Abstract
Leprosy remains an important problem globally. Timely detection of new cases and prompt treatment with MDT continue to be the main intervention strategies. We review the various issues related to classification, treatment, drug resistance and the possible steps to eliminate the disease in the near future. The need for newer anti leprosy agents has been felt and various agents like fluroquinolones, macrolides and minocycline have all been tried in various combinations and duration. Uniform MDT in all leprosy patients might be a logical one too. Drug resistance can be identified by PCR based DNA sequence analysis which saves much time. Drugs like thalidomide analogues, pentoxifylline, selective cytokine inhibitory drugs have proved effective in controlling type-2 reaction in leprosy patients. New drugs for leprosy reactions are still needed. Far from being eliminated as a public health problem, leprosy still causes a considerable long term morbidity in both developing and developed world. New treatment and the optimal length of MDT requires further research. We need genome based technology to address the unresolved issues of transmission of M. leprae.
Keywords: Elimination of leprosy, leprosy therapy, drugs in leprosy, multidrug therapy
Introduction
Leprosy is a chronic infectious disease caused by Mycobacterium leprae (M. leprae) which mainly affects the skin and peripheral nerves. The treatment of leprosy has been revolutionized since the introduction of multidrug therapy (MDT) in 1981, following the recommendation of the World Health Organization (WHO).[1] The global detection of new cases has declined by 4% during 2007 when compared to 2006.[2] Although the figures are highly encouraging, the number of new cases detected annually has remained quite stable during the last 15 years.[3] Timely detection of new cases and prompt treatment with MDT continue to be the main intervention strategies. Although these regimens are generally satisfactory, limitations in terms of persisting activity and late reactions/relapses in paucibacillary (PB) leprosy, along with the persistence of dead and/or live organisms in multibacillary (MB) forms of the disease have been observed.[4] This article elaborates the recent trends in the management of leprosy and future plans to eliminate this disease.
WHO Therapy and Its Impact
Issues related to classification
The need for an internationally accepted classification for leprosy was recognized long ago. In 1981, WHO recommended a classification for operational purpose as pauci- and multi-bacillary (PB and MB).[1] This was introduced to simplify disease recognition and to ensure that all patients were apparently treated with MDT. Though WHO classification and treatment have become much simpler for health workers, the expertise needed to recognize a wide range of presentations of symptoms and signs of the disease is gradually vanishing. Initially, WHO incorporated slit skin smears in the classification and patients with bacterial index (BI) of 2+ were only included in MB category of disease. In 1988, WHO further modified its recommendations that a positive smear at any site was sufficient to include in MB category.[5] Much later the need for skin smear was dropped altogether so that the current classification includes anyone with six or more skin lesions under MB.[6] There is a chance of under treatment according to this criteria. In a study from Philippines, it was concluded that 38–51% of patients may have the risk of under treatment if Ridley Jopling's (RJ) classification is incorporated into WHO classification.[7] Hence, the two classifications should be seen as complementary and not as exclusive. However, it may considered that WHO classification may be useful for treatment and RJ classification for predicting the complications in a given patient.[8] Another pitfall of WHO classification is that neuritic leprosy is also not included. In India, the proportion of neuritic leprosy cases has been found to be as high as 18%.[9] It would also be important to consider how newer tests such as PGL-1, T-cell-based tests and skin tests using new antigens fit into the RJ and WHO classification in future.[8]
Issues related to therapy
Therapy of leprosy has also been constantly revised. In the beginning of 1981, WHO recommended to treat MB cases till two consecutive skin smear negative results were achieved.[1] Subsequently, in 1992, fixed duration therapy (FDT) was introduced where MB patients were given treatment for 2 years or 24 pulses in a period of 36 months by which time the dependability of skin smear was removed.[10] Later, in 1995, WHO redefined the therapy with 12 pulses of MDT–MB.[11] Hence, it was recommended to stop treatment at the end of 12 months or if 12 pulses were completed in a period of 18 months. Skin smear examination was not mandatory. Since 1998, the great majority of MB patients has been treated with MDT for only 12 months. However, there is no information about the 5-year relapse rate. Apparently, determination of the relapse rate after 12-month MDT is highly relevant, and should be considered as a top priority for research.[6]
In contrast, the duration of PB therapy has not changed since WHO's first recommendation and remains for 6 months, or six pulses to be completed within 9 months. As most patients in this group are lepromin-positive, it was opined that residual organisms which remain after stoppage of therapy would be tackled by the immunity of the host.
A third category has been introduced as single lesion paucibacillary leprosy (SLPB), where single dose rifampicin 600 mg, ofloxacin 400 mg, and minocycline 100 mg (ROM) therapy was recommended for cure. The recommendation was on the basis of a multicentric double-blind field trial conducted in India.[12] This regimen was applied widely in India, Bangladesh, and Brazil, but patients should be under surveillance for 2 years after administration of therapy. Preliminary results showed only a marginal advantage over the conventional therapy. Report of the international leprosy association technical forum recommended this regimen only in those countries where single lesion leprosy cases are at large.[13]
Need for newer agents
The reason for the need of new drugs and regimens are: (1) From the operational point of view, the recommended duration of treatment, particularly for MB leprosy is still too long. (2) Two of the components of currently administered drugs for MB leprosy, i.e., dapsone and clofazimine are only weakly bactericidal against M. leprae.[14] Hence further shortening the duration of treatment by this regimen might result in high relapse rate. (3) Administration of the daily components, dapsone, and clofazimine cannot be supervised. (4) Patients who cannot tolerate any of the drugs in MDT–MB need a safer and effective alternative.
Over recent years, many important advances have been made in developing molecular diagnostics, in identifying highly effective drugs and designing multidrug regimens for treatment. Several effective regimens have been developed, which are generally satisfactory. Limitations exist in terms of persisting activity and late reactions or relapses in PB leprosy and persistence of dead and/or live organisms in MB forms of the disease.
Fluoroquinolones
This group of compounds exerts their antimicrobial effect by inhibiting α subunit of DNA gyrase (an enzyme which is not affected by any other therapeutic agent in use) and thereby interfering with bacterial DNA replication. Several fluoroquinolones such as ofloxacin, pefloxacin, sparfloxacin, and moxifloxacin have all been found to be effective against M. leprae.[15] Ofloxacin is preferred as the other two drugs need to be given in a very high dose to achieve the same effect as ofloxacin at a dose of 400 mg. It is bactericidal against M. leprae although less so than a single dose of rifampicin. When given daily for 22 days, it killed 99% of the viable organisms.[16] Moxifloxacin has been found to be more effective than the other fluoroquinolones.
Macrolides
Several members of this group have been evaluated, and clarithromycin appears to be promising. Clarithromycin is a semi-synthetic macrolide antibiotic structurally related to erythromycin. Mouse footpad studies showed potent bactericidal activity of this drug, but it is less effective than rifampicin. At a dose of 500 mg per day, the drug is reported to kill 99% of M. leprae by 58 days.[17] Concurrent administration of rifampicin decreases the serum concentration of clarithromycin by 80%.
Minocycline
Minocycline is the only tetracycline which exhibits significant activity against M. leprae. It is because the drug is lipophilic which permits it to penetrate the bacterial cell wall. It is bactericidal against M. leprae but lesser than rifampicin. The drug binds reversibly to the 30 S unit of the ribosome, thus blocks the binding of aminoacyl transfer RNA to the messenger RNA-ribosomal complex and thus inhibits protein synthesis.
Combination regimens
A highly desirable new regimen is one that would permit all of the components to be administered once monthly under supervision, which would significantly reduce the risk of emergence of rifampicin–resistant mutants, caused by irregular administration of the daily dapsone–clofazimine component, and would also simplify the treatment. ROM is the first fully supervisable, monthly administered regimen. Its efficacy of monthly doses for treatment of MB and PB leprosy has been tested in field trials in three different countries.[18,19]
Rifapentine, a rifampicin derivative, is another drug which has pharmacokinetic properties far superior than rifampicin with significantly higher peak serum concentrations and much longer serum half-life.[20] Recent findings from mouse experiments indicate that rifapentine and moxifloxacin are significantly more bactericidal, respectively, than rifampicin and ofloxacin, which is far more bactericidal than ROM. Combination of rifapentine-moxifloxacin-minocycline (PMM) is found to be more superior.[21,22]
Rifampicin 600 mg is also combined with once a month clarithromycin at a dose of 1000 mg and minocycline 200 mg. This regimen has caused significant delay in growth of M. leprae in mice.[23] Similarly, a combination of daily ofloxacin with minocycline along with monthly rifampicin has also been tried. Another regimen included usual MDT–MB along with addition of ofloxacin and minocycline once monthly.[24] Intensive short course regimens consisting of daily doses of rifampicin with ofloxacin for 1 month in MB patients have produced high relapse rates.[25]
Uniform MDT
A common regimen for treatment of PB and MB leprosy is desirable. However, the size of the bacterial populations and the underlying immunological responses are different in two groups. Hence, a common regimen would appear likely to result in over treatment of PB patients or under treatment of MB patients. Recently, the WHO technical Advisory Group (TAG) on Elimination of Leprosy proposed “implementation of a uniform 6-month MB–MDT regimen for all patients.”[26] As there is no proper information on the follow-up of 1 year FDT in MB patients, there is no justification to test the possibility of further shortening the duration of MDT for MB patients to 6 months. Addition of clofazimine in PB regimen in 44 patients has improved the clinical outcome and produced disappearance of lesions and regression of granuloma.[27]
Quadruple regimen
In a study at Belgium, MB patients were given weekly supervised doses of rifampicin, ofloxacin, clofazimine, and minocycline for 6 weeks. Initial results are highly encouraging. Relepse rate was only 2%. However, long-term follow-up is needed in all these shortened regimens.[23]
A-MDT
Adherence to dapsone self-administration in leprosy patients became apparent only when urinary dapsone/cratinine ratio for monitoring ingestion of dapsone was established and tested in many leprosy centers. A review of the results showed that only one-half of the prescribed dapsone was ingested.[28] With MDT, the adherence was only 70–80%.[29] However, to accelerate leprosy elimination process, recently the WHO leprosy programme and its TAG have changed dramatically their position on supervised therapy. They concluded that after the first dose of MDT “supervision of the subsequent monthly component of the MDT regimens is no longer essential.“[30] Further, on the basis of this conclusion they recommended large-scale implementation of accompanied multidrug therapy (AMDT), which refers to a policy that patients are provided the entire supply of MDT drugs at the time of diagnosis, when choosing someone close to them to accompany them with their treatment.[30] The problems which are yet to be solved are: (1) Whether AMDT is to be applied routinely or only in special situations. (2) Who may be chosen to supervise? (3) How does AMDT help? (4) How to train health workers to supervise AMDT? With large-scale implementation of AMDT, the quality of treatment is a matter of real concern. Without a guarantee of quality, quantitative achievement or a declaration of leprosy elimination is meaningless. Therefore, it is time to replace wishful thinking with evidence-based practice, and discontinue the implementation of AMDT as a routine in the field.[31]
Drug resistance
Emergence of rifampicin resistance would create lot of difficulties for an individual patient, and its widespread dissemination would pose a problem to the community and a threat to leprosy control. Rifampicin resistance could emerge rapidly in a very few patients. Although rifampicin resistance was not reported in more than 10 million patients who completed MDT, this could be due to two reasons. (1) Post-MDT surveillance for relapse has been discontinued. (2) Rifampicin susceptibility testing is difficult to carryout. Polymerase chain reaction (PCR)-based DNA sequence analysis of the rpoB gene of M. leprae was in full concordance with those of the susceptibility testing in mouse footpad system.[32] This approach may lead to the diagnosis of 80% rifampicin-resistant strains of M. leprae. Secondary rifampicin resistance could probably exist in patients who have relapsed after completion of MDT. Recently, there have been reports on multidrug resistance to M. leprae. Besides resistance to rifampicin, the strains were also resistant to at least one or more drugs other than dapsone, including ofloxacin and sparfloxacin. Although the number of multidrug-resistant strains is small, their occurrence is indeed an alarm bell and must be closely scrutinized.[33–35] In a recent study, across three countries it was found that from new cases 3% were dapsone-resistant and 2% were rifampicin-resistant. In samples from relapsed patients, 15% were dapsone-resistant and 8% were rifampicin-resistant.[36]
Chemoprophylaxis
Recently, because the new case detection rate (NCDR) has not diminished following implementation of MDT, there has been renewed interest in chemoprophylaxis.[31] Both dapsone and acedapsone were used earlier for this purpose. Two basic principles are followed in chemoprophylaxis. They are: (1) Rifampicin should be one of the components. (2) The treatment should be administered only in a single dose. Rifampicin alone or ROM therapy has been proposed for this. Both have been found to have an equal efficacy and hence single dose of rifampicin alone has been proposed. Only one trial report is available on this aspect which shows that the protective effect was only 35-40%.[37] In this trial, a dose of 1500 mg of rifampicin was administered and this was not compared with 600 mg of rifampicin. The limitations of chemoprophylaxis are: (1) If chemoprophylaxis is confined to only household contacts, the benefit will range up to 15%. (2) The effect of chemoprophylaxis may only be transitory and has to be repeated.
Reactions in leprosy
Reactions in leprosy pose greater problems than the disease itself. For type-1 reaction steroids, chloroquine and cyclosporine are used. Treatment with thalidomide provides an effective alternative to steroid therapy gives better long-term control and avoids the adverse effects of prolonged steroid therapy. The results of cyclosporine varies from the modest to highly effective.[38]
Type-2 reaction has generally declined due to the addition of clofazimine in MB–MDT. Treatment of reaction is on the basis of suppression of inflammation and its consequences. Drugs such as corticosteroids, non-steroidal anti-inflammatory drugs (NSAIDS), chloroquine, antimonials, pentoxiphylline, and thalidomide are used in type-2 reaction. Controlled clinical trials have demonstrated that thalidomide rapidly controls ENL and is superior to aspirin and pentoxifylline. However, thalidomide is teratogenic when taken in early pregnancy and is unavailable in many leprosy endemic countries.[39] Thalidomide analogs, which are chemically similar to thalidomide but appear to lack its side effects are being pursued. Revimid and actimid are promising in this category.[40] They are currently in phase I and II trials.
Pentoxifylline, a methyl xanthine derivative has been used in one study but found to be less effective in the control of type-2 reaction.[41] Though several new drugs are available, steroids and thalidomide are the mainstay in the control of type-2 reaction.[42,43]
Selective cytokine inhibitory drugs (Sel CIDs) are phospho-diesterase type-4 inhibitors with potent TNFα activity but without T-cell co-stimulatory effect. It is currently under investigation.
On the basis of earlier studies on the prevention of deformities following recommendations were made.[3]
Steroids were recommended to treat reactions and nerve function impairments (NFIs) of recent onset; the expected recovery rate for NFI is approximately 60%.
MB patients and those with existing NFIs would be carefully monitored for new nerve function loss, as they are the groups at the greatest risk.
Assessment of nerve function using standard methods every month during MDT is recommended.
Research is recommended to identify the optimal steroid regimen, to develop alternative and more effective for the reactions and recent nerve function loss.
Further research is recommended on the use of prophylactic steroids, in prevention of NFI.
Major points for future investigation are:
Mechanisms of Schwann cell injury in leprosy: TNFα, cytotoxic T-cell, and apoptosis.
The definition of tissue markers as indicative of nerve damage: myelin components, ninjurin adhesions, and ECM components.
Establishment of therapeutic interventions for nerve regeneration: Matrix metalloproteinases inhibitors, inhibitors of apoptosis, adhesion inhibitors, and methycobalamine.
Role of immunotherapy
Besides the presence of a small population of viable organisms (“persisters’) after therapy, the problem of persistence of a large pool of dead bacilli is often encountered. Immunomodulators that can stimulate CMI have been applied to reduce this pool. These agents can be divided into three broad categories.
Drugs such as levamisole and zinc.
Antigenically related mycobacteria such as BCG, ICRC bacillus, BCG plus killed M. leprae, Mycobacterium w (Mw), and M. vaccae.
Other immunomodulators such as transfer factor, recombinant interferon γ(IFN γ), and interleukin-2.
Transfer factor-induced transient effects such as lepromin conversion, granuloma formation, and increased influx of lymphocytes locally.[44] Intralesional administration of IFN γ in leprosy patients induced accumulation of lymphocytes and monocytes at the local site of injection. There was a distinct fall in bacillary index at the local site, with the formation of epithelioid granuloma and occurrence of reversal reaction in some cases. Enhanced bacterial clearance with IFNγ has been reported.[45] The administration of interleukin-2 has accelerated bacterial clearance. However, these effects were seen only at the local site.[46]
With regard to vaccine development for leprosy, the explosion of genomic information from both the pathogen (M. leprae) and its natural hosts (man and armadillo) has been revolutionary. Gene cloning and recombinant protein expression tools can be used for the subsequent immunologic characterization and vaccine efficacy trials of high-priority antigens. Potent vaccines have the added advantage of producing long-lived immunological memory, which can block multiple exposures over host's lifetime. Vaccines may be very useful to take us to the next level disease eradication. The following are ongoing research with future potentials.[47]
Protein make-up of armadillo-derived M. leprae has been studied using two-dimensional polyacrylamide gel electrophoresis. There are 391 cell-associated proteins. These proteins may encompass activities involved in virulence or capable of being immunogenic during various stages of infection.
Testing for vaccine efficacy in the armadillo would also allow for the assessment of efficacy across the disease spectrum as the armadillo manifests similar bacterial growth characteristics and histopathological features observed in humans infected with M. leprae.
Can We Hope to Eliminate Leprosy?
Definition of terms
The principles of disease elimination and eradication have been clearly described by Dowdle in the Bulletin of WHO in 1998.[48] Control of disease is defined as the reduction of disease incidence, prevalence, morbidity, or mortality to a locally acceptable level as a result of deliberate efforts; continued intervention measures are required to maintain the reduction.
Elimination of disease is defined as the reduction to zero of the incidence of a specified disease in a defined geographical area as a result of deliberate efforts; continued intervention measures are required. Similarly, elimination of infection is defined as a reduction to zero of the incidence of infection caused by a specific agent in a defined geographical area as a result of deliberate efforts; continued measures to prevent reestablishment of transmission are required.
Eradication is defined as the permanent reduction to zero of the worldwide incidence of infection caused by a specific agent as the result of deliberate efforts; intervention measures are no longer needed.
Extinction is defined as in which the specific infectious agent no longer exists in nature or in the laboratory.[49]
Milestones in leprosy elimination
In leprosy, WHO limited elimination to control instead of transmission, using prevalence instead of incidence.[50] Elimination in leprosy was defined as reducing the global prevalence to less than 1 per 10,000. The 44th World Health Assembly in 1991 passed a resolution to “eliminate leprosy as a public health problem” by the year 2000. In the year 2000, WHO announced the elimination was achieved globally, i.e., a world prevalence of less than 600,000 leprosy patients. In 2005, a strategic plan for the elimination of leprosy was introduced. By the end of 2005, all but six countries reported a prevalence of less than 1 per 10,000. The six countries are Brazil, Republic of Congo, Madagascar, Mozambique, Nepal, and Tanzania. For the period 2006–2010, WHO introduced the “Global Strategy for Further Reducing the Leprosy Burden and Sustaining Leprosy Control Activities” to address the remaining challenges in providing services for leprosy patients under conditions of low prevalence.
Impact of MDT on trends of transmission
It was assumed that MDT would reduce the transmission of M. leprae through a reduction of the number of contagious individuals in the community, but unfortunately there is no convincing evidence for this hypothesis.[51–54] There were two large-scale studies on trend analysis available to interpret the impact of MDT globally.[51,55] In the first study published by Meima in 1997, the author concluded that factors such as case detection and treatment would reduce leprosy transmission is reasonable, but the reality may be more complicated. Individuals incubating the disease may already harbor many bacilli, and it is possible that those individuals already transmit M. leprae to others long before the onset of the disease. More interestingly, a general acceleration of downward trends in the NCDR after the introduction of MDT has not occurred. In the more recent study, the same author showed no general decline in case detection at global level up to 2000.
There are no suitable tests to detect the subclinical mycobacterial infections reliably, including M. leprae. Assessment of results of leprosy control depends on information about disease and not infection. Disease statistics are expressed in terms of prevalence and new case detection. Disease prevalence in leprosy is measured by counting all patients receiving MDT at a given time and expressing this as a ratio using the population as the denominator (in leprosy, it is per 10,000). Therefore, the figures are linked to the length of treatment. As the duration of treatment is reduced from 24 to 12 months, the prevalence also is reduced by 50%.[50]
For PB group, there is a chance that patients might be missed for that year as those patients on roll only on December, 31 are taken into account. Hence, those patients who completed in the first half of the year may not be included into the figures.[50]
Indian scenario
Detection of new cases in leprosy in 2007 and the number of new cases detected previously:
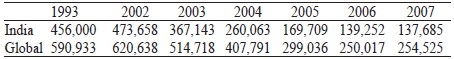
In India, it was found that the NCDR dropped by 75% from 559,938 in 2000 to 139,252 in 2006. The factors which may be responsible are operational influence and BCG vaccination. Protective efficacies against leprosy ranged from 24% to 34% in randomized control trials in India.[56] At the same time, proportion of new cases with WHO grade-2 disability increased from 1.6% to 2.2% between 2004 and 2007 which is an increase by 38%. This is an alarming situation.[54]
Decline in the transmission of incidence (i.e., onset of disease) of leprosy may be related to several factors.[57–59]
The period during which M. leprae is transmitted, which can be reduced by early case detection and chemotherapy.
BCG vaccination, which is not only widely administered as a preventive measure for many against tuberculosis, but also appears to afford protection against leprosy.
Improvement in socioeconomic conditions such as housing conditions, member of persons per household per room, family size, and nutritional factors.
Lessons learnt
The assumption that case detection and treatment would reduce leprosy transmission is reasonable, but the reality may be more complicated. Individuals incubating the disease may already harbor many bacilli, and these individuals might have transmitted M. leprae to others long before the onset of the disease, given its long incubation period. Such transmission could be prevented by early case detection and treatment.
Second problem is delay between the onset of disease and detection. Leprosy is a quiescent disease and hence there may be substantial delay before the patient seeks treatment. The average detection delay exceeded 2 years in a study in Ethiopia.[60] It is possible that close contacts of a leprosy patient become infected rapidly.[60] Other factors which could limit the impact of control are carriage of infection in the nose, persistence of M. leprae in the soil and even in animal reservoirs.[61]
Global leprosy situation at the beginning of 2008-South-east Asia statistics is given below.[62]
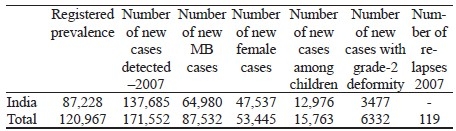
Cure rates % for PB and MB not known.
Leprosy's Global Statistics — Fallacies
Leprosy statistics pose particular problems for surveillance for several reasons.[63]
There are problems with diagnosis and classification of the disease in the field even in good programmes.
Problems in stigmata and confidentiality, which affect reporting practices and official data.
There have been major operational changes in the recent years in many countries.
There are often delays in reporting from some countries.
Statistics have emphasized only prevalence, which is difficult to interpret.
Political pressures associated with elimination initiative, which appear to have influenced the manner of reporting statistics.
The most striking trend in global leprosy in recent years is the decline in India, which reported 137,685 new cases in 2007 when compared to 559,938 in the year 2000. This implies that India's contribution to the global leprosy burden has declined from 73% to 54% of the world's newly detected leprosy cases over these years. It is unclear the extent to which this decline reflects changes in ascertainment and criteria for new cases to be counted in India. It is doubtful whether single lesion cases are being systematically counted.[64] Without such information, this important trend in India's statistics remain difficult to interpret.
Relapse statistics are of considerable interest, because of their potential relevance to drug resistance. Three countries provide more than 80% of the world's total of 2355 reported relapses. India reported no relapses. It is apparent that vast majority of relapses in the world go unreported with present systems.
Again, cure rates should be substituted with treatment completion rate, which is not available for many countries including India. Overall, the global prevalence statistics are not comparable. Due to these problems, they are of little use in monitoring global leprosy trends. WHO has recognized this problem, and the current (2005–2010) Global Strategy urges a concentration upon new case detection.[65]
Scenario Analysis of Leprosy Trends Up to 2020
Now a days, the diagnosis and treatment of leprosy are easy and most endemic countries are striving to fully integrate leprosy services into existing general health services. This is especially important for those under-served and marginalized communities mostly at risk from leprosy, often the poorest of the poor. Access to information, diagnosis, and treatment with MDT remain the key elements in the strategy to eliminate the disease as a public health problem. Most previously, highly endemic countries have now reached elimination (defined as a registered prevalence rate of <1 case/10,000 population). However, pockets of high-endemicity still remain in some areas of Angola, Brazil, Central African Republic, Democratic Republic of Congo, India, Madagaskar, Mozambique, Nepal, and the United Republic of Tanzania. These countries remain highly committed to eliminating the disease, and continue to intensify their control activities.[66]
The need for an internationally accepted classification system for leprosy was recognized long ago. Many classifications have been proposed each with its merits and demerits. It would now be timely to establish agreed clinical and histological case definitions for classifications together with protocols for patient classification. New diagnostic tests such as T-cell-based tests should fit into the new classification and protocols.
MDT-based control appears to reduce transmission. The pace of reduction is highly uncertain, but in any case slow. BCG may enhance the pace, but its impact is also uncertain. M. leprae genome can now be explored using in silico analysis to identify new immuno-dominant genes which can be used for developing new leprosy vaccines. The gray areas in leprosy control are the role of close contact transmission, the speed of transmission, and the extent of contagiousness during the incubation period. Research addressing these questions is essential to narrow down the uncertainty regarding the impact of MDT-based control. There are newer regimens available to treat leprosy, but rifampicin is still considered as the sheet anchor in the treatment of leprosy. Although rifampicin resistance has not yet been reported to occur in a larger scale, we should be aware of this possibility in future and evolve alternative strategies.
More than 13 million cases were detected and treated with MDT between 1982 and 2002. Nevertheless, many new cases are still detected yearly and future projections of the global leprosy burden indicate that at least 5 million new cases will arise between 2000—the year of leprosy “elimination”—and 2020. To attain leprosy elimination in future effective intervention is needed to interrupt the transmission of M. leprae. BCG vaccination does not offer full protection and in the absence any other specific vaccination against the bacillus, other strategies such as chemoprophylaxis of subclinically infected people at risk must be developed. Similarly, the other possibilities such as nonhuman or environmental reservoirs of M. leprae need to be explored. There are considerable numbers of people who are compelled to live under the threat of deteriorating function due to established leprosy-related impairments. It is predicted that there will be still approximately one million cases with WHO grade-2 deformities in the year 2020.[66] With the expertise that is available to intensify research, we continue to anticipate that a more effective treatment will eventually be developed to address the risk of NFI. Prevention of deformities does not require advanced technology but will require advanced thinking.[67]
When elimination of the disease is defined as the reduction to zero of the incidence, leprosy is definitely not eliminated. We need genomic-based technologies to address the unresolved issues of transmission and infection with M. leprae.[68]
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.WHO study group. Chemotherapy of leprosy for control programs. Report of WHO study. World Health Organ Tech Rep Ser. 1982:675. [PubMed] [Google Scholar]
- 2.WHO Global leprosy situation. Wkly Epidemiol Rec. WHO. 2008 Aug 15th; [Google Scholar]
- 3.Report of the International Leprosy Association Technical Forum. Lepr Rev. 2002;73:513–6. [PubMed] [Google Scholar]
- 4.Lockwood DN. Steroids in leprosy type-1 reactions: Mechanism of actions and effectiveness. Lepr Rev. 2000;71:111–4. doi: 10.5935/0305-7518.20000080. [DOI] [PubMed] [Google Scholar]
- 5.WHO Expert Committee on Leprosy. World Health Organ Tech Rep Ser. 1988;768:1–51. [PubMed] [Google Scholar]
- 6.WHO/CDS/CPE/CEE/2002/29. Geneva: WHO; Report of the third meeting of the WHO technical advisory group on the elimination of leprosy. [Google Scholar]
- 7.Pardillo FE, Fajarado TT, Abalos RM. Methods for the classification of leprosy for treatment purposes. Clin Infect Dis. 2007;44:1096–9. doi: 10.1086/512809. [DOI] [PubMed] [Google Scholar]
- 8.Lockwood DN, Sarno E, Smith WC. Classifying leprosy patients - searching for the perfect solution? Lepr Rev. 2007;78:317–20. [PubMed] [Google Scholar]
- 9.Noordeen SK. Epidemiology of poly neuritic type of leprosy. Lepr India. 1972;44:85–93. [Google Scholar]
- 10.Leprosy division. Directorate General of Health Services. New Delhi: 1992. Operational guidelines on case detection, treatment, followup and reporting forms NLEP. [Google Scholar]
- 11.WHO Expert Committee on Lprosy. World Health Organ Tech Rep Ser. 1998;874:1–43. [PubMed] [Google Scholar]
- 12.Efficacy of single dose multi drug therapy for treatment of single lesion paucibacillary leprosy. Single lesion multi centered trial group. Indian J Lepr. 1997;69:121–9. [PubMed] [Google Scholar]
- 13.WHO document. 2nd. Geneva: WHO; 2003. Final push strategy to elimination of leprosy as a public health problem. Questions and Answers. [Google Scholar]
- 14.Shepard CC. A brief review of experience with short-term clinical trials monitored by mouse foot pad inoculation. Lepr Rev. 1981;52:299–308. doi: 10.5935/0305-7518.19810040. [DOI] [PubMed] [Google Scholar]
- 15.Venkatesan K. Pharmacokinetics and drug interactions of newer anti-leprosy drugs. Indian J Dermatol Venereol Leprol. 1997;63:148–52. [PubMed] [Google Scholar]
- 16.Katoch K. New emerging drug regimens for leprosy. Indian J Dermatol Venereol Leprol. 1997;63:130–47. [PubMed] [Google Scholar]
- 17.Jacobson RR. Needed research in chemotherapy of leprosy related to the individual patient. Int J Lepr Other Mycobact Dis. 1996;64:S16–20. [PubMed] [Google Scholar]
- 18.Ji B. Bactericidal activity of a single-dose com-bination of ofloxacin plus minocycline, with or without rifampin, against Mycobacterium leprae in mice and in lepromatous patients. Antimicrob Agents Chemother. 1998;42:1115–20. doi: 10.1128/aac.42.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daumerie D. Current World Health Organization-sponsored studies in the chemotherapy of leprosy. Lepr Rev. 2000;71:88–90. doi: 10.5935/0305-7518.20000075. [DOI] [PubMed] [Google Scholar]
- 20.Ji B, Truffort-Pernot C, Lacriox C, Raviglione MC, O’Brien RJ, Olliaro P, et al. Effectiveness of rifampicin, rifabutin and rifapentine for preventive therapy for tuberculosis in mice. Am Rev Respir Dis. 1993;148:1541–6. doi: 10.1164/ajrccm/148.6_Pt_1.1541. [DOI] [PubMed] [Google Scholar]
- 21.Consigny S. Bactericidal activities of HMR 3647, moxifloxicin and rifapentines against Mycobacterium leprae in mice. Antimicrob Agents Chemother. 2000;44:2919–21. doi: 10.1128/aac.44.10.2919-2921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grosset JH. Study of 39 documented relaps-es of multibacillary leprosy after treatment with rifampin. Int J Lepr Other Mycobact Dis. 1989;57:607–14. [PubMed] [Google Scholar]
- 23.World Health Organisation. Report of the ninth meeting of the WHO technical advisory group on leprosy control: Cairo, Egypt, 6-7 March 2008. Lepr Rev. 2008;79:452–70. [PubMed] [Google Scholar]
- 24.Katoch K. Chemotherapy trials in MB leprosy using conventional and newer drugs Ofloxacin and minocycline. Indian J Dermatol Venereol Leprol. 2000;66:18–25. [PubMed] [Google Scholar]
- 25.Ganapati R, Pai VV, Shroff HJ, Gandewar K. Rate of decline in bacterial index in leprosy; observations after three different chemotherapeutic interventions. Int J Lepr Other Mycobact Dis. 1997;65:264–6. [PubMed] [Google Scholar]
- 26.Geneva: World Health Organization; 2002. Report on the Third Meeting of the WHO Technical Advisory Group on Elimination of Leprosy. (document number WHO/CDS/CPE/CEE/200229) [Google Scholar]
- 27.Prasad PV, Babu A, Kaviarasan PK, Viswanathan P, Tipoo R. MDT–MB therapy in paucibacillary leprosy: A clinico pathological assessment. Indian J Dermatol Venereol Leprol. 2005;71:242–6. doi: 10.4103/0378-6323.16614. [DOI] [PubMed] [Google Scholar]
- 28.Ellard GA. Drug compliance in the treatment of leprosy. Lepr Rev. 1981;52:201–13. doi: 10.5935/0305-7518.19810030. [DOI] [PubMed] [Google Scholar]
- 29.Ellard GA. Clofazimine and dapsone compliance in leprosy. Lepr Rev. 1988;59:205–13. doi: 10.5935/0305-7518.19880026. [DOI] [PubMed] [Google Scholar]
- 30.1st ed. Geneva: World Health Organization; 2000. Guide to eliminate leprosy as a public health problem. [PubMed] [Google Scholar]
- 31.Ji B. Chemotherapy and chemoprophylaxis of leprosy. Report of Scientific Working Group on leprosy TDR/SWG. 2002 [Google Scholar]
- 32.Honoré N, Cole ST. Molecular basis of rifampin resistance in Mycobacterium leprae. Antimicrob Agents Chemother. 1993;37:414–8. doi: 10.1128/aac.37.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cambau E. Molecular detection of rifampin and ofloxacin resistance for patients who experience relapse of multibacillary leprosy. Clin Infect Dis. 2002;34:39–45. doi: 10.1086/324623. [DOI] [PubMed] [Google Scholar]
- 34.Matsuoka M, Kashiwabara Y, Namisato M. A Mycobacterium leprae isolate resistant to dapsone, rifampin and sparfloxacin. Int J Lepr Other Mycobact Dis. 2000;68:452–5. [PubMed] [Google Scholar]
- 35.Maeda S. Multidrug resistant Mycobacterium lep-rae from patients with leprosy. Antimicrob Agents Chemother. 2001;45:3635–9. doi: 10.1128/AAC.45.12.3635-3639.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuoka M, Budiawan T, Aye KS, Kyaw K, Tan EV, Cruz ED, et al. The frequency of drug resistance mutations in Mycobacterium leprae isolates in untreated and relapsed patients from Myanmar, Indonesia and the Philippines. Lepr Rev. 2007;78:343–52. [PubMed] [Google Scholar]
- 37.Nguyr LN, Cartel JL, Grosset JH. Chemoprophylaxis of leprosy in the Southern Marquises with a single 25 mg/kg dose of rifampicin. Results after 10 years. Lepr Rev. 2000;71:S33–6. doi: 10.5935/0305-7518.20000064. [DOI] [PubMed] [Google Scholar]
- 38.Marlowe SN, Leekassa R, Bizuneh E, Knuutilla J, Ale P, Bhattarai B, et al. Response to ciclosporin treatment in Ethiopian and Nepali patients with severe leprosy Type 1 reactions. Trans R Soc Trop Med Hyg. 2007;101:1004–12. doi: 10.1016/j.trstmh.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Walker SL, waters MF, Lockwood DN. The role of thalidomide in the management erythema nodosum leprosum. Lepr Rev. 2007;78:197–215. [PubMed] [Google Scholar]
- 40.Kaplan G. Potential of thalidomide and thalidomide analogues as immuno modulatory drugs in leprosy and leprosy reactions. Lepr Rev. 2000;71:S117–20. doi: 10.5935/0305-7518.20000082. [DOI] [PubMed] [Google Scholar]
- 41.Sales AM, de Matos HJ, Nery JA, Duppre NC, Sampaio EP, Sarno EN. Double blind trial of the efficacy of pentoxifylline vs.thalidomide for the treatment of type-II reaction in leprosy. Braz J Med Biol Res. 2007;40:243–8. doi: 10.1590/s0100-879x2007000200011. [DOI] [PubMed] [Google Scholar]
- 42.Girdhar BK, Girdhar A, Chakma JK. Advances in the treatment of reactions in leprosy. Indian J Lepr. 2007;79:121–34. [PubMed] [Google Scholar]
- 43.Feuth M, Wim Brandsma J, Faber WR, Bhattarai B, Feuth T, Anderson AM. Erythema nodosum leprosum in Nepal: A retrospective study of clinical features and response to treatment with prednisolone or thalidomide. Lepr Rev. 2008;79:254–69. [PubMed] [Google Scholar]
- 44.Hastings RC, Job CK. Reversal reactions in lepromatous leprosy following transfer factor therapy. Am J Trop Med Hyg. 1978;27:995–1004. doi: 10.4269/ajtmh.1978.27.995. [DOI] [PubMed] [Google Scholar]
- 45.Mathur NK. Long term follow up of lepromatous leprosy patients receiving intra lesional recombinant gamma interferon. Int J Lepr Other Mycobact Dis. 1992;60:98–100. [PubMed] [Google Scholar]
- 46.Kaplan G. The systemic influence of recombinant interlekin-2 on the manifestations of lepromatous leprosy patients. J Exp Med. 1991;173:993–1006. doi: 10.1084/jem.173.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gillis T. Is there a role for vaccine in leprosy control? Lepr Rev. 2007;78:338–42. [PubMed] [Google Scholar]
- 48.Dowdle WR. The principles of disease elimination and eradication. Bull World Health Organ. 1998;76:S22–5. [PMC free article] [PubMed] [Google Scholar]
- 49.Richardus JH, Habbema JD. The impact of leprosy control on the transmission of M.leprae: Is elimination being attained? Lepr Rev. 2007;78:330–7. [PubMed] [Google Scholar]
- 50.Meima A, Gupte MD, van Oortmarssen GJ, Habbema JD. Trends in leprosy case detection rates. Int J Lepr Other Mycobac Dis. 1997;65:305–19. [PubMed] [Google Scholar]
- 51.Fine PE, Warndorff DK. Leprosy by the year 2000- what is being eliminated? Lepr Rev. 1997;68:201–2. doi: 10.5935/0305-7518.19970027. [DOI] [PubMed] [Google Scholar]
- 52.Smith WC. We need to know what is happening to the incidence of leprosy. Lepr Rev. 1997;68:195–200. doi: 10.5935/0305-7518.19970026. [DOI] [PubMed] [Google Scholar]
- 53.Lepr Rev. Vol. 73. Paris, France: 2002. Report of the International Leprosy Association Technical Foum. 25-28 February 2002; pp. S3–61. [PubMed] [Google Scholar]
- 54.Meima A, Richardus JH, Habbema JD. Trends in leprosy case detection worldwide since 1985. Lepr Rev. 2004;75:19–33. [PubMed] [Google Scholar]
- 55.Global leprosy situation, 2007. Wkly Epidemiol Rec. 2007;82:225–32. [PubMed] [Google Scholar]
- 56.Fine PE, Smith PG. Vaccination against leprosy – the view from 1996. Lepr Rev. 1996;67:249–52. doi: 10.5935/0305-7518.19960025. [DOI] [PubMed] [Google Scholar]
- 57.International Leprosy Association Technical Forum. Report. Lepr Rev. 2002;73:S1–62. [PubMed] [Google Scholar]
- 58.Fine PE. Leprosy: The epidemiology of slow bacterium. Epidemiol Rev. 1982;4:161–88. doi: 10.1093/oxfordjournals.epirev.a036245. [DOI] [PubMed] [Google Scholar]
- 59.Meima A. The impact of multi drug therapy on trends in transmission of leprosy. Report of the Scientific Working Group on Leprosy. 2002 TDR/SWG/02. [Google Scholar]
- 60.van Beers SM, Hatta M, Klatser PR. Patient contact is the major determinant in incident leprosy: Implications for future control. Int J Lepr Other Mycobact Dis. 1999;67:119–28. [PubMed] [Google Scholar]
- 61.Deps PD, Alves BL, Gripp CG, Aragao RL, Guedes BV, Filho JB, et al. Contact with armadillos increases the risk of leprosy in Brazil: A case control study. Indian J Dermatol Venereol Leprol. 2008;74:338–42. doi: 10.4103/0378-6323.42897. [DOI] [PubMed] [Google Scholar]
- 62.Global leprosy situation, beginning of 2008. [last cited on 2008 Dec 15];Wkly Epidemiol Rec. 2008 83:293–300. Available from: http://www.who.int/wer . [PubMed] [Google Scholar]
- 63.Fine PE. Leprosy's global statistics- still room for improvement. Lepr Rev. 2008;79:235–8. [PubMed] [Google Scholar]
- 64.Rao PN, Lakshmi TS. Final push of leprosy in India: What is being pushed? Indian J Dermatol Venereol Leprol. 2005;71:226–9. doi: 10.4103/0378-6323.16611. [DOI] [PubMed] [Google Scholar]
- 65.World Health Organization Regional Office for South-East Asia New Delhi. Lepr Rev. 2006;77:1–50. Global strategy for further reducing the leprosy burden and sustaining leprosy control activities 2006-2010 Operational Guidelines. [Google Scholar]
- 66.Richardus JH, Habbema JD. The impact of leprosy control on the transmission of M.leprae; is elimination being attained? Lepr Rev. 2007;78:330–7. [PubMed] [Google Scholar]
- 67.Scollard DM. Leprosy research declines, but most of the basic questions remain unanswered. Int J Lepr Other Mycobact Dis. 2005;73:25–7. doi: 10.1489/1544-581X(2005)73[25:LRDBMO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 68.Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbio Rev. 2006;19:338–81. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


