Abstract
Background
The opioid peptide β-Endorphin (β-E) is synthesized and released in response to stressful stimuli as well as acute alcohol administration. The release of β-E following exposure to an inescapable aversive situation may mediate behaviors that contribute to allostasis of the stress response. The present study examines the effects of β-E on immobility in assays involving inescapable stress, both under basal conditions as well as after acute administration of EtOH.
Methods
Female and male transgenic mice with varying capacities to translate β-E were subject to either the forced swim (FST, Experiment 1) or tail suspension test (TST, Experiment 2). In Experiment 3, mice were divided into three groups based on hormonal status (male, female-estrous and female-nonestrous) and injected with either 1 g/kg EtOH or equivolume saline 14 min prior to behavioral assessment on the TST.
Results
Experiments 1 and 2 demonstrated a direct relationship between β-E levels and immobility. There were also sex differences in behavior in these tests, with males displaying more immobility than females. A main effect of genotype in Experiment 3 replicated findings in Exp 1 & 2. There was also an effect of EtOH (increasing immobility) and a significant interaction reflecting a particularly robust effect of the drug in mice with low β-E. In addition, there were interactions between β-E, EtOH effects and hormonal status.
Conclusions
These findings support the contention that β-E moderates behavioral responses to stressful stimuli and suggest a role for this peptide in coping behavior. Furthermore, the effects of EtOH on the response to stress may be mediated by β-E. Sex differences in this influence may contribute to sex differences in disease susceptibility and expression.
INTRODUCTION
The endogenous opioid peptide β-Endorphin (β-E) is synthesized and released in response to stress and alcohol (EtOH) administration through activation of the hypothalamic-pituitary-adrenal (HPA) axis (Constanopoulos et al., 1995; Gianoulakis, 1990; Marinelli et al., 2004; Schedlowski et al., 1995). A member of the large family of endogenous opioids, β-E possesses potent analgesic and addictive properties and serves a role in homeostatic functions (e.g., appetite, temperature) as well as in the rewarding and reinforcing properties of drugs of abuse, such as alcohol (Froehlich et al., 2000; Gianoulakis, 2004; Racz et al., 2008).
Following stimulation by corticotrophin releasing hormone, β-E is cleaved from the Proopiomelanocortin (POMC) gene along with Adrenocorticotropin Hormone (ACTH). β-E contributes to the behavioral responses to stress (Amat et al., 2005; Grisel et al., 2008; Hunt and Zakhari, 1995; Janssen and Arntz, 2001) perhaps in part, by inhibiting secretion of corticotrophin releasing hormone (CRH; Buckingham 1986; Plotsky, 1991). Thus, release of β-E by acute activation of the HPA axis following exposure to an inescapable aversive situation may moderate the stress response and thereby facilitate endocrine and behavioral allostasis (McEwen, 2002).
Likewise, variations in sensitivity of the HPA axis and subsequent β-E production in response to stress exposure may underlie individual differences in coping behavior (Gianoulakis, 1998; Hunt and Zakhare, 1995). We have shown, for example, an inverse relationship between β-E levels and anxious behavior in mice (Grisel et al., 2008) suggesting decreased ability to behaviorally manage stressful stimuli with lower β-E levels, along with, physiologically, a blunted attenuation of the stress response (Gianloukais, 1998; McEwen, 2002; Sarkar et al., 2007).
Compromised regulation of the stress response in low β-E subjects may have implications for a longstanding ‘opioid deficiency hypothesis’ suggesting that those with low basal levels of β-E may be especially inclined to self-medicate with drugs of abuse (Gianoulakis, 2001; Koob and LeMoal, 2008; Zalewska-Kaszubska and Czarnecka, 2004). A number of experimental reports indicate that alcoholics, at-risk non-alcoholics, and animal models for these clinical groups have low β-E levels (Aguirre et al., 1995; Dai et al., 2005; Gianoulakis 2001, 2004; Gianoulakis et al., 1996; Grisel et al., 1999; Zalewska-Kaszubska, 2005). Acute alcohol administration increases levels of pituitary β-E through activation of the HPA axis (de Waele and Gianoulakis, 1993; Gianoulakis, 2001; Gianoulakis and Barcomb, 1987; Herz, 1997; Thiagarajan et al., 1989). So by drinking, individuals with low β-E may be self-medicating a hyperactive stress axis along with its behavioral sequelae (Gianoulakis et al., 1989; Khantzian, 1985; Markou et al., 1998; Zalewska-Kaszubska and Czarnecka, 2004). Thus, it is hypothesized that in the absence of sufficient β-E for effective means of coping with stress, alcohol serves as an alternative coping mechanism.
In the present study, we investigated the role of β-E in moderating behavioral responses to an inescapable aversive stressor as well as the relationship between β-E and EtOH in the same situation. Because it is well-documented that alcohol alters the opioid system (Gianoulakis, 1998; Gianoulakis et al., 1989) and that subjects with varying levels of β-E display varying effects of, as well as preference for, EtOH, (de Waele et al., 1992; Gianoulakis et al., 1992; Grisel et al., 1999, Grisel et al., 2008; see Herz 1997 for review) our study was aimed at determining the effects of β-E on coping capabilities in mice after acute administration of EtOH, using the forced swim and tail suspension tests.
MATERIALS AND METHODS
Subjects
Adult naïve male and female β-E deficient (B6.129S2-Pomctm1Low/J; KO), heterozygous (HT) and wildtype (C57BL/6J; B6) mice were used in these experiments. These mice were developed over a decade ago in the laboratory of Malcolm Low (Rubinstein et al. 1996) by insertion of a premature stop codon into the Pomc gene. Homozygotes (KO) can not synthesize β-E, though all other Pomc products show normal expression. Opioid receptor expression also remains unchanged (Rubinstein et al. 1996). HT mice produce 50% of B6 levels of β-E. Mice for these studies were bred in-house from stock purchased from Jackson Laboratories (Bar Harbor, ME). The gene mutation has been fully backcrossed to the C57BL/6J strain (> 20 generations). HT mice were bred from KO males and B6 females; others were bred under identical conditions from genotype-matched pairs. They were group housed by sex with 4–5 per Plexiglas cage following weaning at 20–21 days and maintained in a at 21±2 °C colony room with ad lib food and water on a reverse 12:12 light: dark cycle with lights on at 7 PM. Because β-E is known to help regulate energy homeostasis and to contribute to weight differences with increasing age (Low, 2004) all subjects were between 50 and 90 days old at time of testing, and matched (within sex) for body weight. Pilot studies in our lab have shown no genotypic differences in brain or blood EtOH concentrations following a range of EtOH doses. Testing always occurred between 1000 and 1600 hr during the dark phase of the light/dark cycle, after at least 30 min habituation in a dimly lit testing room. During habituation, mice were weighed and tail marked according to experimental group. Testing order was counterbalanced across genotype, sex, and drug condition and experimenters were blind to genotype, and (as much as possible) drug injection. There were 8–10 subjects per genotype and sex, in each experiment, unless otherwise noted.
All procedures were carried out in accordance with the National Institutes of Health guidelines and were approved by the Animal Care and Use Committee of Furman University.
Experiment 1 and 2, Basal Immobility
Forced Swim Test
Mice were subject to a modified version of Porsolt’s (1977) forced swim test (FST) for 15 min in a white plastic five gallon bucket measuring 30 cm in diameter by 40 cm in height containing 20 cm of water maintained at 23°C. Mice were judged immobile when making no movements other than that required to stay afloat, for at least 5 s. Latency to immobility, total time spent immobile, and number of immobile segments were recorded.
Tail Suspension Test
Mice were hung by threading their tails through a 1cm hole in a board measuring 10 cm by 20 cm. The board was suspended from a stand 30 cm in height. Tails were secured with lab tape, approximately 2 cm from the base of the tail to the opposite side of the board. Latency to immobility, number of immobile segments, and total time spent immobile were recorded during the 6 min test.
Experiment 3, Tail Suspension Test with EtOH
Interactions between β-E and EtOH were evaluated using the Tail Suspension Test (TST). The FST and TST are thought to reflect the same substrates (Cryan et al., 2005; Kulkami and Dhir, 2007) but at least in our hands, the TST is less variable and we were interested in minimizing the number of subjects required. During habituation, at the time of weight determination, females were visually checked for estrous by two independent experimenters using the basic strategy of Champlain et al., (1973). This procedure takes practice, but is relatively straightforward; the size, shape and color of the vaginal opening differs between pro-estrous/estrous and non-estrous females. During experimenter training we corroborated the validity of the visual method in our laboratory using cytology (vaginal smears) and demonstrating significant differences in body weight and behavior following experimenter-blind assessment. Only female mice that were scored identically (over 90%) were used in the study. There were 15–19 subjects per genotype and drug condition (saline or EtOH) in order to get a minimum of 5 subjects per genotype, condition, and hormonal state (male, estrous female and non-estrous female). An intraperitoneal injection (i.p.) of either 1g/kg EtOH (20% vol:vol) or equivolume saline was administered 14 min prior to TST evaluation, in order to evaluate behavior during the period when EtOH brain concentrations and effects are approaching peak levels, Mice were individually housed between injection and testing in order to control for differential interactions depending upon drug or saline. Latency to immobility, number of immobile segments and total time spent immobile were recorded for 6 min.
Statistical Analysis
Data were analyzed separately for each experiment by factorial analysis of variance (ANOVA) in SPSS: first by genotype, sex or hormonal status, and drug (where appropriate) and then, in the absence of significant interactions with sex/hormone status, collapsing across this factor. Significant main effects and interactions were investigated further using Tukey’s HSD test for post hoc comparisons. In all cases, the criterion for significance (α level) was set at p ≤ 0.05.
RESULTS
Experiment 1 and 2, Basal Immobility
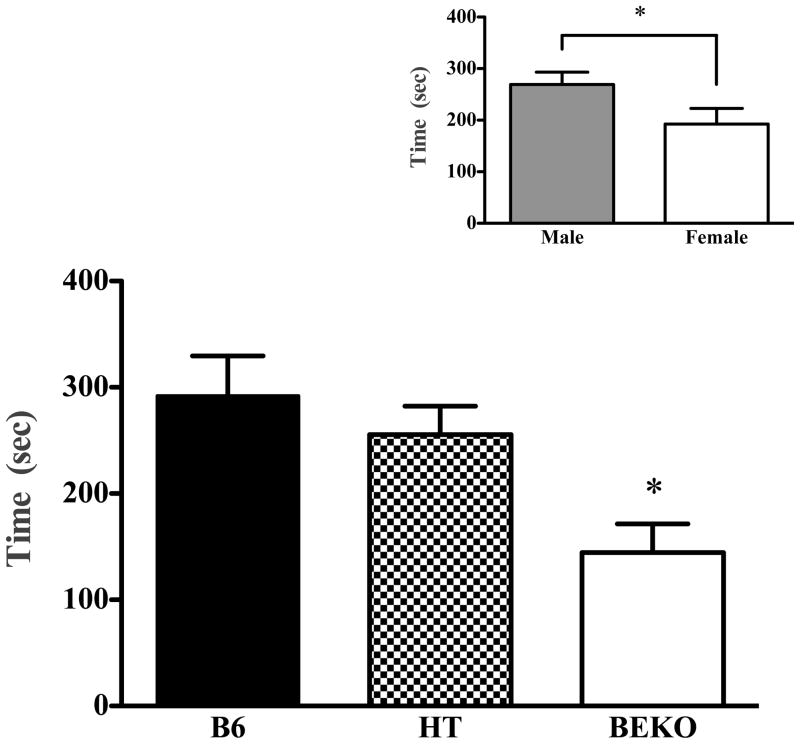
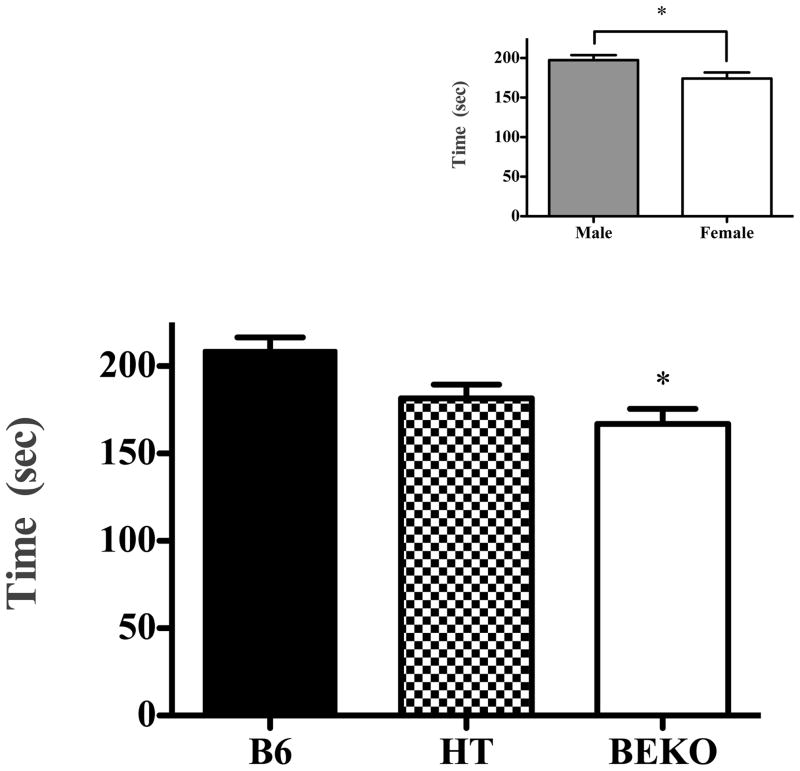
β-endorphin genotype was directly related to immobility in both the FST and TST. In the FST (see Fig. 1), B6 mice spent the most time immobile and the KO mice spent the least time immobile (F(2,50) = 6.244; p < 0.005). Likewise, in the tail suspension test, increasing β-E increased immobility (Fig. 2; F(2,49) = 8.77; p < 0.001). There were also significant effects of sex in both the FST (F(1,50) = 4.469; p < .05) and the TST (F(1,49) = 6.658; p < 0.01) with males spending more time immobile, though there were no significant interactions between genotype and sex on either of these tests (p > 0.05; see insets, Fig. 1 and 2). Despite a tendency toward increased latency to immobility as β-E levels decreased, neither this, nor the total number of immobile segments, depended upon genotype, sex, or their interaction (data not shown, p > 0.05).
Fig. 1.
Experiment 1 evaluated immobility in wildtype C57BL6/J (B6), heterozygote (HT) and β-E ‘KO’ mice in the forced swim test. The lower panel shows the amount of time mice spent immobile during the 15 min test (data show mean ±SE). βEKO mice differed significantly from B6’s. The inset panel shows sex differences in time immobile, collapsed across strain. Significant differences were determined following ANOVA by post hoc analysis (Tukey’s HSD) test and are designated by an asterisk (all p values ≤ 0.05).
Fig. 2.
Experiment 2 evaluated immobility of B6, HT and βEKO mice in the tail suspension test. The lower panel shows the amount of time spent immobile during the 6- min test (data show mean ± SE). There was a tendency for decreased immobility in HTs (p = .068 in Tukey’s post hoc test). The inset panel shows sex differences in immobility time, collapsed across strain. Significant differences from control (B6) or between groups are designated with an asterisk following ANOVA by post hoc analysis (Tukey’s HSD) test.
Experiment 3
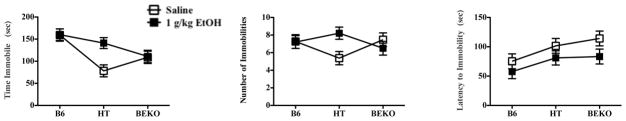
β-endorphin level was again directly correlated with the total time immobile in the TST (Fig. 3, left panel; F(2,97) = 9.258; p < 0.001) replicating the results of Experiment 1. There was also a main effect of drug on immobility, in that EtOH increased total time spent immobile (F(1,97) = 3.888; p = 0.052). Moreover, the effect of EtOH depended upon genotype (F(2,97) = 3.675; p < 0.05) reflecting particularly robust drug effects in HT but not other genotypes (Tukey’s HSD p ≤ 0.05). These data are depicted in Fig. 3. There was no significant effect of sex (M/F) or hormone status (male, estrous female and non-estrous female) nor was there any interaction with these factors (p’s all > 0.05).
Fig. 3.
Experiment 3 evaluated behavior of B6, HT and βEKO mice in the tail suspension test (data show mean ±SE). The left panel shows time spent immobile during the 6-min test in all genotypes following either saline or 1 g/kg EtOH, in which there were main effects of genotype and drug, as well as a significant interaction, reflecting enhanced drug efficacy in HT mice. The middle panel shows an the number of immobilities in these groups during the same test, which did not depend upon genotype or drug, but again, a significant interaction between these factors reflects a drug effect in HT mice. The right panel shows the latency to become immobile during this test, which did depend upon genotype and drug. Significant differences between groups were determined following ANOVA by post hoc analysis (Tukey’s HSD) and are discussed more fully in the text.
In this study, although the number of immobile segments during the 6 min test were no more prevalent as β-E levels increased (F(2,97) = .181; p > 0.05) or following EtOH (F(1,97) = .966; p > 0.05), EtOH increased the number of immobile segments in HT mice, as demonstrated by a significant interaction between genotype and drug (F(2,97) = 3.594; p ≤ 0.05, and confirmed by Tukey’s HSD; see Fig. 3, middle panel). There were no significant effects of sex hormonal status or interactions with sex or hormonal status on the number of immobile segments displayed.
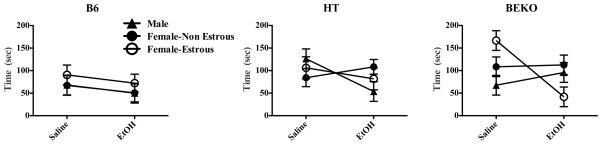
In terms of the latency to first demonstrate immobility (Fig. 3, right hand panel), there was a main effect of genotype (F(2,95) = 3.914; p ≤ 0.05) and drug (F(1,95) = 5.691; p ≤ 0.05) but no interaction (F(2,95) = .141; p > 0.05). Thus, decreased β-E was associated with increased latency to become immobile, and EtOH generally decreased the latency to display immobility,. However, for this measure, despite no significant main effect of sex or hormone status, there was a triple interaction between genotype, EtOH, and hormone/sex (F(4,95) = 3.380; p ≤ 0.05; see Fig. 4). In the left hand panel of Fig 4, B6 mice demonstrate a tendency to assume immobility slightly more quickly with the administration of EtOH, independent of hormone status (though there is a non-significant trend for females in estrous to take longer to first become immobile than either males or non-estrous females who are virtually identical each other). The far right panel of this Figure shows the relationship between hormone status and drug effect in β-E deficient (KO) mice. Female mice in estrous that are entirely lacking β-E struggle the longest in the inescapable situation after saline injections but also show the most profound effect of EtOH in precipitating immobility.
Fig. 4.
A triple interaction between genotype, drug and hormonal status on latency to first show immobility in Experiment 3 is shown here (data show mean ±SE). Effects of drug and genotype (depicted in Fig. 3) depended upon hormonal state; with decreased levels of β-E, EtOH effects became more genotype-dependent.
DISCUSSION
Transgenic mice engineered with a modified capacity to transcribe the opioid peptide β-E showed altered behavioral responses in two murine assays of behavioral despair. The direct relationship found between β-E levels and amount of immobility in both the tail suspension and forced swim tests (TST, FST) supports the idea that β-E contributes to the behavioral consequences of stress (Gianoulakis, 1998; Hunt and Zakhari, 1995; see Yamada and Nabeshima 1995 and Ribiero et al., 2005 for reviews). B6 mice assumed an immobile posture sooner and for a longer period of time in these assays than their counterparts with low or absent β-E. The TST and FST tests were used to subject mice to an inescapable aversive situation whereby failure to exhibit actions aimed at escape may represent an effective coping strategy in a despairing situation. Indeed, experimenters noted that mice deficient in β-E displayed frequent and intense struggling behavior rather than passive coping. Combined with previous results using the plus maze and light dark box assays, (Grisel et al., 2008) these data support the contention that β-E moderates behavioral responses to stressful stimuli.
A significant effect of low dose EtOH (1 g/kg) on immobility in these tests was found primarily in the heterozygous (HT) line of mice, suggesting a β-E-dependent behavioral response to the effects of EtOH. While HT mice behaved similarly to KO mice following a saline injection, HT mice injected with EtOH behaved similarly to B6 mice. This ability of EtOH to normalize behavior in mice with low levels of β-E may be related to their capacity to synthesize and release β-E in response to the drug. These results are consistent with previous findings that EtOH-induced anxiolysis is particularly strong in β-E deficient mice (Grisel et al., 2008) and may help explain why HT mice, producing 50% of the wildtype amount of β-E, consistently self-administered more EtOH than β-E KO or B6 control mice (Grisel et al., 1999; Williams et al., 2007). The alcohol-seeking behavioral trend seen in mice with low levels of β-E (Graham et al., 1998; Grisel et al., 1999) correlates with data showing that alcoholics and at-risk non-alcoholics have lower plasma and basal levels of β-E (de Waele et al., 1992; Gianoulakis, 2001 and 2004; Gianoulakis et al., 1996b). These data support the hypothesis that increased preference for EtOH in HT mice may be due in part to increased sensitivity to the effects of EtOH (Gianoulakis et al., 1989; Froehilch et al., 1990; Williams et al., 2007; Zalewska-Kaszubska, 2004). Furthermore, our data suggests that a β-E deficiency may be indicative of a coping deficiency whereby insufficient attenuation of the stress response may be ameliorated by administration of EtOH. Thus, an individual who is less able to cope with stress (perhaps as a result of a genetic inability to produce sufficient β-E for allostasis of the stress response) may be inclined to self-medicate with alcohol as a substitute coping mechanism.
Transgenic models like the ones used in this study can provide insight into neural substrates of behavior, but data interpretation should evince an appreciation (if not understanding) of the complex, interactive brain systems underlying behavior. Indeed, we have argued elsewhere (Grisel et al., 2008) that a constitutive lack of β-E leads to an over-active stress axis (demonstrated behaviorally and physiologically) and that compensatory adaptation may involve changes in gene expression, chemical signaling, and consequent sensitivity to drugs and behavioral tests. In the present study, selective effects of EtOH in HT mice suggest a direct effect of β-E. This contrasts with our previous results showing augmented effects of EtOH in both HT and KO mice (Grisel et al., 2008). Contributions of both direct and indirect effects of β-E are probable and will be revealed with more empirical studies, but it is worth noting that transgenic models may enable insight about the functional interplay between contributing neural factors (e.g. Mogil and Grisel 1998).
The combination of rewarding and positively reinforcing effects of EtOH are theorized to prompt alcohol drinking, while the negatively reinforcing effects of drinking are theorized to play a larger role in maintaining chronic alcohol drinking, leading in some cases to the development of alcoholism (see Koob and Le Moal, 2008 for a recent review). Acute administration of EtOH induces an increase in the synthesis and release of β-E in the hypothalamus and pituitary gland (de Waele et al., 1993; Keith et al., 1986) as well as dopamine in the nucleus accumbens leading to positive reinforcement (de Waele et al., 1993, Gianoulakis 1998; Goldowitz et al., 2006; Koob and Le Moal 1997; Markou et al., 1998). Negative reinforcing effects have been linked to anxiety-reduction following exposure to alcohol (Goldowitz et al., 2006; Kiefer et al., 2002). Thus, increased release of β-E by acute EtOH administration may encourage the acquisition of alcohol drinking, but decreased synthesis and release of β-E with chronic use may be partly responsible for the maintenance of alcohol drinking (Aguirre et al., 1990; de Waele and Gianoulakis, 1993; Genazzani et al., 1982; Kiefer et al., 2002; Sarkar et al., 2007; Scanlon et al., 1992). Chronic alcohol drinking has been hypothesized to produce tolerance to the effects of alcohol and desensitize the β-E system (Goldowitz et al., 2006; Koob 2003b). Chronic exposure, therefore, may lower levels of β-E and subsequently induce overactivation of the HPA axis (Wand, 2001) such that alcohol withdrawal induces anxiety. Thus, in order to reduce this anxiety and discomfort, alcohol drinking becomes negatively reinforcing, and is maintained by the need to remove the aversive stimulus resulting, perhaps in part, from downregulation of β-E (Aguirre et al., 1995; Diana et al., 1993; Scanlon et al., 1992; Valdez et al., 2004; Becker and Lopez, 2004).
Of the many factors influencing the development of alcoholism in humans (Froehlich et al., 1990) the genetically determined amount of β-E produced (Froelich et al., 1990; Gianoulakis et al., 1996b; Wand et al., 1998) as well as environmental stressors and the ability to cope with those stressors, are modeled in our study. In addition, though the majority of animal studies employ only male subjects, we included both sexes in order to assess hormonal effects that may underlie sex differences. For instance, the statistic that females have significantly lower alcoholism rates than males (Hettema et al., 2003; Hunt and Zakhari, 1995) suggests the possibility of sex-dependent coping mechanisms. Our experiments found that female mice were significantly less immobile than males in both the FST and TST. After acute administration of EtOH, however, we found a triple interaction between strain, drug, and hormonal status (we evaluated three groups: male, female non-estrous, and female estrous) on the latency to become immobile in the TST. In general, with decreasing levels of β-E, EtOH’s effect in the TST became more hormone-dependent (Figure 4). Female β-E deficient mice in estrous were by far the most sensitive to EtOH, showing about a 4-fold reduction in latency to immobility relative to saline-injected controls. While the mechanisms underlying the relationship between EtOH, β-E, and sex hormones are unclear, our data suggests a role for sex hormones in coping abilities depending on levels of β-E. Numerous epidemiological and genetic studies (Grant et al., 2008; Hasin et al., 2007; Hettema et al., 2003; Hunt and Zakhari, 1995; Racz et al., 2008) show significant sex differences in the risk and prevalence of disorders associated with chronic stress; for men, alcohol abuse and dependence, whereas for women, major depressive disorder. These findings suggest sexually dimorphic means of coping; however, future research is needed to elucidate the mechanisms responsible, which may lead to sex-specific approaches to understanding and treatment of anxiety, alcoholism, and depression.
The comorbidity of alcohol abuse and dependence, anxiety disorders, and major depressive disorder has long been observed (Brook et al., 2002; Grant and Harford, 1995; Helzer et al., 1988; Hettema, 2003; Kushner et al., 2000; Regier et al., 1990), but less is known of the shared neural mechanisms involved in all three disorders. While correlation does not prove causation, epidemiological studies (Grant et al., 2005; Hassin et al., 2007; Nunes and Rounsaville, 2006; Nurnberger et al., 2001; Schuckit, 2006) consistently reveal links between genetic risk factors, substance use, and psychiatric disorders. For example, alcohol dependence reliably precedes the onset of major depression for males while anxiety disorders reliably precede the onset of alcoholism and depression (across both sexes; Hettema et al., 2003). Moreover, genetic studies have elucidated similar origins in the development of alcoholism and depression (Hettema et al., 2003; Nurneberger et al., 2001; Todd et al., 1996). Because stress and the ability to cope with stressful stimuli are implicated as causal factors in the development of anxiety, alcoholism and depression (Bastürk et al., 2000; Brown et al., 1995; Darko et al., 1992; Koob, 2006; Racz et al., 2008 for example) β-E may be a common mediator, either directly or indirectly, of these disorders. Our data supports the notion that those with low β-E may be less able to effectively cope with stressful stimuli as a result of insufficient attenuation of the stress response, and thus be more inclined to suffer from anxiety, self-administer alcohol, and develop alcoholism and depression. The fact that most alcoholics are men and that most sufferers of major depression are women, in light of the present findings of sex-dependent influences of β-E, suggests that this peptide may help mediate these differences. Overall, the results of our study support the contention that β-E plays an active role in coping behavior and may be implicated in the complex interplay of stress-related disorders.
Acknowledgments
This publication was made possible by NIH Grant Numbers P20 RR-016461 from the National Center for Research Resources, AA13259 (through the INIA Stress Consortium) and AA13641 from the National Institute on Alcohol Abuse and Alcoholism.
References
- Aguirre JC, del Arbol JL, Rico J, Raya J, Mirand MT. Classification of alcoholics on the basis of plasma b-endorphin concentration. Alcohol. 1995;12:531–534. doi: 10.1016/0741-8329(95)00039-9. [DOI] [PubMed] [Google Scholar]
- Aguirre JC, del Arbol JL, Raya J, Ruiz-Requena ME, Rico IJ. Plasma beta-endorphin levels in chronic alcoholics. Alcohol. 1990;7:409–412. doi: 10.1016/0741-8329(90)90024-7. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature Neuroscience. 2005;8:117–120. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amir S. Involvement of endogenous opioids with forced swimming-induced immobility in mice. Physiology & Behavior. 1981;28:249–251. doi: 10.1016/0031-9384(82)90070-1. [DOI] [PubMed] [Google Scholar]
- Bastürk M, Muhtaroglu S, Karaaslan M, Oguz A, Simsek A, Reyhancan M. The relationship between basal plasma β-endorphin levels and the severity of major depressive episode. Bull Clin Psychopharmacol. 2000;10:117–120. [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;12:1829–38. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance abuse disorders. Psychiatr Clin North Am. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Brooke DW, Brook JS, Zhang C, Cohen P, Whiteman M. Drug use and the risk of major depressive disorder, alcohol dependence, and substance use disorders. Arch Gen Psychiatry. 2002;59:1039–44. doi: 10.1001/archpsyc.59.11.1039. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–45. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Buckingham JC. Stimulation and inhibition of corticotrophin releasing factor secretion by endorphin. Neuroendocrinology. 1986;42:148–152. doi: 10.1159/000124266. [DOI] [PubMed] [Google Scholar]
- Champlain AK, Dorr DL, Gates AH. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol Reprod. 1973;8(4):491–494. doi: 10.1093/biolreprod/8.4.491. [DOI] [PubMed] [Google Scholar]
- Constanopoulos A, Papadaki-Papandreou U, Papaconstantinou E. Increased beta-endorphin but not Leu-enkephalin in plasma due to preoperative stress. Experientia. 1995;51:16–18. [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Darko DF, Risch SC, Gillin JC, Golshan S. Association of beta-endorphin with specific clinical symptoms of depression. Am J Pyschiatry. 1992;149:1162–1167. doi: 10.1176/ajp.149.9.1162. [DOI] [PubMed] [Google Scholar]
- Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: Electrophysiological and biochemical evidence. Proc Natl Acad Sci U S A. 1993;90:7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waele JP, Papachristou D, Gianoulakis C. The alcohol preferring- C57BL/6 mice present an enhanced sensitivity of the hypothalamic b-endorphin system to ethanol than the alcohol avoiding DBA/2 mice. J Pharmacol Exp Ther. 1992;261:788–794. [PubMed] [Google Scholar]
- de Waele JP, Gianoulakis C. Effects of single and repeated exposure to ethanol on hypothalamic b-endorphin and CRH release by the C57Bl/6 and DBA/2 strains of mice. Neuroendocrinology. 1993;57:700–709. doi: 10.1159/000126428. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, Li T-K. Naloxone attenuates voluntary ethanol intake in rats selectively bred for high ethanol preference. Pharmacol Biochem Behav. 1990;35:385–390. doi: 10.1016/0091-3057(90)90174-g. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Zink RW, Li TK, Christian JC. Analysis of heritability of hormonal responses to alcohol in twins: beta-endorphin as a potential biomarker of genetic risk for alcoholism. Alcohol Clin Exp Res. 2000;24:265–277. [PubMed] [Google Scholar]
- Genazzani AR, Nappi G, Facchinetti F. Central deficiency of beta-endorphin in alcohol addicts. J Clin Endocrin Metab. 1982;55:583–586. doi: 10.1210/jcem-55-3-583. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Characterization of the effects of acute ethanol administration on the release of beta-endorphin peptides by the rat hypothalamus. Eur J Pharmacol. 1990;180:21–29. doi: 10.1016/0014-2999(90)90588-w. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. The roles of the hypothalamic-pituitary-adrenal axis and the endogenous opioid system. Alcohol Health & Research World. 1998;22:202–210. [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C. Influence of the endogenous opioid system on high alcohol consumption and genetic predisposition to alcoholism. J Psychiatry Neurosci. 2001;26:304–318. [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Current Topics in Medicinal Chemistry. 2004;4:39–50. doi: 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Barcomb A. Effect of acute ethanol in vivo and in vitro on the b-endorphin system in the rat. Life Sci. 1987;40:19–28. doi: 10.1016/0024-3205(87)90247-5. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Beliveau D, Angelogianni P, Meaney M, Thavundayil J, Tawar V, Dumas M. Different pituitary beta-endorphin and adrenal cortisol response to ethanol in individuals with high and low risk for future development of alcoholism. Life Sci. 1989;45:1097–1109. doi: 10.1016/0024-3205(89)90167-7. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, De Waele JP, Thanvundayil J. Implication of the endogenous opioid system in excessive ethanol consumption. Alcohol. 1996a;13:19–23. doi: 10.1016/0741-8329(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Krishnan B, Thavumdayil J. Enhanced sensitivity of pituitary b-endorphin to ethanol in subjects at high risk of alcoholism. Arch Gen Psychiatry. 1996b;53:250–257. doi: 10.1001/archpsyc.1996.01830030072011. [DOI] [PubMed] [Google Scholar]
- Goldowitz D, Matthews DB, Hamre KM, Mittleman G, Chesler EJ, Becker HC, Lopez MF, Jones SR, Mathews TA, Miles MF, Kerns R, Grant KA. Progress in using mouse inbred strains, consomics, and mutants to identify genes related to stress, anxiety, and alcohol phenotypes. Alcohol Clin Exp Res. 2006;30:1066–1078. doi: 10.1111/j.1530-0277.2006.00125.x. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Low MJ, Cunningham CL. Intravenous self-administration of ethanol in the b-endorphin deficient mice. Alcohol Clin Exp Res. 1998;22:1093–1098. [PubMed] [Google Scholar]
- Grant BF, Hartford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend. 1995;39:197–206. doi: 10.1016/0376-8716(95)01160-4. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Blanco C, Stinson FS, Chou P, Goldstein RB, Dawson DA, Smith S, Shara TD, Huang B. The epidemiology of social anxiety disorder in the United States: Results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2005;66:1351–1361. doi: 10.4088/jcp.v66n1102. [DOI] [PubMed] [Google Scholar]
- Grisel JE, Bartels JL, Allen SA, Turgeon VL. Influence of β-Endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacol. 2008;200:105–115. doi: 10.1007/s00213-008-1161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisel JE, Mogil JS, Grahame NJ, Rubinstein M, Bellknap JK, Crabbe JC, Low MJ. Ethanol oral self-administration is increased in mutant mice with decreased beta endorphin expression. Brain Res. 1999;835:62–67. doi: 10.1016/s0006-8993(99)01384-0. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Helzer JE, Pryzbeck TR. The co-occurrence of alcoholism with other psychiatric disorders in the general population and it impact on treatment. J Stud Alcohol. 1988;49:219–24. doi: 10.15288/jsa.1988.49.219. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology. 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Kendler KS. The effects of anxiety, substance abuse and conduct disorders on risk of major depressive disorder. Psych Med. 2003;33:1423–1432. doi: 10.1017/s0033291703008365. [DOI] [PubMed] [Google Scholar]
- Hunt WA, Zakhari S. Stress, Gender, and Alcohol-Seeking Behavior. NIH; Bethesda: 1995. [Google Scholar]
- Janssen SA, Arntz A. Real-life stress and opioid mediated analgesia in novice parachute jumpers. J Psychophysiol. 2001;15:106–113. [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Horntrich M, Jahn H, Wiedemann K. Is withdrawal-induced anxiety in alcoholism based on b-endorphin deficiency? Psychopharmacol. 2002;162:433–437. doi: 10.1007/s00213-002-1118-y. [DOI] [PubMed] [Google Scholar]
- Keith LD, Crabbe JC, Robertson LM, Kendall JW. Ethanol stimulated endorphin and corticotropin secretion in vitro. Brain Res. 1986;367:222–229. doi: 10.1016/0006-8993(86)91595-7. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism and beyond. Alcohol Clin Exp Res. 2003a;21:232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003b;6:442–52. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101:23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal ML. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal ML. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Dhir A. Effect of various classes of antidepressants in behavioral paradigms of despair. Prog Neurospychopharmacol Biol Psychiatry. 2007;31:1248–1254. doi: 10.1016/j.pnpbp.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–171. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Low MJ. Role of proopiomelanocortin neurons and peptides in the regulation of energy homeostasis. J Endocrinol Invest. 2004;27(6 Suppl):95–100. [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. An in vivo profile of beta-endorphin release in the arcuate nucleus and nucleus accumbens following exposure to stress or alcohol. Neuroscience. 2004;127:777–784. doi: 10.1016/j.neuroscience.2004.05.047. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: A self-medication hypothesis. Neuropyschopharmacol. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurbiol of Aging. 2001;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Rounsaville BJ. Comorbidity of substance use with depression and other mental disorders: from Diagnostic and Statistical Manual of Mental Disorder, fourth edition (DSM-IV) to DS-V. Addiction. 2006;101:89–96. doi: 10.1111/j.1360-0443.2006.01585.x. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, JR, Foroud T, Flury L, Su J, Meyer ET, Hu K, Crowe R, Edenburg H, Goate A, Bierut L, Reich T, Schuckit M, Reich W. Evidence for a locus on chromosome 1 that influences the vulnerability to alcoholism and affective disorder. Am J Psychiatry. 2001;158:718–24. doi: 10.1176/appi.ajp.158.5.718. [DOI] [PubMed] [Google Scholar]
- Plotsky PM. Pathways to the secretion of adrenocorticotropin: a review from the portal. J Endocrinol. 1991;3:1–18. doi: 10.1111/j.1365-2826.1991.tb00231.x. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–32. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Racz I, Schürmann B, Karpushova A, Reuter M, Cichon S, Montag C, Fürst R, Schütz C, Franke PE, Strohmaier J, Wienker TF, Terenius L, Ösby U, Gunnar A, Maier W, Bilkei-Gorzó, Nöthen M, Zimmer A. The opiod peptides enkephalin and b-endorphin in alcohol dependence. Bio Psychiatry. 2008;64:989–997. doi: 10.1016/j.biopsych.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiger DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disordes with alcohol and other drugs of abuse: Results from the Epidemiological Catchment Area (ECA) study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK. Interface of physical and emotional stress regulation through the endogenous opioid system and mu-opioid receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1264–1280. doi: 10.1016/j.pnpbp.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Kuhn P, Marano J, Chen C, Boyadjieva N. Alcohol exposure during the developmental period induces beta-endorphin neuronal death and causes alteration in the opioid control of stress axis function. Endocrinology. 2007;148:2828–2834. doi: 10.1210/en.2006-1606. [DOI] [PubMed] [Google Scholar]
- Scanlon MN, Lazar WE, Grant KA, Kunos G. Proopiomelanocortin messenger RNA is decreased in medio-basal hypothalamus of rats made dependent upon ethanol. Alc Clin Exp Res. 1992;16:1147–1151. doi: 10.1111/j.1530-0277.1992.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Schedlowski M, Flüge T, Richter S, Tewes U, Schmidt RE, Wagner TO. Beta-endorphin, but not substance-P, is increased by acute stress in humans. Psychoneuroendocrinology. 1995;20:103–110. doi: 10.1016/0306-4530(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006;101:76–88. doi: 10.1111/j.1360-0443.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- Thiagarajan AB, Mefford IN, Eskay RL. Single-dose ethanol administration activates the hypothalamic-pituitary-adrenal axis; exploration of the mechanism of action. Neuroendocrinology. 1989;50:427–432. doi: 10.1159/000125259. [DOI] [PubMed] [Google Scholar]
- Todd RD, Gellar B, Neuman R, Fox LW, Hickok J. Increased prevalence of alcoholism in relatives of depressed and bipolar children. J Am Acad Child Adolesc Psychiatry. 1996;35:716–724. doi: 10.1097/00004583-199606000-00011. [DOI] [PubMed] [Google Scholar]
- Ulm RR, Volpicelli JR, Volpicelli LA. Opiates and alcohol self-administration in animals. J Clin Psychiatry. 1995;56:5–14. [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotrophin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Wand GS, Mangold D, El Deiry S, McCaul ME, Hoover D. Family history of alcoholism and hypothalamic opioidergic activity. Arch Gen Psychiatry. 1998;55:1114–1119. doi: 10.1001/archpsyc.55.12.1114. [DOI] [PubMed] [Google Scholar]
- Williams SB, Holloway A, Karwan K, Allen SS, Grisel JE. Oral self-administration of Ethanol in transgenic mice lacking b-endorphin. Impulse Online Journal 2007 [Google Scholar]
- Yamada K, Nabeshima T. Stress-induced behavioral responses and multiple opioid systems in the brain. Behav Brain Res. 1995;67:133–145. doi: 10.1016/0166-4328(94)00150-e. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Toda K, Hayashi Y. Stressful training changes endogenous neurotransmitters in human plasma. Stress and Health. 2004;20:159–163. [Google Scholar]
- Zalewska-Kaszubska J, Czarnecka E. Deficit in beta-endorphin peptide and tendency to alcohol abuse. Peptides. 2004;26:701–705. doi: 10.1016/j.peptides.2004.11.010. [DOI] [PubMed] [Google Scholar]