Abstract
Background:
Direct immunofluorescence (DIF) is the gold standard in the diagnosis of immunobullous diseases. However, it cannot reliably differentiate various subtypes of subepidermal immune- bullous diseases (SIBD). Salt split technique (SST) could be used under such circumstances to differentiate them. There is paucity of reports in the Indian literature regarding the SST.
Aim:
This study was designed to evaluate the utility of direct SST in subepidermal blistering diseases.
Materials and Methods:
Fourteen clinically diagnosed cases of subepidermal blistering diseases were included in the study. Two perilesional punch biopsies were taken one each for DIF and salt split study.
Results:
Linear basement membrane zone band with IgG and/or C3 was seen in 14 cases of patients BP. Salt split study showed epidermal or mixed pattern of deposits in 12 patients and exclusive floor pattern in two patients. The diagnosis was revised in these two patients to epidermolysis bullosa acquisita.
Conclusion:
SST is a simple, inexpensive procedure and should be routinely employed in the diagnosis of subepidermal bullous diseases.
Keywords: Immunofluorescence, salt-split technique, subepidermal bullous diseases
Introduction
The subepidermal immunobullous diseases (SIBD) are a group of disorders characterized clinically by the presence of cutaneous and/or mucosal blisters and histologically by subepidermal blister formation. It encompasses bullous pemphigoid (BP), epidermolysis bullosa acquisita (EBA), cicatricial pemphigoid (CP), pemphigoid gestationis (PG), linear IgA dermatosis (LAD), dermatitis herpetiformis (DH), and bullous systemic lupus erythematosus (BLSE). Another common feature to these conditions is the deposition of immunoglobulins and complement at the basement membrane zone (BMZ), with the exception of DH where IgA deposits are found in the dermal papillae.[1]
BP is the commonest type of SIBD; clinical and histopathological features of BP may often be confused with EBA. Direct immunofluorescence (DIF) and indirect immunofluorescence (IIF) typically demonstrate linear deposition of IgG and C3 in the BMZ in both conditions.[2–4] These features have resulted in the misdiagnosis of EBA as BP or vice versa, in many patients. Although immunoelectron microscopy (IEM), immunoprecipitation, and immunoblotting have been employed to distinguish between these conditions, they are expensive, technically demanding and not widely available.[5–8] To overcome this problem, salt split technique (SST) using 1 mol/L NaCl was introduced in 1984 to differentiate pemphigoid group from other SIBD.[9] This study was undertaken with the aim of evaluating the utility of direct SST and comparing it with the routine method of immunofluorescence in the diagnosis of SIBD in a tertiary care hospital in South India.
Materials and Methods
Patients attending skin OPD of a tertiary care hospital, between October 2007 and September 2008 with a provisional diagnosis of SIBD were enrolled in the study. Detailed history and thorough clinical examination was carried out for each case, and the findings were recorded in a predesigned format. The study was approved by the institutional ethical committee review board. Written informed consent was obtained from all the patients. Two perilesional punch biopsies (each 3.5 mm), one for DIF and other for direct salt split, were collected after prior anesthetization with xylocaine 2%. DIF and IIF (in two dilutions of 1:10 and 1:80, using normal skin as substrate) were done in all cases as per standard reference. Results were recorded by the same observer and intensity of staining were graded subjectively as strong (+++), moderately strong (++), weak (+), or negative (-). Additional biopsy for histopathological study (H and E) was carried out in five patients who had fresh intact vesicle.
Salt split technique
Punch biopsy samples were incubated in 5 mL of NaCl (1 mol/L) at 4ºC for 24 h. The epidermis was then teased from the dermis with the use of a fine forceps. The specimens were then processed in the same manner and treated with IgG and C3 conjugates as in DIF.
Results
Fourteen (five males and nine females) clinically suspected cases of SIBD were enrolled in the study. Average age of presentation was 57.3 years (range, 10-80 years). Duration of the disease varied from 2 weeks to 3 years (mean duration 7.8 months). All cases were diagnosed clinically as BP; histopathological study (Hematoxyline and Eosine) from the fresh blister in five patients was consistent with the diagnosis of BP.
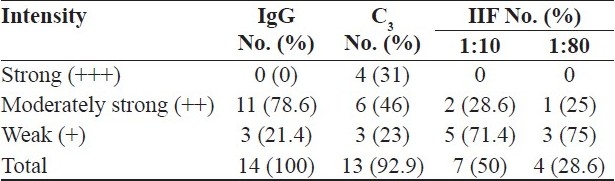
DIF was positive in all the 14 (100%) patients, whereas IIF was positive in only seven patients. A linear BMZ band with IgG and/or C3 was seen in all patients clinically diagnosed as BP [Table 1]. In addition, five patients showed weak BMZ deposition with fibrin. Other immunoreactants deposition was not seen in patients with BP.
Table 1.
Results of DIF and IIF in clinically suspected BP (n = 14)
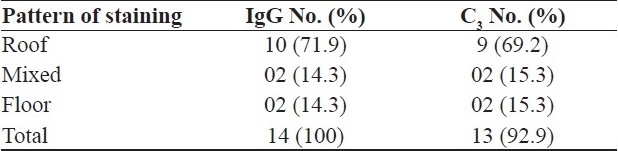
Results of direct salt split study [Figures 1 and 2] are depicted in Table 2. Two patients showed exclusive floor pattern on salt split study; diagnosis was then revised and they were labeled as EBA.
Figure 1.
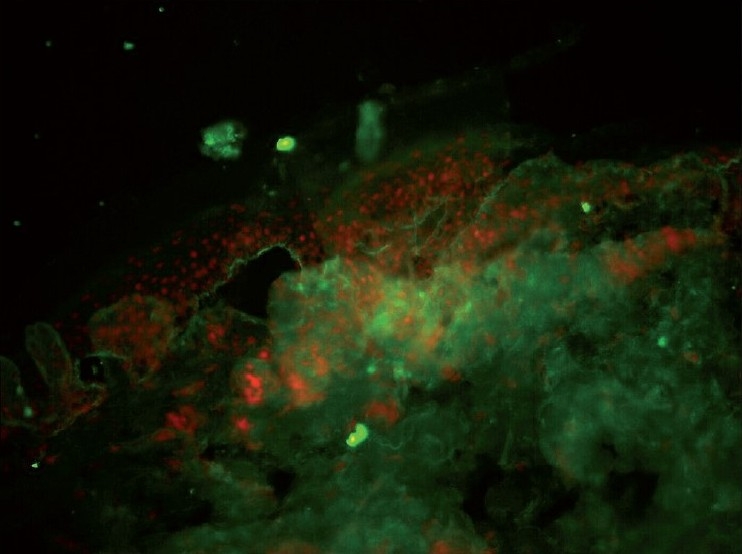
Linear C3 band at BMZ showing roof pattern (DIF ×20)
Figure 2.
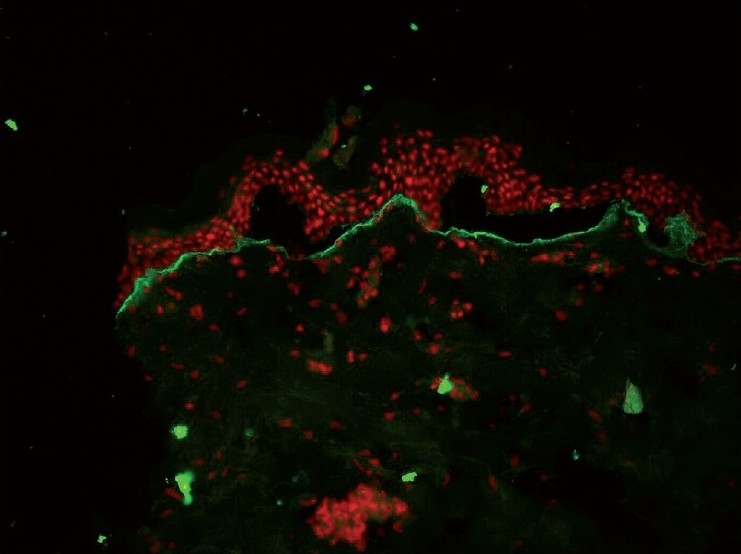
Floor pattern of BMZ band with C3 in EBA (DIF ×20)
Table 2.
Results of salt split study (n = 14)
Therefore, with the help of DIF, IIF, and salt split studies, we were able to confirm the diagnosis of BP in 12 out of 14 cases and rectify the diagnosis of two cases. DIF was found to be 100% sensitive and specific whereas sensitivity of IIF was 50% in our patients with SIBD.
Discussion
BP was the commonest type (85.7%) of SIBD in our study followed by EBA (14.3%). Wong and coworkers made similar observations; however, Nanda and coworkers reported high prevalence of PG in Kuwait.[1,10] We did not encounter any other types of SIBD during the study period.
DIF is considered as the gold standard for the diagnosis of SIBD. Sensitivity of DIF and direct salt split was 100% in our patients. Beutner et al. have made similar observations.[11] IIF had low sensitivity (50%) in our study. Hence, indirect SST would have resulted in lower sensitivity than that of direct technique. Domloge-Hultsch et al. have expressed similar apprehension.[12] Further, there was no correlation between the disease activity and titer of circulating antibodies in our study. IgG was positive in 100% cases both by DIF and direct salt split whereas C3 was found positive in 13 of the 14 (92.9%) samples of clinically diagnosed cases of BP. This is consistence with the findings of Satyapal et al. who found C3 alone or in combination with other immunoreactants in 90% of cases. [13]
EBA has three modes of presentation; clinically, inflammatory form of EBA masquerades as BP.[4] These patients will have tense blisters on erythematous or urticarial background which heals without scarring or milia formation. However, these two disorders are distinct biochemically. It is imperative for the clinician to make every effort to distinguish BP from EBA for two simple reasons. EBA has been known to be associated with various systemic diseases such as inflammatory bowel disease (IBD); so, necessary investigation should be done in all cases of EBA to rule out such underlying conditions. Another compelling reason to establish the correct diagnosis is to predict the course of the disease, as EBA is relatively resistant to treatment.[14] SST using 1 M NaCl is a simple tool which aides in the differentiation of BP from EBA.[15,16] In this study, we have shown that 24 h (as against 72 h) incubation could reliably split the skin at dermoepidermal junction, thereby avoiding the longer hours of incubation. The majority of our cases took a roof (epidermal) pattern, suggesting the diagnosis of BP. This is in contrast to the findings of Satyapal et al. who observed that mixed type to be the common pattern of staining in their patients.[13] Gammon et al. noted that BP patients consistently took epidermal or mixed staining.[8] Patients with IgG on the floor pattern of SSS represent about 12% of patients with IgG at dermal-epidermal junction.[17] It is generally believed that floor pattern of staining corresponds to the diagnosis of EBA. Same criterion was adapted by earlier studies when assessing the prevalence of SBID in various parts of the world.[1,10] This was further supported by Zhu et al. who had shown that four out of five serum samples with dermal reactivity on indirect IF and SSS reacted with EBA antigens.[18] On the basis of these facts, we revised the diagnosis in two of our cases to EBA who showed only floor pattern. However, Ghohestani et al. using the SSS on indirect IF and immunoblotting methods have shown that 2% of their patients with floor pattern of staining were suffering from BP.[19] They argued that dermal pattern of staining is not specific for EBA autoantibodies as it may be seen with antibodies to type IV, laminin 5, or type XVII (i.e., BPAg2). In contrast, they concluded that there was a good correlation between roof (epidermal) pattern and combined pattern of staining with BP.
We conclude that direct SST is a simple, cheap, and easy tool and should be routinely employed in immunofluorescence study of patients with SIBD. Roof pattern of staining is highly suggestive of BP; one needs to be careful when dealing with floor pattern. Although it generally indicates the diagnosis of EBA, further testing (such as immunoblotting, immunoelectron microscope, or indirect IF using toad skin[20] ) may be necessary to confirm the diagnosis.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Wong SN, Chua SH. Spectrum of subepidermal immuno-bullous disorders seen at the National skin centre, Singapore: A 2-year review. Br J Dermatol. 2002;147:476–80. doi: 10.1046/j.1365-2133.2002.04919.x. [DOI] [PubMed] [Google Scholar]
- 2.Bernard P, Vaillant L, Bedani C. Incidence and distribution of subepidermal autoimmune bullous disease in 3 French regions. Arch Dermatol. 1995;131:48–52. [PubMed] [Google Scholar]
- 3.Briggaman RA, Gammon WR, Woodley DT. Epidermolysis bullosa acquisita of the immunopathological type (dermolytic pemphigoid) J Invest Dermatol. 1985;85:79s–84s. doi: 10.1111/1523-1747.ep12275505. [DOI] [PubMed] [Google Scholar]
- 4.Gammon WR, Briggaman RA, Woodley DT, Heald PW, Wheeler CE., Jr Epidermolysis bullosa acquisita: A pemphigoid like disease. J Am Acad Dermatol. 1984;11:820–3. doi: 10.1016/s0190-9622(84)80459-4. [DOI] [PubMed] [Google Scholar]
- 5.Yaoita H, Briggaman RA, Lawley TJ, Provost TT, Katz SI. Epidermolysis bullosa acquisita: Ultrastructural and immunological studies. J Invest Dermatol. 1981;76:288–92. doi: 10.1111/1523-1747.ep12526124. [DOI] [PubMed] [Google Scholar]
- 6.Mueller S, Klaus-Kovtum V, Stanley JR. A 230-kD basic protein is the major bullous pemphigoid antigen. J Invest Dermatol. 1989;92:33–8. doi: 10.1111/1523-1747.ep13070476. [DOI] [PubMed] [Google Scholar]
- 7.Labib RS, Anhalt GJ, Patel HP, Mutasim DF, Diaz LA. Molecular heterogenicity of the bullous pemphigoid antigens as detected by immunoblotting. J Immunol. 1986;136:1231–5. [PubMed] [Google Scholar]
- 8.Gammon WR, Kowalewski C, Chorzelski TP, Kumar V, Briggaman RA, Beutner EH. Direct immunofluorescence studies of sodium chloride- separated skin in the differential diagnosis of bullous pemphigoid and epidermolysis acquisita. J Am Acad Dermatol. 1990;22:640–70. doi: 10.1016/0190-9622(90)70094-x. [DOI] [PubMed] [Google Scholar]
- 9.Gammon WR, Briggaman RA, Inman AO, Queen LL, Wheeler CE Jr. Differentiating anti-lamina lucida and anti-sublamina densa anti-BMZ antibodies by direct immunofluorescence on 1.0 M sodium chloride- separated skin. J Invest Dermatol. 1984;82:139–44. doi: 10.1111/1523-1747.ep12259692. [DOI] [PubMed] [Google Scholar]
- 10.Nanda A, Dvorak R, Al-Saeed K, Al-Sabah H, Alsaleh QA. Spectrum of autoimmune bullous disease in Kuwait. Int J Dermatol. 2004;43:876–81. doi: 10.1111/j.1365-4632.2004.02292.x. [DOI] [PubMed] [Google Scholar]
- 11.Beutner EH, Jordon RE, Chorzelski TP. The immunopathology of pemphigus and bullous pemphigoid. J Invest Dermatol. 1989;292:166–8. [PubMed] [Google Scholar]
- 12.Domloge-Hultsch N, Bisalbutra P, Gammon WR, Yancey KB. Direct immunofluorescence microscopy of 1 mol/L sodium chloride-treated patient skin. J Am Acad Dermatol. 1991;24:946–51. doi: 10.1016/0190-9622(91)70151-q. [DOI] [PubMed] [Google Scholar]
- 13.Satyapal S, Amladi S, Jerajani HR. Evaluation of salt split technique of immunofluorescence in bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2002;68:330–3. [PubMed] [Google Scholar]
- 14.Woodley DT. Immunofluorescence on salt-split skin for the diagnosis of epidermolysis bullosa acquisita. Arch Dermatol. 1990;126:229–31. [PubMed] [Google Scholar]
- 15.Woodley DT, Sauder D, Talley MJ, Silver M, Grotendorst G, Qwarnstrom E. Localization of basement membrane components after dermal-epidermal junction separation. J Invest Dermatol. 1983;81:149–53. doi: 10.1111/1523-1747.ep12543517. [DOI] [PubMed] [Google Scholar]
- 16.Logan RA, Bhogal B, Das AK, McKee PM, Black MM. Localization of bullous pemphigoid antibody: An indirect immunofluorescence study of 228 cases using a split-skin technique. Br J Dermatol. 1987;117:471–8. doi: 10.1111/j.1365-2133.1987.tb04927.x. [DOI] [PubMed] [Google Scholar]
- 17.Barnadas MA, Gelpi C, Currel R, de Moragas JM, Alomar A. Repeat direct immunofluorescence (DIF) test, using 1 M NaCl treated skin, in the subepidermal autoimmune bullous diseases that contain IgG at the dermal-epidermal junction. J Cutan Pathol. 1999;26:37–41. doi: 10.1111/j.1600-0560.1999.tb01788.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhu XJ, Niimi Y, Bystryn JC. Epidermolysis bullosa acquisita: Incidence in patients with basement membrane antibodies. Arch Dermatol. 1990;126:171–4. doi: 10.1001/archderm.126.2.171. [DOI] [PubMed] [Google Scholar]
- 19.Ghohestani RF, Nicolas JF, Rousselle P, Claudy A. Diagnostic value of indirect immunofluorescence on sodium chloride- split skin in differential diagnosis of subepidermal autoimmune bullous dermatoses. Arch Dermatol. 1997;133:1102–7. [PubMed] [Google Scholar]
- 20.Pang RK, Lee YS, Ratnam KV. Floor pattern in salt split can not distinguish bullous pemphigoid from epidermolysis bullosa acquisita.Use of toad skin. Arch Dermatol. 1993;129:744–6. [PubMed] [Google Scholar]


