Abstract
BACKGROUND
Collaterals sustain the penumbra prior to recanalization and offset infarct growth, yet the influence of baseline collateral flow on recanalization following endovascular therapy remains relatively unexplored.
METHODS
We analyzed consecutive patients who received endovascular therapy for acute cerebral ischemia from two distinct study populations. We assessed the relationship between pretreatment collateral grade and vascular recanalization (TIMI scale). In addition, we assessed infarct growth on serial MRI.
RESULTS
A total of 222 patients were included; 138 from the United States and 84 from South Korea. Complete revascularization occurred in 14.1% (11 of 78) patients with poor pretreatment collateral grades, whereas it was observed in 25.2% (26 of 103) patients with good collaterals and 41.5% (17 of 41) patients with excellent collaterals (p<0.001). This relationship was consistently observed in both study populations, though the mode of endovascular therapy was different between them. After adjustment for other factors, including mode of endovascular therapy, prior use of intravenous tPA, and site of occlusion, pretreatment collateral grade was independently associated with recanalization. When revascularization was achieved, greater infarct growth occurred in patients with poor collaterals than in those with good collaterals (p=0.012).
CONCLUSION
Our data indicate that angiographic collateral grade determines the recanalization rate after endovascular revascularization therapy. When therapeutic revascularization was achieved, beneficial effects were not observed in patients with poor collaterals. Angiographic collateral grade provides may therefore help guide treatment decision-making in acute cerebral ischemia.
Keywords: Stroke, Ischemic, Collaterals, Magnetic resonance imaging, Thrombolysis, Angiography
INTRODUCTION
In the setting of acute ischemic stroke, arterial revascularization to restore antegrade perfusion to the ischemic territory remains the principal therapeutic approach. Although the pathophysiologic recruitment of cerebral collateral circulation in the setting of chronic hemodynamic insufficiency has been explored,1 relatively little attention has been devoted to the role of pre-treatment collateral circulation in patients with acute ischemic stroke who are candidates for recanalization therapy.2, 3
In a previous transcranial Doppler study of a small series with acute stroke, early recanalization of embolic middle cerebral artery (MCA) occlusions within up to 8 hours, in conjunction with good transcortical collateralization, has a favorable impact on infarct size and outcome.4 A retrospective computed tomogram (CT)-based volumetric analysis reported that infarct volume and clinical severity at discharge were lower for patients with better pre-treatment pial collateral formation.5 A recent perfusion-diffusion MRI-based study has shown that angiographic collateral grade and penumbral volume interactively shape tissue fate in patients undergoing endovascular recanalization therapy.6 However, the relationship between baseline collateral flow and the rate of revascularization remains unknown.
In the present study, we evaluated the relationship between baseline angiographic collaterals and recanalization after endovascular therapy. For external validation, we compared the data from two study populations where the mode of revascularization therapy differs. In addition, we assessed the infarct growth on serial MRI.
PATIENTS AND METHODS
Patient selection
We analyzed demographic, clinical, laboratory, and radiographic data prospectively collected on consecutive patients who received endovascular therapy (intra-arterial thrombolytic therapy, mechanical thrombectomy device [MERCI Retrieval System, Concentric Medical, Inc.], or other mechanical therapy such as guide-wire manipulation) for acute cerebral ischemia. This study analyzed consecutive patients encountered at two university hospital stroke centers; Los Angeles, CA, from May 2002 through July 2007 and Seoul, South Korea, from July 2005 through October 2009.
Patients were included in this study if (1) they presented with symptoms of acute cerebral ischemia within the MCA territory, and (2) they underwent conventional angiography for endovascular therapy. The local Institutional Review Boards approved the study.
Angiography
All patients underwent comprehensive diagnostic cerebral angiography, including injection of both ICAs and the dominant vertebral artery, with assessment through the late venous phase to assess collateral flow from all possible sources. Angiographic collateral grade was evaluated with the ASITN/SIR Collateral Flow Grading System on pre-treatment angiography.7 This angiographic scale assigns patients to grade 0 (no collaterals visible to the ischemic site), 1 (slow collaterals to the periphery of the ischemic site with persistence of some of the defect), 2 (rapid collaterals to the periphery of ischemic site with persistence of some of the defect and to only a portion of the ischemic territory), 3 (collaterals with slow but complete angiographic blood flow of the ischemic bed by the late venous phase), and 4 (complete and rapid collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion). Vascular reperfusion was based on the Thrombolysis in Myocardial Infarction (TIMI) scale, with assignments of 3 (complete recanalization), 2 (partial recanalization), 1 (minimal recanalization), and 0 (no recanalization). Two reviewers independently assessed the pretreatment collateral grade and TIM scale. κ-coefficient for interobserver agreement was 0.896 for collateral grade and 0.79 for TIMI grade. The opinion of third neuroradiologist was sought to resolve disagreements. The authors were blinded to the patient information through the processes.
Diagnostic evaluation
All patients underwent routine blood tests, electrocardiography, cardiac telemetry for at least 24 hours, and echocardiography. All patients underwent MRI before revascularization therapy if not contraindicated. Diffusion-weighted imaging (DWI) lesion volume measurements were performed by one of authors blinded to the clinical information. For each patient, DWI lesion volumes were outlined automatically with subsequent manual correction and volumes were calculated with a computer-assisted volumetric analysis program (Medical Image Processing, Analysis and Visualization, version 2.1, CIT, NIH).
Statistical analysis
Continuous data are shown as mean ± standard deviation, while categorical variables are presented as absolute and relative frequencies. We analyzed the differences between the groups using Pearson’s chi-square test or linear by linear association for categorical variables, and a student t-test, a one-way analysis of variance, or Kruskall-Wallis test for continuous variables. Spearman’s correlation coefficient was used to analyze the association of collateral grade with the degree of infarct growth. In addition, independent factors for a patient having a good recanalization were evaluated using a logistic regression. In this study, a score of 0–1 was designated as poor, and 2–3 as good (therapeutic) recanalization. Age, gender, atrial fibrillation, time from onset of symptoms to endovascular therapy, site of occlusion, the NIHSS score on admission, the mode of endovascular therapy, IV tPA prior to endovascular therapy, and pretreatment collateral grade all served as predictor variables. Variables from univariate analyses at P<0.2 were considered to represent explanatory variables and were evaluated together by multivariate analysis. All statistical analyses were performed using commercially available software (SPSS for windows, version 13.0; SPSS Inc, Chicago, Il). A P< 0.05 was considered statistically significant.
RESULTS
Patient characteristics
A total of 222 patients were included; 138 from U.S. and 84 from South Korea. Baseline characteristics for the U.S. and South Korean subjects are listed in Table 1. The NIHSS score on admission was lower in the South Korean subjects compared to the U.S. subjects. Most U.S. subjects received endovascular thrombectomy (93 of 138 patients) and the rest of the patients received IA fibrinolytics or other endovascular therapy. On the contrary, 28 South Korean subjects received IA fibrinolytics alone and 53 patients received other endovascular therapy with/without IA fibrinolytics. The time from last known well to the onset of endovascular therapy was shorter in the South Korean subjects compared to the U.S. subjects (P=0.001). South Korean subjects more frequently received IV tPA prior to endovascular therapy than the U.S. subjects (P<0.001).
Table 1.
Patients’ characteristics
| U.S. subjects (N=138) | South Korean subjects (N=84) | P | |
|---|---|---|---|
| Female gender (%) | 65 (47.1) | 38 (45.2) | 0.787 |
| Age (years) | 66.1 ± 18.0 | 63.9 ± 13.5 | 0.324 |
| Atrial fibrillation | 52 (40.0) | 34 (40.5) | 0.454 |
| Initial NIH Stroke Scale (score) | 17.8 ± 6.9 | 14.4 ± 5.3 | <0.001 |
| Mode of endovascular therapy (%) | <0.001 | ||
| IA fibrinolysis alone | 26 (20.6) | 28 (34.6) | |
| Mechanical alone | |||
| Merci clot retrieval | 79 (62.7) | 0 (0) | |
| Other mechanical methods* | 6 (4.8) | 14 (17.3) | |
| Mechanical + IA fibrinolysis | 15 (11.9)† | 39 (48.1) | |
| Time from onset to endovascular therapy (h) | 5.9 ± 2.4 | 4.2 ± 1.7 | 0.001 |
| IV tissue plasminogen activator prior to endovascular therapy (%) | 27 (19.6) | 53 (63.1) | <0.001 |
including mechanical clot disruption, angioplasty with/without stent
including 14 patients who received Meci clot retrieval
Factors affecting revascularization rates
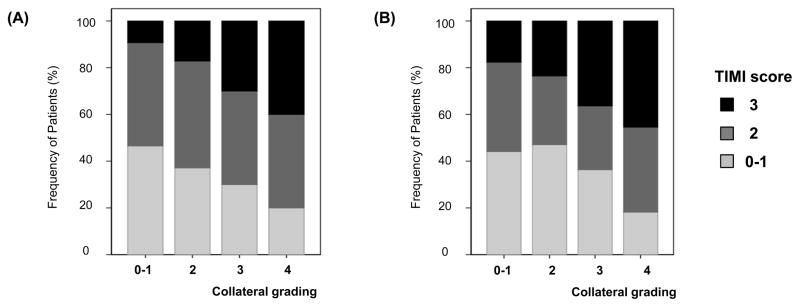
Patient characteristics depending on the treatment response (TIMI scale) are illustrated in Table 2. Complete recanalization (TIMI 3) occurred in 54 patients, and 87 patients showed partial recanalization (TIMI 2), 81 poor recanalization (TIMI 0–1). Patients with greater extent of pretreatment collaterals more frequently achieved therapeutic recanalization. Among patients with poor pretreatment collateral grading (0–1 scores), complete recanalization (TIMI 3) was achieved in only 14.1% (11 of 78) patients, whereas it was observed in 25.2% (26 of 103) patients with good collaterals (2–3 score) and 41.5% (17 of 41) patients with excellent collaterals (4 score) (P<0.001). This relationship was consistently observed in both study populations, though the mode of endovascular therapy was greatly different between them (Figure 1). When the analysis was performed separately in the U.S. and South Korean subjects, this association remained significant (P for trend=0.001 for the U.S. subjects and P for trend=0.045 for the South Korean subjects).
Table 2.
Patient characteristics depending on the TIMI score
| TIMI score |
P-value | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| No. of patients | N=42 | N=39 | N=87 | N=54 | |
| Female gender (%) | 22 (52.4) | 17 (43.6) | 44 (50.6) | 20 (37.0) | 0.356 |
| Age | 67.4 ± 17.2 | 62.1 ± 15.0 | 66.0 ± 16.0 | 64.6 ± 17.6 | 0.487 |
| Atrial fibrillation (%) | 17 (40.5) | 16 (44.4) | 38 (45.8) | 15 (28.3) | 0.215 |
| NIHSS score | 16.7 ± 6.5 | 15.6 ± 7.3 | 17.1 ± 6.0 | 16.5 ± 7.0 | 0.744 |
| Site of occlusion (%) | 0.249 | ||||
| Carotid bifurcation | 7 (18.9) | 2 (6.5) | 5 (7.9) | 9 (19.1) | |
| Carotid intracranial | 10 (27.0) | 5 (16.1) | 8 (12.7) | 10 (21.3) | |
| Proximal M1 | 8 (21.6) | 14 (45.2) | 28 (44.4) | 15 (31.9) | |
| Distal M1 | 5 (13.5) | 2 (6.5) | 11 (17.5) | 6 (12.8) | |
| Distal MCA | 7 (18.9) | 8 (25.8) | 11 (17.5) | 7 (14.9) | |
| Pretreatment collateral grading (%) | <0.001* | ||||
| 0 | 8 (19.0) | 4 (10.3) | 6 (6.9) | 3 (5.6) | |
| 1 | 11 (26.2) | 12 (30.8) | 26 (29.9) | 8 (14.8) | |
| 2 | 9 (21.4) | 12 (30.8) | 21 (24.1) | 9 (16.7) | |
| 3 | 11 (26.2) | 6 (15.4) | 18 (20.7) | 17 (31.5) | |
| 4 | 3 (7.1) | 5 (12.8) | 16 (18.4) | 17 (31.5) | |
| Mode of endovascular treatment (%) | 0.084 | ||||
| IA fibrinolytics alone | 14 (38.9) | 6 (18.2) | 23 (27.1) | 11 (20.8) | |
| Mechanical | 4 (11.1) | 4 (12.1) | 3 (3.5) | 9 (17.0) | |
| Merci clot retrieval | 14 (38.9) | 12 (36.4) | 36 (42.4) | 17 (32.1) | |
| Combined IA fibrinolytics and endovascular therapy | 4 (11.1) | 11 (33.3) | 23 (27.1) | 16 (30.2) | |
| IV tPA prior to endovascular therapy (%) | 16 (38.1) | 10 (25.6) | 28 (32.2) | 26 (48.1) | 0.116 |
| Time from onset to endovascular therapy (min) | 274.4±108.7 | 328.3±190.6 | 357.7±147.3 | 346.9±126.9 | 0.193 |
P for trends
Figure 1.
Degree of recanalization depending on pretreatment collateral grading; (a) U.S. subjects and (b) South Korean subjects.
However, recanalization rate was not different depending on the mode of treatment. In addition, no association was found between intravenous tPA use prior to endovascular treatment and recanalization rate. Age/gender, atrial fibrillation and NIHSS score on admission were not different depending on the TIMI score.
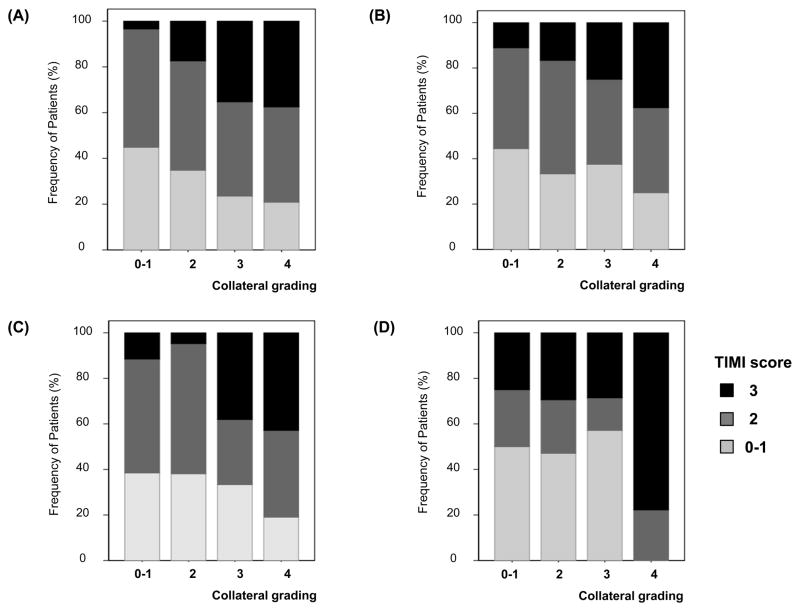
Because the recanalization rate may differ depending on the site of occlusion and the mode of treatment, subgroup analyses were performed. The relationship between the collateral grade and TIMI score were consistent observed in patients with ICA occlusion and MCA occlusion (P for trend=0.012 for ICA occlusion and P for trend=0.008 for MCA occlusion) (Figure 2A–B). The same is true for patients who received Merci mechanical clot retrieval (n=93); the degree of recanalization differed depending on the pretreatment collateral grading although patients received the same treatment modality (P=0.002) (Figure 2C).
Figure 2. Degree of recanalization depending on pretreatment collateral grading.
The association between pretreatment collaterals and the degree of recanalization was consistently observed regardless of mode of treatment modality (A–B) and the site of occlusion (C–D). Patients who received (A) mechanical embolectomy and (B) intra-arterial fibrinolytics alone. Patients with occlusion at (C) ICA and (D) proximal MCA.
Multiple regression analysis was performed to further evaluate independent predictors for therapeutic recanalization (TIMI 2–3) (Table 3). Pretreatment collateral grade was independently associated with recanalization rate, adjusting for other variables. Other factors including the mode of endovascular therapy, prior use of intravenous tPA, and site of occlusion did not significantly add value to pretreatment collateral grade. There was no significant correlation between the individual factors.
Table 3.
Multiple logistic regression for good (therapeutic) recanalization (TIMI 2–3)
| Odds ratio (95% confidence interval |
|||
|---|---|---|---|
| Crude | Multivariate | P | |
| Mode of recanalization | |||
| IA fibrinolytics alone | Reference | - | |
| Mechanical | 1.101 (0.358–3.386) | - | |
| Merci clot retrieval | 1.275 (0.561–2.897) | - | |
| Combined | 1.660 (0.715–3.852) | - | |
| IV tPA prior to endovascular therapy | 1.378 (0.698–2.720) | - | |
| Time from onset to endovascular therapy | 1.001 (0.999–1.003) | - | |
| Carotid Intracranial occlusion | 0.469 (0.0211–1.045) | - | |
| Pretreatment collateral grading | |||
| 0 | Reference | Reference | |
| 1 | 1.969 (0.680–5.703) | 1.972 (0.690–5.633) | 0.205 |
| 2 | 2.191 (0.728–6.596) | 2.322 (0.789–6.830) | 0.126 |
| 3 | 3.049 (0.996–9.331) | 3.005 (1.001–9.023) | 0.050 |
| 4 | 4.321 (1.302–14.341) | 4.645 (1.424–15.153) | 0.011 |
Infarct growth after therapeutic recanalization
Day 7 follow-up MRI was performed in 115 patients. The volume of infarct growth on serial DWI was different depending on the pretreatment collaterals and recanalization (TIMI scale). It was largest in patients who had poor collaterals and achieved partial/complete recanalization (TIMI 2–3) (65.9 ± 94.3 ml) than in those of other groups; 19.9 ± 31.2 ml in patients with good collaterals and TIMI 2–3, 41.0 ± 54.1 ml in patients with good collaterals and TIMI 0–1, and 38.4 ± 56.5 ml in patients with poor collaterals and TIMI 0–1 (P=0.012). When therapeutic recanalization was achieved, a greater infarct growth occurred more frequently in patients with poor collaterals (group 2) than in those with good collaterals (group 1) (P=0.002). Spearman’s correlation analysis showed that the volume of infarct growth was correlated with TIMI score in patients who had good collaterals (r=−0.268, P=0.017) but not in those with poor collaterals (r=−0.109, P=0.527).
DISCUSSION
Heterogeneity of collateral status in acute stroke
Collateral status differs among patients with acute ischemic stroke.6, 8 Some patients may have excellent collaterals, whereas others may show poor collaterals. Despite these influential aspects of collateral flow, collaterals at angiography are often only considered as an interesting curiosity and are not typically used for decision-making in endovascular management.3 In clinical practice, stroke neurologists or interventionalists often do not pay attention to the pretreatment collateral status, or they tend to restore antegrade flow more aggressively if patients have poor retrograde filling via collaterals.
The main findings of this study are that (a) pretreatment collaterals greatly influence the recanalization rate after endovascular therapy and (b) favorable responses may not be expected after therapeutic recanalization in patients with poor collaterals.
Pretreatment collateral status and recanalization rate
Revascularization restoring antegrade flow is crucial for favorable outcome in patients undergoing revascularization therapy. Final recanalization status represents a strong predictor of clinical outcomes in meta-analysis9 and recent pooled analysis of thrombectomy trials.10 The more aggressive and faster treatment may result in better recanalization. New revascularization modalities with a higher revascularization rate, such as suction device, are now under development. However, collateral status was not considered in previous studies of endovascular therapy. Our data showed that the collateral status was the strongest predictor for therapeutic revascularization. On the contrary, the mode of endovascular therapy, prior use of intravenous tPA, and the time from onset to endovascular therapy were not independently associated with the recanalization rate. The strength of our study is rooted in our analyses from two centers evaluating distinct study populations to control for therapeutic modes. In addition, our results showed that the relationship between pretreatment collaterals and TIMI score was consistently observed when the site of occlusion and mode of endovascular treatment was considered.
There may be several explanations for better recanalization in patients with good collaterals. First, retrograde collateral filling may allow thrombolytic and neuroprotective agents (extrinsic, intrinsic, or both) access to distal aspects of clot.11 Robust collaterals may also be important for dissolution of fragmented proximal thrombi. Second, beside the enhanced delivery of fibrinolytics agents to the occlusion site, other beneficial mechanisms of retrograde collateral flow may exist. Our previous results illustrate that collaterals and perfusion-diffusion mismatch represent related, yet distinct aspects of ischemic pathophysiology.6 Our present results showed that the recanalization rate was higher in patients who showed good collaterals and received mechanical revascularization therapy (Merci clot retrieval). During focal cerebral ischemia, cerebral blood vessel damage occurs early and in a progressive fashion.12 The degree of ischemic vascular injury can be minimized by collateral supply to vessels as well as brain tissue within the oligemic regions; thus, the response to endovascular therapy could be augmented in patients with good collaterals. The importance of pharmacological agents designed to protect the vasculature has been suggested.13 Further studies about the role of collaterals on vascular protection are warranted.
Our results are in good agreement with the previous studies which showed a correlation between pretreatment perfusion status and recanalization rates.14, 15 Higher blood flow values in the affected hemisphere assessed by xenon-CT were the predictors of recanalization in patients with proximal MCA occlusion treated with intra-arterial thrombolysis.14 In the present study, the perfusion status assesses at the time of endovascular treatment through a collateral grading score. Our results suggest the possibility of selecting patients for endovascular therapy based on perfusion information derived from collateral assessment obviating the need for other time consuming tests.
Achieving therapeutic recanalization in patients with poor collaterals
Revascularization is main strategy of acute ischemic stroke, and favorable outcome can be expected only in recanalized patients. However, not all patients with therapeutic revascularization showed the favorable outcome.10 A recent review showed that despite higher rates of recanalization, the mechanical thrombectomy studies have demonstrated substantially lower rates of good outcomes compared with IV and/or intra-arterial thrombolytic trials.16 Such analyses disregard important differences in clot location and burden, baseline stroke severity, time from stroke onset to treatment, and patient selection in these studies.16
Our results showed that recanalization despite of poor collaterals may result in unexpected results. We have previously reported that pretreatment collateral grade may influence the severity of ischemic injury over the hypoperfused region and associated with infarct growth.6 Collaterals have previously been shown to be potentially more important than recanalization in some cases; poor collaterals are an important determinant, irrespective of the degree of recanalization.5, 6 Chronic mild reduction of cerebral perfusion pressure induces ischemic tolerance in focal cerebral ischemia.17 A smaller infarct growth within hypoperfused region in patients with good collaterals may due to ischemic preconditioning. In the present study, infarct growth was not correlated with TIMI score in patients with poor collaterals. Moreover, when revascularization was achieved, more frequent symptomatic hemorrhagic transformation occurred in patients with poor collaterals than in those with good collaterals (data not shown). A higher frequency of infarct growth and symptomatic hemorrhagic transformation in patients with poor collaterals in whom therapeutic recanalization was achieved may support the concept of reperfusion injury.
A recent guideline for the early management of ischemic stroke recommended that unlike to intravenous use of tPA in patients within therapeutic time window, the use of intra-arterial fibrinolytics or endovascular therapy should be used in selected patients.18 Thus, personalized approach is needed. Our data raise the possibility that although patients who showed a baseline poor collateral are expected to have unfavorable outcome, the efforts to revascularization may not be beneficial, even harmful. In the present study, when complete recanalization was not achieved, infarct growth was usually not observed if good collaterals were noted.
Limitations
The results of this study should be interpreted with caution because this study is not a randomized controlled trial. Patients were treated with a variety of revascularization therapies, including fibrinolytics and various type of endovascular therapy. However, the association between pretreatment collaterals and the degree of recanalization was consistently observed regardless of mode of treatment modality and the site of occlusion. In addition, our data do not provide the answer to the question about what should we do for patients with poor collaterals. In cases where collateral flow is marginal, evolving hemodynamic strategies to improve ischemia through augmentation of collateral perfusion may be warranted.19 Therapeutic strategies enhancing collaterals may ultimately be as important as recanalization. Infarct growth may be minimized with the use of collateral therapeutic strategies, particularly in the setting of persistent arterial occlusion despite attempted recanalization. Collateral therapeutics may entail use of readily available hemodynamic manipulations such as head positioning, hypervolemia, hypertensive therapy, or partial aortic obstruction in selected cases.19 Considering that recanalization is often achieved in only a fraction of cases undergoing endovascular therapy, further research on collateral circulation and related therapeutic approaches is warranted.
Conclusions
Our data indicated that personalized approach is needed to maximize the effect of endovascular therapy. Angiographic collaterals should be considered individually in such patients, and the decision for more aggressive endovascular treatment should be tailored with the information of angiographic collateral status. Absence or relative paucity of collaterals may be used to predict unlikely recanalization despite aggressive therapeutic endovascular strategies. Alternatively, the presence of robust collateral flow at angiography may persuade clinicians to attempt further recanalization efforts, as collateral flow may be a marker for eventual success of such approaches. Our findings support the inclusion of collateral status in future efficacy trial of endovascular therapy.
Supplementary Material
Acknowledgments
The UCLA-Samsung Stroke Collaborators: Oh Young Bang; Suk Jae Kim; Gyeong-Moon Kim; Keon Ha Kim; Pyoung Jeon; Chin-Sang Chung; Kwang Ho Lee; Jeffry R. Alger; Sidney Starkman; Bruce Ovbiagele; Doojin Kim; Latisha K. Ali; Samir H. Shah; Paul M. Vespa; Reza Jahan; Noriko Salamon; Gary R. Duckwiler; J. Pablo Villablanca; Fernando Viñuela; David S. Liebeskind; and Jeffrey L. Saver.
Funding: This study was supported by the National Institutes of Health (K23NS054084 and P50NS044378) to D.S.L. and the National Research Foundation of Korea, the Ministry of Education, Science and Technology (2010-0007979) and the Samsung Medical Center Clinical Research Development Program grant CRS110-13-1 to O.Y.B.
Footnotes
Disclosures: None
References
- 1.Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29:231–240. doi: 10.1002/ana.410290302. [DOI] [PubMed] [Google Scholar]
- 2.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 3.Liebeskind DS. Collaterals in acute stroke: Beyond the clot. Neuroimaging Clin N Am. 2005;15:553–573. x. doi: 10.1016/j.nic.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Ringelstein EB, Biniek R, Weiller C, Ammeling B, Nolte PN, Thron A. Type and extent of hemispheric brain infarctions and clinical outcome in early and delayed middle cerebral artery recanalization. Neurology. 1992;42:289–298. doi: 10.1212/wnl.42.2.289. [DOI] [PubMed] [Google Scholar]
- 5.Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2005;26:1789–1797. [PMC free article] [PubMed] [Google Scholar]
- 6.Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, Vinuela F, Liebeskind DS. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, Sacks D. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 8.Bang OY, Saver JL, Alger JR, Starkman S, Ovbiagele B, Liebeskind DS. Determinants of the distribution and severity of hypoperfusion in patients with ischemic stroke. Neurology. 2008;71:1804–1811. doi: 10.1212/01.wnl.0000335929.06390.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: A meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 10.Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: Pooled analysis of the mechanical embolus removal in cerebral ischemia (merci) and multi merci trials. Stroke. 2009;40:3777–3783. doi: 10.1161/STROKEAHA.109.561431. [DOI] [PubMed] [Google Scholar]
- 11.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 12.Cipolla MJ, McCall AL, Lessov N, Porter JM. Reperfusion decreases myogenic reactivity and alters middle cerebral artery function after focal cerebral ischemia in rats. Stroke. 1997;28:176–180. doi: 10.1161/01.str.28.1.176. [DOI] [PubMed] [Google Scholar]
- 13.Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35:2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- 14.Jovin TG, Gupta R, Horowitz MB, Grahovac SZ, Jungreis CA, Wechsler L, Gebel JM, Yonas H. Pretreatment ipsilateral regional cortical blood flow influences vessel recanalization in intra-arterial thrombolysis for mca occlusion. AJNR Am J Neuroradiol. 2007;28:164–167. [PMC free article] [PubMed] [Google Scholar]
- 15.Tsivgoulis G, Saqqur M, Sharma VK, Lao AY, Hoover SL, Alexandrov AV. Association of pretreatment aspects scores with tpa-induced arterial recanalization in acute middle cerebral artery occlusion. J Neuroimaging. 2008;18:56–61. doi: 10.1111/j.1552-6569.2007.00169.x. [DOI] [PubMed] [Google Scholar]
- 16.Nogueira RG, Yoo AJ, Buonanno FS, Hirsch JA. Endovascular approaches to acute stroke, part 2: A comprehensive review of studies and trials. AJNR Am J Neuroradiol. 2009;30:859–875. doi: 10.3174/ajnr.A1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitagawa K, Yagita Y, Sasaki T, Sugiura S, Omura-Matsuoka E, Mabuchi T, Matsushita K, Hori M. Chronic mild reduction of cerebral perfusion pressure induces ischemic tolerance in focal cerebral ischemia. Stroke. 2005;36:2270–2274. doi: 10.1161/01.STR.0000181075.77897.0e. [DOI] [PubMed] [Google Scholar]
- 18.Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF. Guidelines for the early management of adults with ischemic stroke: A guideline from the american heart association/american stroke association stroke council, clinical cardiology council, cardiovascular radiology and intervention council, and the atherosclerotic peripheral vascular disease and quality of care outcomes in research interdisciplinary working groups: The american academy of neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007;115:e478–534. doi: 10.1161/CIRCULATIONAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 19.Liebeskind DS. Collateral therapeutics for cerebral ischemia. Expert Rev Neurother. 2004;4:255–265. doi: 10.1586/14737175.4.2.255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.