swim speeds that were within the range of vehicle-treated rats. Interestingly, Alleva et al (32) administered different doses of haloperidol to adult rodents in an effort to determine catalepsy, as measured by latency between paw placement and movement on a wooden bar. They found that the minimal dose to induce complete catalepsy was 10 mg/kg, while 0.6 mg/kg, a dose very close to that used in the current study (0.5 mg/kg), had a virtually non-existent cataleptic response (32). One neurobehavioral explanation for the reoccurrence of motor and spatial acquisition deficits is that antipsychotic drugs can significantly affect attention and this can lead to deficit in performance in an already learned task.
Alterations in neural transmission as reported in seminal studies by Feeney and colleagues (2,6,36) and Goldstein et al (27,38) provide additional explanations for the reinstatement of deficits. Antagonism of dopamine D2 receptors by chronic high dose treatments of haloperidol and risperidone is a viable choice given the plethora of data that exist showing that the administration of D2 receptor agonists such as amantadine (16), bromocriptine (9–10), and methylphenidate (11) improve functional outcome after cortical impact injury in rodents. Furthermore, beneficial effects with these same agents have also been observed in human TBI patients (18–21). In addition to D2 receptor antagonism, both haloperidol and risperidone also affect α-1 and α-2 noradrenergic (NA) receptors (33). An abundance of experimental studies strongly implicate this neurotransmitter system in mediating many of the functional effects (deficits and recovery of function) observed after TBI. Briefly, Feeney and colleagues have shown that drugs with antagonistic effects on NA, such as haloperidol and clonidine, impede recovery after brain injury and can reinstate clinical deficits long after full recovery has occurred [see 2,6,28,34–37 for excellent reviews]. Haloperidol and risperidone also have a high affinity for 5-HT2A and 5-HT2C receptors. Serotonergic pathways originating in the raphé nuclei project extensively to brain areas involved in cognitive functions, and serotonin (5-HT) receptor agonists and antagonists alter these processes (38,39). While we have studied the effect of 5-HT1A receptor agonists in learning and memory and have found improved spatial acquisition (8), other groups studying different 5-HT receptors have reported either beneficial or detrimental effects (38,39). Taken together, these pharmacological studies indicate that motor and cognitive function can be affected by manipulating various neurotransmitter systems. Furthermore, because both haloperidol and risperidone affect these systems, it is difficult to ascribe the deleterious effects observed in our study, as well as others, to a single neurotransmitter system.
In conclusion, the data indicate that neither a single administration of the antipsychotics haloperidol and risperidone nor multiple low doses impair motor and cognitive function after experimental TBI. However, multiple administrations of haloperidol and high dose risperidone lead to a reoccurrence of motor and spatial learning deficits. These findings replicate laboratory and clinical data suggesting that some commonly prescribed drugs have the ability to produce a reoccurrence of symptoms in fully recovered animals (36) and patients (36,37) and thus challenges the accepted hypothesis that rewiring underlies recovery. Furthermore, the data indicate that recovery is a fragile state and physicians should be aware of the possible harmful effects of some drugs on outcome. However, we cannot rule out the possibility that multiple treatments were deleterious because of a confounding influence of previous drug exposure. These findings may have significant relevance for the physician who is faced with the decision of whether to administer antipsychotics to attenuate TBI-induced aggression or agitation so that patient care can resume. However the implications may be quite different for single use in the critical care setting when compared with chronic use in a rehabilitation environment.
Acknowledgements
This work was supported, in part, by National Institutes of Health grants HD043851 and HD046700 awarded to AEK
References
- 1.Dunn-Meynell A, Pan S, Levin BE. Focal traumatic brain injury causes widespread reductions in rat brain norepinephrine turnover from 6 to 24 h. Brain Res. 1994;660:88–95. doi: 10.1016/0006-8993(94)90842-7. [DOI] [PubMed] [Google Scholar]
- 2.Feeney DM, Weisend MP, Kline AE. Noradrenergic pharmacotherapy, intracerebral infusion and adrenal transplantation promote functional recovery after cortical damage. J Neur Transplant Plast. 1993;4:199–213. doi: 10.1155/NP.1993.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huger F, Patrick G. Effect of concussive head injury on central catecholamine levels and synthesis rates in rat brain regions. J Neurochem. 1979;33:89–95. doi: 10.1111/j.1471-4159.1979.tb11710.x. [DOI] [PubMed] [Google Scholar]
- 4.Massucci JL, Kline AE, Ma X, et al. Time dependent alterations in dopamine tissue levels and metabolism after experimental traumatic brain injury in rats. Neurosci Lett. 2004;372:127–131. doi: 10.1016/j.neulet.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 5.McIntosh TK, Yu T, Gennarelli TA. Alterations in regional brain catecholamine concentrations after experimental brain injury in the rat. J Neurochem. 1994;63:1426–1433. doi: 10.1046/j.1471-4159.1994.63041426.x. [DOI] [PubMed] [Google Scholar]
- 6.Feeney DM. From laboratory to clinic: noradrenergic enhancement of physical therapy for stroke or trauma patients. Adv Neurol. 1997;73:383–394. [PubMed] [Google Scholar]
- 7.Hamm RJ, Dixon CE, Gbadebo DM, et al. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- 8.Kline AE, Massucci JL, Dixon CE, et al. The therapeutic efficacy conferred by the 5-HT(1A) receptor agonist 8-Hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) after experimental traumatic brain injury is not mediated by concomitant hypothermia. J Neurotrauma. 2004;21:175–185. doi: 10.1089/089771504322778631. [DOI] [PubMed] [Google Scholar]
- 9.Kline AE, Massucci JL, Ma X, et al. Bromocriptine reduces lipid peroxidation and enhances spatial learning and hippocampal neuron survival in a rodent model of focal brain trauma. J Neurotrauma. 2004;21:1712–1722. doi: 10.1089/neu.2004.21.1712. [DOI] [PubMed] [Google Scholar]
- 10.Kline AE, Massucci JL, Marion DW, et al. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J Neurotrauma. 2002;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- 11.Kline AE, Yan HQ, Bao J, et al. Chronic methylphenidate treatment enhances water maze performance following traumatic brain injury in rats. Neurosci Lett. 2000;280:163–166. doi: 10.1016/s0304-3940(00)00797-7. [DOI] [PubMed] [Google Scholar]
- 12.Wagner AK, Sokoloski JE, Ren D, et al. J Neurochem. 2005;95:457–465. doi: 10.1111/j.1471-4159.2005.03382.x. [DOI] [PubMed] [Google Scholar]
- 13.Wilson MS, Chen X, Ma X, et al. Synaptosomal dopamine uptake in rat striatum following controlled cortical impact. J Neurosci Res. 2005;80:85–91. doi: 10.1002/jnr.20419. [DOI] [PubMed] [Google Scholar]
- 14.Yan HQ, Kline AE, Ma X, et al. Tyrosine hydroxylase, but not dopamine beta-hydroxylase, is increased in rat frontal cortex after traumatic brain injury. NeuroReport. 2001;12:2323–2327. doi: 10.1097/00001756-200108080-00009. [DOI] [PubMed] [Google Scholar]
- 15.Yan HQ, Kline AE, Ma X, et al. Traumatic brain injury reduces dopamine transporter protein expression in the rat frontal cortex. NeuroReport. 2002;13:1899–1901. doi: 10.1097/00001756-200210280-00013. [DOI] [PubMed] [Google Scholar]
- 16.Dixon CE, Kraus MF, Kline AE, et al. Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor Neurol Neurosci. 1999;14:285–294. [PubMed] [Google Scholar]
- 17.Kline AE, Chen MJ, Tso-Olivas DY, et al. Methylphenidate treatment following ablation-induced hemiplegia in rat: experience during drug action alters effects on recovery of function. Pharmacol Biochem Beh. 1994;48:773–779. doi: 10.1016/0091-3057(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 18.Kraus MF, Maki PM. Effect of amantadine hydrochloride on symptoms of frontal lobe dysfunction in brain injury: case studies and review. J Neuropsychiat Clin Neurosci. 1997;9:222–320. doi: 10.1176/jnp.9.2.222. [DOI] [PubMed] [Google Scholar]
- 19.McDowell S, Whyte J, D'Esposito M. Differential effect of a dopaminergic agonist on prefrontal function in traumatic brain injury patients. Brain. 1998;121:1155–1164. doi: 10.1093/brain/121.6.1155. [DOI] [PubMed] [Google Scholar]
- 20.Plenger PM, Dixon CE, Castillo RM, et al. Subacute methylphenidate treatment for moderate to moderately severe traumatic brain injury: a preliminary double-blind placebo-controlled study. Arch Phys Med Rehab. 1996;77:536–540. doi: 10.1016/s0003-9993(96)90291-9. [DOI] [PubMed] [Google Scholar]
- 21.Whyte J, Hart T, Schuster K, et al. Effects of methylphenidate on attentional function after traumatic brain injury. a randomized, placebo-controlled trial. Am J Phys Med Rehabil. 1997;76:440–450. doi: 10.1097/00002060-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Parton A, Coulthard E, Husain M. Neuropharmacological modulation of cognitive deficits after brain damage. Curr Opin Neurol. 2005;18:675–680. doi: 10.1097/01.wco.0000189872.54245.13. [DOI] [PubMed] [Google Scholar]
- 23.Elovic EP, Lansang R, Li Y, et al. The use of atypical antipsychotics in traumatic brain injury. J Head Trauma Rehabil. 2003;18:177–195. doi: 10.1097/00001199-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Levy M, Berson A, Cook T, Bollegala N, et al. Treatment of agitation following traumatic brain injury: a review of the literature. NeuroRehabil. 2005;20:279–306. [PubMed] [Google Scholar]
- 25.Lombard LA, Zafonte RD. Agitation after traumatic brain injury: considerations and treatment options. Am J Phys Med & Rehabil. 2005;84:797–812. doi: 10.1097/01.phm.0000179438.22235.08. [DOI] [PubMed] [Google Scholar]
- 26.Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein LB, Bullman S. Differential effects of haloperidol and clozapine on motor recovery after sensorimotor cortex injury in rats. Neurorehabil Neural Repair. 2002;16:1–5. doi: 10.1177/154596830201600402. [DOI] [PubMed] [Google Scholar]
- 28.Feeney DM, Westerberg VS. Norepinephrine and brain damage: alpha noradrenergic pharmacology alters functional recovery after cortical trauma. Can J Psychol. 1990;44:233–252. doi: 10.1037/h0084243. [DOI] [PubMed] [Google Scholar]
- 29.Wilson MS, Gibson CJ, Hamm RJ. Haloperidol, but not olanzapine, impairs cognitive performance after traumatic brain injury in rats. Am J Phys Med Rehabil. 2003;82:871–879. doi: 10.1097/01.PHM.0000091982.33232.CB. [DOI] [PubMed] [Google Scholar]
- 30.Kline AE, Dixon CE. Contemporary in vivo models of brain trauma and a comparison of injury responses. In: Miller LP, Hayes RL, editors. Head Trauma: Basic, Preclinical and Clinical Directions. John Wiley & Sons; NY: 2001. pp. 65–84. [Google Scholar]
- 31.Rosengarten H, Quartermain D. The effect of chronic treatment with typical and atypical antipsychotics on working memory and jaw movements in three-and eighteen-month-old rats. Prog Neuro-Psychopharmacol & Biol Psychiat. 2002;26:1047–1054. doi: 10.1016/s0278-5846(02)00221-x. [DOI] [PubMed] [Google Scholar]
- 32.Alleva E, Della-Seta D, Cirulli F, et al. Haloperidol treatment decreases nerve growth factor levels in the hypothalamus of adult mice. Prog Neuro-Psychopharmacol & Biol Psychiat. 1996;20:483–489. doi: 10.1016/0278-5846(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 33.chotte A, Janssen PF, Megens AA, et al. Occupancy of central neurotransmitter receptors by risperidone, clozapine and haloperidol, measured ex vivo by quantitative autoradiography. Brain Res. 1993;631:191–202. doi: 10.1016/0006-8993(93)91535-z. [DOI] [PubMed] [Google Scholar]
- 34.Feeney DM, Sutton RL. Pharmacotherapy for recovery of function after brain injury. Crit Rev Neurobiol. 1987;3:135–197. [PubMed] [Google Scholar]
- 35.Phillips JP, Devier DJ, Feeney DM. Rehabilitation pharmacology: bridging laboratory work to clinical application. J Head Trauma Rehabil. 2003;18:342–356. [PubMed] [Google Scholar]
- 36.Feeney DM, DeSmet AM, Rai S. Noradrenergic modulation of hemiplegia: facilitation and maintenance of recovery. Restor Neurol Neurosci. 2004;22:175–190. [PubMed] [Google Scholar]
- 37.Goldstein LB. Common drugs may influence motor recovery after stroke. the sygen in acute stroke study investigators. Neurology. 1995;45:865–871. doi: 10.1212/wnl.45.5.865. [DOI] [PubMed] [Google Scholar]
- 38.Meneses A. 5-HT system and cognition. Neurosci Biobehav Rev. 1999;23:1111–1125. doi: 10.1016/s0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 39.Meneses A, Hong E. Role of 5-HT1B, 5-HT2A and 5-HT2C receptors in learning. Behav Brain Res. 1997;87:105–110. doi: 10.1016/s0166-4328(96)02266-8. [DOI] [PubMed] [Google Scholar]