Abstract
Extracellular thiol/disulfide redox environments are highly regulated in healthy individuals and become oxidized in disease. This oxidation affects the function of cell surface receptors, ion channels, and structural proteins. Downstream signaling due to changes in extracellular redox potential can be studied using a redox clamp in which thiol and disulfide concentrations are varied to obtain a series of controlled redox potentials. Previous applications of this approach show that cell proliferation, apoptosis, and proinflammatory signaling respond to extracellular redox potential. Furthermore, gene expression and proteomic studies reveal the global nature of redox effects, and different cell types, for example, endothelial cells, fibroblasts, monocytes, and epithelial cells, show cell-specific redox responses. Application of the redox clamp to studies of different signaling pathways could enhance the understanding of redox transitions in many aspects of normal physiology and disease.
1. Introduction
Recognition of the highly regulated nature of extracellular thiol/disulfide couples, measured as GSH/GSSG and cysteine/cystine (Cys/CySS) redox potentials ( Jones et al., 2000), prompted studies to determine whether variation in extracellular redox state changed with cell differentiation (Nkabyo et al., 2002) or affected cell growth and proliferation ( Jonas et al., 200, 2003). The latter studies revealed that human cells in culture regulate the Cys/CySS redox potential (EhCySS) of the culture medium to the same value as found in plasma of young health adults (Fig. 10.1). Subsequent human research has revealed that EhCySS values are more oxidized in association with disease risk factors and specific diseases ( Jones and Liang, 2009). In the present chapter, we describe the redox clamp approach which is useful to study mechanisms whereby variation in extracellular Eh value contributes to disease.
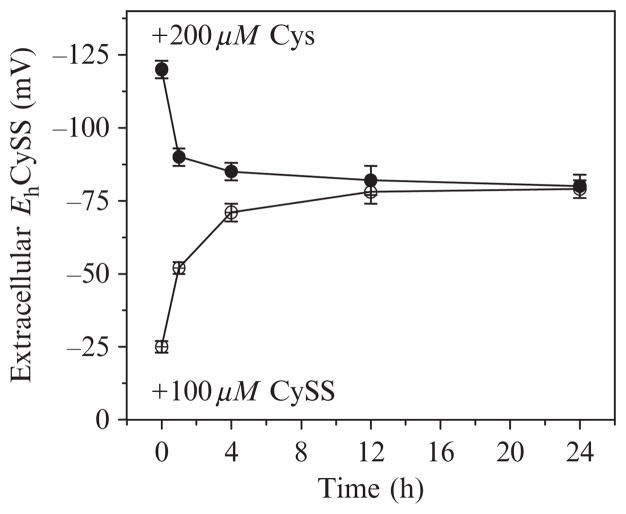
Figure 10.1.
Changes in cysteine/cystine redox potential in culture medium of HT29 cells. Cells were grown to 90% confluence, culture media was removed, and cells were washed once with PBS. Cys and CySS-free DMEM with 10% neonatal calf serum was added with Cys and CySS concentrations calculated as in Table 10.1 to give either −150 or 0 mV. Samples were collected over a 24-h time course and Cys and CySS concentrations were measured by HPLC. Eh was calculated using the Nernst equation.
2. Key Concepts for Use
The GSH/GSSG and Cys/CySS couples represent distinct redox signaling nodes ( Jones et al., 2004) which have not been fully delineated. The initial observations showed that the GSH/GSSG redox potential (EhGSSG) in human plasma is maintained at a more reducing steady-state value (approximately −140 mV) compared to the EhCySS value (approximately −80 mV). Importantly, the Cys/CySS pool size (>90 μM in Cys equivalents) in human plasma is at least 10-fold greater than the GSH/GSSG pool (<9 μM in GSH equivalents). Cell culture media commonly contain CySS, and only some specialized media contain Cys, GSH, or GSSG. Consequently, the most commonly used redox clamp has been created by varying Cys and CySS concentrations.
Under cell culture conditions, cells slowly release GSH into the culture media, and GSH reacts with CySS to produce the disulfide of Cys and GSH, CySSG, and a small amount of GSSG (Reed and Beatty, 1978). The rates of these processes are relatively slow in tissue culture so that unlike the in vivo situation for plasma, EhCySS and EhGSSG are equilibrated in the culture medium. Thus, studies with a Cys/CySS redox clamp do not discriminate between effects of EhCySS and EhGSSG. One can anticipate that systematic variation in EhGSSG could yield cellular responses which are distinct from those of EhCySS due to differences in accessibility and reactivity of the couples with specific protein thiols but this has not been explicitly shown. Differences are expected because GSH and GSSG are negatively charged at physiologic pH while Cys and CySS are neutral. Moreover, in lung lining fluid and bile, the GSH/GSSG pools are in higher abundance than the Cys/CySS pools. Consequently, in this description of the redox clamp, we include conditions for systematic variation of both Cys/CySS and GSH/GSSG pools to facilitate studies to discriminate effects of each couple (see Tables 10.1, 10.2, 10.3). Similar studies with a homocysteine/homocystine clamp could be useful to understand possible mechanisms of total homocysteine in cardiovascular disease (CVD).
Table 10.1.
Cys and CySS concentrations and respective redox potentials in pool size of 200 μM Cys equivalents
| Cys (μM) | CySS (μM) | EhCySS (mV) |
|---|---|---|
| 0.6 | 99.7 | 0.9 |
| 4.0 | 98.0 | −49.8 |
| 14.0 | 93.0 | −82.5 |
| 40.0 | 80.0 | −111.3 |
| 80.0 | 60.0 | −132.8 |
| 130.0 | 35.0 | −152.1 |
Table 10.2.
Cys and CySS concentrations and respective redox potentials in different pool sizes
| Total pool size of Cys equivalents (μM) | Cys (μM) | CySS (μM) | EhCySS (mV) |
|---|---|---|---|
| 100 | 0.4 | 49.8 | 0.5 |
| 10.0 | 45.0 | −83.2 | |
| 78.0 | 11.0 | −153.9 | |
| 200 | 0.6 | 99.7 | −0.9 |
| 14.0 | 93.0 | −82.5 | |
| 130.0 | 35.0 | −152.1 | |
| 400 | 0.8 | 199.6 | 0.6 |
| 20.0 | 190.0 | −82.5 | |
| 220.0 | 90.0 | −153.6 |
Table 10.3.
GSH and GSSG concentrations and respective redox potentials in different pool sizes
| Total pool size of GSH equivalents (μM) | GSH (μM) | GSSG (μM) | EhGSSG (mV) |
|---|---|---|---|
| 100 | 0.2 | 49.9 | 4.3 |
| 5 | 47.5 | −78.8 | |
| 55 | 22.5 | −149.8 | |
| 99.4 | 0.3 | −220.3 | |
| 200 | 0.4 | 99.8 | −4.5 |
| 8 | 96 | −81.8 | |
| 92 | 54 | −151.8 | |
| 198 | 1 | −222.5 | |
| 400 | 0.5 | 199.8 | −1.4 |
| 10 | 195 | −78.4 | |
| 140 | 130 | −151.3 | |
| 391 | 4.5 | −220.7 |
Human studies show that some disease risks associate with plasma EhCySS while others associate with plasma EhGSSG ( Jones and Liang, 2009). As summarized below, several studies using a Cys/CySS redox clamp show cell responses to variation in EhCySS. In addition, Essex and Li (2003) found that platelet activation was maximal with a mixture of GSH and GSSG. The optimal value for EhGSSG calculated from their experimental conditions corresponded to the physiologic EhGSSG found in human plasma. These results highlight the need to consider cellular responses to EhGSSG separately from EhCySS.
3. Principles for Experimental Design
Desired Eh values are obtained by addition of selected concentrations of thiol and disulfide
A redox clamp is obtained by using a relatively large pool of Cys plus CySS in which the concentrations of Cys and CySS are set to values which give desired Eh values according to the Nernst equation:
In this equation, Eo is the standard half-cell potential (− 250 mV for pH 7.4) for the Cys/CySS couple relative to a standard hydrogen electrode, R is the gas constant, T is absolute temperature, and F is Faraday’s constant. The value for n is 2 for a two-electron transfer, so that an alternative form of the equation, with combined constants is
where concentrations are expressed in molar values and EhCySS is expressed in millivolts.
A total cysteine pool of 200 μM provides a reasonable compromise condition for most experiments
A redox clamp can be obtained with different pool sizes. We typically use a pool size of 200 μM Cys equivalents, that is, CySS concentration is multiplied by 2 to express in Cys equivalents, and this is added to Cys concentration (Table 10.1). This provides an initial pool which is at the upper limit of physiologic concentrations but is useful because the concentrations are too high for most cell lines to normalize the Eh within 12 h (Fig. 10.2A). Consequently, with this condition, culture media is changed every 12 h to maintain the clamp (Fig. 10.2B) or more frequently if necessary. It is important to note that rapidly proliferating cells tend to adjust the Eh of the culture medium more rapidly than slowly proliferating cells, and that addition of growth factors increases this rate ( Jonas et al., 2003). Thus, the redox clamp as described does not provide precisely clamped values but rather Eh ranges (see Fig. 10.2A). More precise control can be obtained with a biofermenter which provides continuous control of Eh, along with control of pH and pO2 (Hwang and Sinske, 1991).
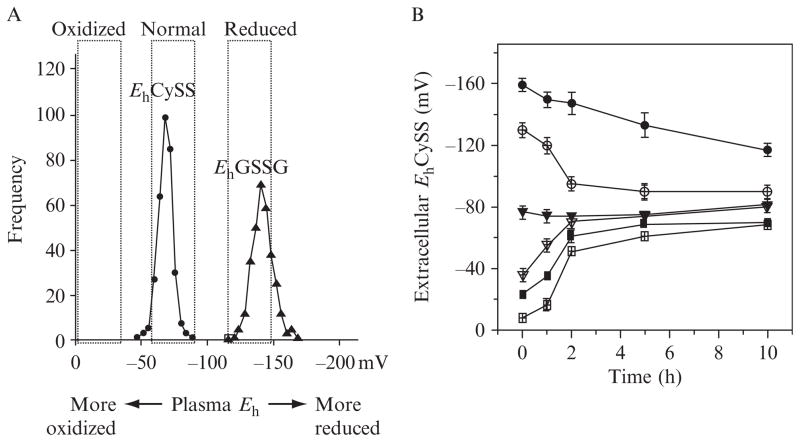
Figure 10.2.
Human plasma redox states of thiol/disulfide couples, GSH/GSSG, and Cys/CySS, and control of extracellular EhCySS by cultured cells. (A) Histogram showing frequency of EhCySS and EhGSSG in human plasma. Data are fasting values for young, healthy, and physically fit individuals. More reduced, normal and more oxidized ranges of plasma redox potential are indicated by the labels at the top. (B) Changes in extracellular EhCySS with time were measured in cultured bovine aortic endothelial cells (Go and Jones, 2005), human retinal pigment epithelial cells ( Jiang et al., 2005), and human colonic cancer cells, HT-29 (Anderson et al., 2007). Cells were incubated with different initial EhCySS (−159, −130, −77, −35, −23, −8 mV) and concentrations of Cys and CySS were quantified as function of time (0, 1, 2, 4, 10 h). EhCySS values calculated from the Nernst equation are shown as mean ± S.E.M., n = 3.
Verification of Eh is obtained by analysis of respective thiol and disulfide concentrations or measurement with a potentiometric electrode
Cys is relatively unstable in solution but CySS is relatively stable. Most cell culture media contains CySS in the range of 200–400 μM without Cys, GSH, or GSSG. Most conditioned medium contains Cys in the low micromolar range so that upon change of culture media, EhCySS values are in the range of 0 to + 10 mV. To perform redox clamp studies involving more negative values which are characteristic of healthy in vivo conditions, cell culture medium without CySS, Cys, GSH, or GSSG is used to allow appropriate additions of thiol and disulfide to create desired Eh values. Verification of values is obtained by measurement of respective concentrations of Cys and CySS and calculation of the Eh value ( Jones and Liang, 2009) or by potentiometric measurements (Ramirez et al., 2007). Values obtained using potentiometric electrodes are not precisely the same as those calculated from the Nernst equation because of electrode response characteristics; however, the systematic variation in Eh with altered concentrations of Cys and CySS can be readily determined and are in agreement.
Serum has small effect on initial Eh value
Many cells are grown in the presence of serum, which contains a substantial amount of CySS with lesser amounts of Cys, GSH, and GSSG. However, by avoiding use of more than 10% serum, redox clamp studies can be performed with only small adjustments for the contributions of serum (0.5% fetal bovine serum for the studies of endothelial cells). On the other hand, serum contains growth factors and affects cell growth rate. Consequently, serum should be maintained constant in experimental designs.
Controls are needed for unambiguous interpretation of redox clamp results
For any given redox clamp experiment, variations in EhCySS result in systematic variation in three other parameters, Cys concentration, CySS concentration, and [Cys]/[CySS] ratio. Thus, although it is convenient to discuss effects of redox clamp experiments in terms of association with EhCySS, the redox clamp results typically do not allow an unambiguous interpretation. For instance, Cys is required for protein synthesis so that variation in Eh could have effects because the conditions alter the Cys concentration and this affects protein synthesis. This may or may not have any relationship to the prevailing Eh value. Similarly, CySS concentration could determine the rate of cysteinylation of a signaling protein, and this could be kinetically limited by the CySS concentration and not determined by the equilibration with the EhCySS. Alternatively, CySS could be used for cysteinylation but in a rapid and reversible manner. In this case, the equilibrium reaction,
would determine the amount of cysteinylation so that the [Cys]/[CySS] would be the most relevant expression. Cysteinylation involving monothiols results in a 10-fold change in reduced:oxidized ratio with a 60 mV change and does not have as much of a gain-of-function per millivolt as the corresponding dithiol interaction,
where a 60 mV change corresponds to a 100-fold change in reduced:oxidized protein ratio (Gilbert, 1990). As is apparent from these multiple possibilities, that is, dependence upon Cys concentration, CySS concentration, Cys/CySS ratio, or EhCySS, careful experimental designs are needed to discriminate possible mechanisms.
Variation in pool size to discriminate effects of Eh from respective thiol and disulfide variations
A relatively straightforward test to distinguish possibilities is to use different pool sizes with adjustments of both Cys and CySS concentrations to obtain the same Eh values (Table 10.2). With this approach, mechanistic links to Cys or CySS concentration will be separated from those due to Eh. Such an approach indicated that Eh, rather than Cys or CySS concentration, was the important parameter in redox signaling of monocyte adhesion to endothelial cells (Go and Jones, 2005).
Semilog plots allow discrimination of functions related to Eh and to thiol/disulfide ratio
To distinguish effects related to EhCySS from those due to [Cys]/[CySS], one can plot cell response as a function of the log of [Cys]/[CySS] (Gilbert, 1990). For functions dependent upon the ratio, the slope will be 1. In contrast, for processes dependent upon EhCySS, the slope will be 2. Such an approach was used as the basis to conclude that nuclear erythroid 2-related factor-2 (Nrf2) signaling in response to cellular EhGSSG operates through a monothiol mechanism (Hansen et al., 2004).
4. Summary of Available Redox Clamp Studies
Hwang and Sinskey (1991) provided an important contribution to the understanding of redox potential and mammalian cell growth in a study designed to optimize in vitro conditions for production of biologic products using cultured mammalian cells. They noted that three parameters were critical to rapidly obtain maximum cell density, i.e., pO2, pH, and redox potential. They found that with pO2 and pH controlled, the redox potential (measured with a potentiometric electrode) could be maintained by controlled supply of cysteine. Although they did not show that the measured potential was related to the EhCySS and did not examine underlying mechanisms, they showed that maximum cell density could be obtained with a measured potential about −60 mV and that this value was similar for over 20 different mammalian cell lines. The studies of Hwang and Sinskey were performed with a biofermenter which allowed continuous control of pO2, pH, and Eh. Studies summarized below using standard cell culture conditions (95% air, 5% CO2, pH buffered at 7.4) confirm that with nominally constant pO2 and pH conditions, cell density varies as a function of Eh. Importantly, these studies indicate that the effects are specifically linked to EhCySS and are mediated through effects on both cell proliferation and cell death.
A study of a human colon carcinoma cell line (Caco-2) showed that cell proliferation was altered by systematic variation in extracellular EhCySS over a range (0 to −150 mV) that occurs in human plasma (Jonas et al., 2002). Caco-2 cells grow slowly in the absence of serum and respond to growth factors with increased rate of cell division. Incorporation of 5-bromo-2-deoxyuridine (BrdU) showed that DNA synthesis was lowest at the most oxidized extracellular Eh (0 mV). Incorporation increased as a function of redox state, attaining a 100% higher value at the most reduced condition (−150 mV). Addition of insulin-like growth factor-1 (IGF-1) or epidermal growth factor (EGF) increased the rate of BrdU incorporation at more oxidizing redox conditions (0 to −80 mV) but had no effect at −150 mV. Cellular GSH was not significantly affected by extracellular Eh. In the absence of growth factors, extracellular Eh values were largely maintained for 24 h. However, IGF-1 or EGF stimulated a change in extracellular redox to values similar to that for EhCySS in plasma of young, healthy individuals. The results showed that extracellular EhCySS modulates cell proliferation rate and that this control interacts with growth factor signaling apparently independent of cellular GSH.
In a follow-up study with Caco-2 cells ( Jonas et al., 2003), the effects of extracellular EhCySS on glutamine (Gln) and keratinocyte growth factor (KGF)-stimulated cell proliferation were studied. Gln (10 mM) or KGF (10 μg/l) did not alter BrdU incorporation at reducing Eh (−131 to −150 mV), but significantly increased incorporation at more oxidizing Eh values (Gln at 0 to −109 mV; KGF at −46 to −80 mV). Cellular EhGSSG was unaffected by Gln, KGF, or variations in extracellular EhCySS. Control cells largely maintained extracellular Eh at initial values after 24 h (− 36 to −136 mV). However, extracellular EhCySS shifted toward a narrow physiological range with Gln and KGF treatment (Gln, −56 to −88 mV; KGF, −76 to −92 mV). The results showed that EhCySS is an important determinant of Caco-2 cell proliferation induced by Gln and KGF, that this control is independent of intracellular GSH redox status, and that both Gln and KGF enhance the capability of Caco-2 cells to modulate extremes of extracellular redox.
A more reduced extracellular EhCySS activates cell proliferation in Caco-2 cells through the mitogenic p44/p42 mitogen-activated protein kinase (MAPK) pathway (Nkabyo et al., 2005). In the absence of added growth factors, −150 mV induced an 80% increase in EGFR phosphorylation, and this was followed by a marked increase in phosphorylation of p44/p42 MAPK. Inhibitors of EGFR (AG1478) and p44/p42 MAPK (U0126) phosphorylation blocked redox-dependent p44/p42 phosphorylation, indicating that signaling occurred by EGFR. These effects were inhibited by pretreatment with a nonpermeant alkylating agent, showing that signaling involved thiols accessible to the extracellular space. The EGFR ligand, transforming growth factor-α (TGF-α), was increased in culture medium at more reduced redox states. Redox-dependent phosphorylation of EGFR was completely prevented by a metalloproteinase inhibitor (GM6001), and an antibody to TGF-α partially inhibited the phosphorylation of p44/p42 MAPK. Together, the data showed that an EhCySS-dependent activation of a metalloproteinase stimulates the mitogenic p44/p42 MAPK pathway by a TGF-α-dependent mechanism.
Redox clamp studies were used with cultured aortic endothelial cells and monocytes to determine whether oxidized values of EhCySS could contribute in a causal way to atherosclerosis development (Go and Jones, 2005). Endothelial cells were exposed to initial EhCySS from − 150 to 0 mV. Compared with the more reduced Eh, oxidized EhCySS stimulated cellular H2O2 but not nitric oxide production, activated nuclear factor-κB, increased expression of adhesion molecules (intercellular adhesion molecule-1, platelet endothelial cell adhesion molecule-1, P-selectin), and stimulated monocyte binding to endothelial cells. Measurement of protein thiols in the extracellular membrane proteins showed that extracellular EhCySS regulated redox states of membrane proteins, indicating that variation in extracellular EhCySS is detected and signaled at the cell surface.
A subsequent study (Go et al., 2009a) showed that mitochondria are a major source of oxidant production in the NF-κB signaling response to a more oxidized extracellular EhCySS. Analyses with mitochondrial ROS-sensitive reagents, MitoSox and MitoTracker showed that oxidant production in response to EhCySS in mouse aortic endothelial cells occurred in mitochondria and that this production was blocked in cells from thioredoxin-2 (Trx2) transgenic mice. Mass spectrometry-based redox proteomics showed that several classes of plasma membrane and cytoskeletal proteins involved in inflammation responded to oxidized EhCySS, including vascular cell adhesion molecule, integrins, actin, and several ras family GTPases. Together, the data show that the proinflammatory effects of oxidized plasma EhCySS in endothelial cells are due to a mitochondrial signaling pathway which is mediated through redox control of downstream effector proteins. Because EhCySS is oxidized in association with risk factors for CVD, including age, smoking, type II diabetes, obesity, and alcohol abuse, the data support a cause–effect relationship of extracellular EhCySS in cell signaling pathways associated with CVD, including those which control monocyte adhesion to endothelial cells.
Microarray analysis and mass spectrometry-based proteomics were used to evaluate global changes in protein redox state, gene expression, and protein abundance in THP1 monocytes in response to EhCySS (Go et al., 2009b). The percentage oxidized protein thiols for −150 and 0 mV were determined using nanoLC-MS/MS with a redox ICAT method (Go et al., 2009c). Oxidized EhCySS in THP1 monocytes resulted in approximately 10% of the peptides detected being more oxidized. Despite the global nature of these proteomic changes, oxidation of the major cytoplasmic thiol systems, GSH and Trx1, was not detected. The relative abundance of proteins for −150 and 0 mV were determined using nanoLC-MS/MS with a quantitative ICAT method. Protein abundance results showed that oxidized extracellular EhCySS stimulated toxicologic and canonical pathways mediated by Nrf2. Abundance of six proteins in this pathway were found to be higher at 0 mV compared with −150 mV, including ACTB (actin-beta, P60709), CAT (catalase, P04040), CCT7 (chaperonin containing TCP1, Q99832), GSTM3 (glutathione S-transferase M3, P21266), HSP90AB1 (heat shock protein 90kD, P08238), and VCP (valosin-containing protein, P55072). Thus, the quantitative proteomics analyses showed that oxidized EhCySS increased abundance of components linked to oxidative stress and glutathione metabolism. Microarray and pathway analysis also showed activation of stress/detoxification pathways (e.g., NADPH:quinone oxidoreductase-1, ferritin, maf) at 0 mV and revealed that interleukin (IL)-1β-related pathways and cell death pathways were also increased by the oxidized EhCySS.
In contrast, components of cell growth and proliferation pathways were increased by −150 mV. The genes expressed highly by the reduced redox state included transcription factors and regulators [early growth response protein (egr1, 2, 3), cyclin-l-1 (ccnl1), nuclear receptor subfamily-4 (nr4a2), polo-like kinase-2 (plk2), and prostaglandin-endoperoxide synthase2 (ptgs2)] supporting positive regulation of cell growth and proliferation. Phenotypic studies confirmed that a cell stress response occurred with oxidized EhCySS and that cell proliferation was stimulated with reduced EhCySS. The results from this study Go et al. (2009b) with THP1 cells support the conclusion that plasma EhCySS provides a control over monocyte phenotype which could contribute to CVD risk and provide a novel therapeutic target for disease prevention.
The effect of oxidized EhCySS on proinflammatory signaling was studied in more detail in U937 monocytes, specifically examining whether oxidized EhCySS is a determinant of IL-1β levels (Iyer et al., 2009). Results showed a 1.7-fold increase in secreted pro-IL-1β levels in U937 monocytes exposed to −46 mV compared to controls exposed to a physiological Eh of −80 mV. This response was directly confirmed in LPS-challenged mice, where preservation of plasma EhCySS from oxidation by dietary sulfur amino acid (SAA) supplementation was associated with a 1.6-fold decrease in plasma IL-1β compared to control mice fed an isonitrogenous SAA-adequate diet. Similarly, analysis of EhCySS and IL-1β in human plasma revealed a significant positive association between oxidized EhCySS and IL-1β after controlling for age, gender, and BMI. Together, the data substantiate the value of the redox clamp approach in showing that oxidized extracellular EhCySS is a determinant of IL-1β levels.
Additional support for stimulation of cell death pathways by an oxidized EhCySS was obtained using the redox clamp in study of oxidant-induced apoptosis in human retinal pigment epithelial (hRPE) cells (Jiang et al., 2005). hRPE cells were incubated in culture medium with EhCySS varied from +16 mV (most oxidized) to −158 mV (most reduced). The hRPE were sensitized to tert-butylhydroperoxide (tBH)-induced apoptosis in the more oxidized extracellular conditions (Eh > − 55 mV) compared with the reduced conditions (Eh < − 89 mV). Loss of mitochondrial membrane potential (Δμm), release of cytochrome c, and activation of caspase 3 after tBH treatments all increased under the more oxidized conditions. In contrast, EhCySS did not affect expression of Fas or FasL in hRPE cells. The results suggest that the oxidized EhCySS, which has been found in patients with age-related macular degeneration (Moriarty-Craige et al., 2005), could contribute to susceptibility of hRPE to oxidant-induced apoptosis through the intrinsic mitochondrial pathway, and thereby contribute to an age-related decline in cell populations. The studies do not exclude a contribution of the Fas pathway to macular degeneration, but suggest that the increased sFasL, which has been found to increase in human plasma with age ( Jiang et al., 2008), is not likely to occur directly in the hRPE in response to an oxidized EhCySS.
Additional support for Nrf2-dependent activation of cell protective systems in response to oxidized EhCySS was obtained by applying the redox clamp to NIH 3T3 fibroblasts (Imhoff and Hansen, 2009). An oxidized extracellular Cys/CySS redox potential was found to activate nuclear factor-erythroid 2-related factor 2 (Nrf2) and induce an antioxidant response. Cellular and mitochondrial oxidant production increased in cells at 0 and −46 mV compared to −80 mV, and mitochondrial thioredoxin (Trx2) became oxidized. The study confirmed the findings described above that oxidized extracellular EhCySS stimulates mitochondrial oxidant generation and activates Nrf2-regulated gene expression.
Studies with the redox clamp also show that oxidized EhCySS activates profibrotic signaling pathways which could contribute to pulmonary fibrosis by stimulating lung fibroblast proliferation and matrix deposition (Ramirez et al., 2007). Such studies are important because mechanisms that link oxidant stress to fibrogenesis remain only partially elucidated. Unlike cells described above, which proliferated more rapidly with more reduced EhCySS (<−80 mV), primary murine lung fibroblasts were stimulated to proliferate when exposed to an oxidized EhCySS (>−46 mV). The oxidized condition also stimulated expression of fibronectin, a matrix glycoprotein highly expressed in fibrotic lung diseases and implicated in lung injury. This stimulatory effect was dependent on protein kinase C activation. Oxidized EhCySS increased the phosphorylation of cAMP response element binding protein, a transcription factor known for its ability to stimulate fibronectin expression, and increased the expression of mRNAs and proteins coding for the transcription factors, nuclear factor, NF-κB and mothers against decapentaplegic homolog 3 (SMAD3). Fibroblasts cultured in normal (−80 mV) or reduced (−131 mV) EhCySS showed less induction. Fibronectin expression in response to an oxidized EhCySS value was associated with expression of TGF-β1 and was inhibited by an anti-TGF-β1 antibody and SB-431542, a TGF-β1 receptor inhibitor. Thus, the redox clamp approach provided important evidence that an oxidized EhCySS could contribute to pulmonary fibrosis by stimulating lung fibroblast proliferation and matrix expression through upregulation of TGF-β1.
5. Perspectives and Conclusion
The redox clamp approach as described provides a straightforward and convenient means to test for redox signaling processes which are dependent upon extracellular EhCySS or EhGSSG. The cumulative evidence indicates that multiple extracellular and cell surface proteins are responsive to thiols and disulfides in the plasma and other extracellular fluids. Although information on Eh values for thiol/disulfide couples in biologic fluids is limited, the available data has been recently reviewed (Go and Jones, 2008) and provides sufficient information to design studies for many cell types. Importantly, the available data show that cell types differ in their responses to extracellular redox potential so that there is a considerable opportunity to apply the redox clamp approach to different biologic research questions.
Presently, there is little information concerning the polarity of redox responses. Given that epithelial surfaces are highly polarized, different effects are likely to occur with oxidative or reductive changes on the poles of the cell. Such effects could be very important under conditions where barrier integrity fails. Indeed, irrigation solutions designed to provide correct Eh values for biologic surfaces may be useful for a range of surgical and other interventional procedures.
A subject of particular interest for cancer prevention is the possibility that the stimulated cell proliferation due to a more negative EhCySS could contribute to tumor growth. Cells with poor vascularization are likely to be more reduced. The studies with Caco2 cells show that more reduced EhCySS stimulates cell proliferation independent of growth factors and to an extent equivalent to growth factor stimulation. Thus, EhCySS-stimulated growth could be relevant to uncontrolled growth in solid tumors.
Finally, the mechanisms which normally control extracellular redox potentials are largely unknown. In vitro and in situ perfused organ studies show that cells have a considerable activity in redox control. Studies in hepatocytes suggested that regulation of extracellular Eh could involve GSH release and thiol–disulfide exchange reactions (Reed and Beatty, 1978). However, subsequent research in colon carcinoma (HT29) cells showed that depletion of GSH by pretreatment with buthionine sulfoximine did not impair the cells capacity to regulate extracellular EhCySS (Anderson et al., 2007). The best available data supports the function of a cysteine–cystine shuttle mechanism (Dahm and Jones, 2000) in which CySS is transported into cells, reduced to Cys, and Cys is released to the extracellular fluid.
In summary, the presently described method for use of a thiol–disulfide redox clamp in cell culture provides a means to study effects of thiol/disulfide redox potential on cell functions. The method is limited by the requirement that cell culture media be periodically changed and the lack of continuous, precise control of Eh values. However, the approach has been used in a number of studies with different cell types and reveals fundamental cell properties, including cell proliferation and cell death, are sensitive to Eh. Moreover, pathways relevant to cardiovascular and lung diseases are clearly responsive to Eh when studied using this model. Consequently, the redox clamp should be considered as a general approach to incorporate into in vitro studies of redox signaling.
Acknowledgments
This work was supported by NIH grants ES011195 and ES009047.
References
- Anderson CL, Iyer SS, Ziegler TR, et al. Control of extracellular cysteine/cystine redox state by HT-29 cells is independent of cellular glutathione. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1069–R1075. doi: 10.1152/ajpregu.00195.2007. [DOI] [PubMed] [Google Scholar]
- Dahm LJ, Jones DP. Rat jejunum controls luminal thiol-disulfide redox. J Nutr. 2000;130:2739–2745. doi: 10.1093/jn/130.11.2739. [DOI] [PubMed] [Google Scholar]
- Essex DW, Li M. Redox control of platelet aggregation. Biochemistry. 2003;42:129–136. doi: 10.1021/bi0205045. [DOI] [PubMed] [Google Scholar]
- Gilbert HF. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- Go YM, Jones DP. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation. 2005;111:2973–2980. doi: 10.1161/CIRCULATIONAHA.104.515155. [DOI] [PubMed] [Google Scholar]
- Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Park H, Koval M, et al. A key role for mitochondria in endothelial signaling by plasma cysteine/cystine redox potential. Free Radic Biol Med. 2009a;48:275–283. doi: 10.1016/j.freeradbiomed.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Craige SE, Orr M, et al. Gene and protein responses of human monocytes to extracellular cysteine redox potential. Toxicol Sci. 2009b;112:354–362. doi: 10.1093/toxsci/kfp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Pohl J, Jones DP. Quantification of redox conditions in the nucleus. Methods Mol Biol. 2009c;464:303–317. doi: 10.1007/978-1-60327-461-6_17. [DOI] [PubMed] [Google Scholar]
- Hansen JM, Watson WH, Jones DP. Compartmentation of Nrf-2 redox control: Regulation of cytoplasmic activation by glutathione and DNA binding by thioredoxin-1. Toxicol Sci. 2004;82:308–317. doi: 10.1093/toxsci/kfh231. [DOI] [PubMed] [Google Scholar]
- Hwang C, Sinskey AJ. The role of oxidation-reduction potential in monitoring growth of cultured mammalian cells. In: Spier RE, Griffiths JB, Meignier B, editors. Production of biologicals from animal cells in culture. Vol. 991. Halley Court; Oxford: 1991. pp. 548–567. [Google Scholar]
- Imhoff BR, Hansen JM. Extracellular redox status regulates Nrf2 activation through mitochondrial reactive oxygen species. Biochem J. 2009;424:491–500. doi: 10.1042/BJ20091286. [DOI] [PubMed] [Google Scholar]
- Iyer SS, Accardi CJ, Ziegler TR, et al. Cysteine redox potential determines pro-inflammatory IL-1beta levels. PloS One. 2009;4:e5017. doi: 10.1371/journal.pone.0005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Moriarty-Craige SE, Orr M, et al. Oxidant-induced apoptosis in human retinal pigment epithelial cells: Dependence on extracellular redox state. Invest Ophthalmol Vis Sci. 2005;46:1054–1061. doi: 10.1167/iovs.04-0949. [DOI] [PubMed] [Google Scholar]
- Jiang S, Moriarty-Craige SE, Li C, et al. Associations of plasma-soluble fas ligand with aging and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:1345–1349. doi: 10.1167/iovs.07-0308. [DOI] [PubMed] [Google Scholar]
- Jonas CR, Ziegler TR, Gu LH, et al. Extracellular thiol/disulfide redox state affects proliferation rate in a human colon carcinoma (Caco2) cell line. Free Radic Biol Med. 2002;33:1499–1506. doi: 10.1016/s0891-5849(02)01081-x. [DOI] [PubMed] [Google Scholar]
- Jonas CR, Gu LH, Nkabyo YS, et al. Glutamine and KGF each regulate extracellular thiol/disulfide redox and enhance proliferation in Caco-2 cells. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1421–R1429. doi: 10.1152/ajpregu.00702.2002. [DOI] [PubMed] [Google Scholar]
- Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47:1329–1338. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP, Carlson JL, Mody VC, et al. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28:625–635. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- Jones DP, Go YM, Anderson CL, et al. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J. 2004;18:1246–1248. doi: 10.1096/fj.03-0971fje. [DOI] [PubMed] [Google Scholar]
- Moriarty-Craige SE, Adkison J, Lynn M, et al. Antioxidant supplements prevent oxidation of cysteine/cystine redox in patients with age-related macular degeneration. Am J Ophthalmol. 2005;140:1020–1026. doi: 10.1016/j.ajo.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Nkabyo YS, Ziegler TR, Gu LH, et al. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco-2) cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1352–G1359. doi: 10.1152/ajpgi.00183.2002. [DOI] [PubMed] [Google Scholar]
- Nkabyo YS, Go YM, Ziegler TR, et al. Extracellular cysteine/cystine redox regulates the p44/p42 MAPK pathway by metalloproteinase-dependent epidermal growth factor receptor signaling. Am J Physiol Gastrointest Liver Physiol. 2005;289:G70–G78. doi: 10.1152/ajpgi.00280.2004. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Ramadan B, Ritzenthaler JD, et al. Extracellular cysteine/cystine redox potential controls lung fibroblast proliferation and matrix expression through upregulation of transforming growth factor-beta. Am J Physiol. 2007;293(4):L972–L981. doi: 10.1152/ajplung.00010.2007. [DOI] [PubMed] [Google Scholar]
- Reed DJ, Beatty P. In: Functions of Glutathione in Liver and Kidney. Sies H, Wendel A, editors. Springer-Verlag; Berlin: 1978. pp. 13–21. [Google Scholar]