Abstract
Normetanephrine-H3 injected into the cisterna magna of rats is rapidly metabolized and disappears from brain with an initial half-life of about 12 min. Monoamine oxidase inhibition prevents almost completely the conversion of normetanephrine-H3 to other metabolites and markedly diminishes the rate of disappearance of radioactivity from brain ( ). These data show that normetanephrine is normally metabolized primarily by monoamine oxidase and that unaltered normetanephrine does not readily pass out of the brain. Free 3-methoxy-4-hydroxyphenylglycol (MHPG) and 3-methoxy-4-hydroxymandelic acid (VMA) formed from intracistemally injected normetanephrine-H3 represent only a small fraction of the radioactivity in brain. The major metabolite was identified as the sulfate conjugate of MHPG. After intracisternal administration of norepinephrine-H3, 3-methoxy-4-hydroxyphenylgIycol sulfate (MHPG-SO4) was also found to be the major metabolite present in brain. These findings suggest that deamination, reduction, and subsequent corrugation with sulfate is the primary route of metabolism of normetanephrine in rat brain and that norepinephrine is also metabolized to this sulfate conjugate.
Normetanephrine is formed from norepinephrine in brain and various other tissues by the enzyme catechol-O-methyl transferase.1 According to current concepts of norepinephrine metabolism, it is thought that norepinephrine discharged from the neuron in physiologically active form, either by nerve stimulation or by sympathomimetic drugs, is inactivated either by reuptake into the presynaptic neuron or by enzymatic O-methylation to form normetanephrine.2
Various psychoactive drugs produce alterations in norepinephrine metabolism which are in part reflected by changes in normetanephrine levels in brain.3–6 Little is known, however, about the metabolism of normetanephrine in the central nervous system. This study was therefore undertaken to investigate some aspects of the turnover and metabolism of normetanephrine in brain.
MATERIALS AND METHODS
According to a technique described elsewhere,7 25 μl of Elliott’s “B” irrigating solution (Baxter) containing normetanephrine-H3 (8·3 μc; sp. act., 2·5 mc/mg) was injected into the cisterna magna of Sprague-Dawley rats weighing 190–210 g. The animals were anesthetized lightly with ether during the procedure and were fully recovered within 3 min. At various times after injection, groups of animals were killed by cervical fracture and decapitated. The brains were removed rapidly, washed, and immediately homogenized in 10 ml cold 0·4 N perchloric acid in an all-glass homogenizer. The homogenates were frozen until analyzed. After centrifugation, a 0·3-ml aliquot of the supernatant fluid was added to 15 ml of a toluene-ethanol solution (10:4) containing phosphor, and the total radioactivity was assayed in a Packard liquid scintillation spectrometer.8 Internal standards of toluene-H3 were used to correct for efficiency of counting.
Assay of normetanephrine-H3
An aliquot of the supernatant fluid was adjusted to pH 5 and passed through a 0·3 × 4 cm Dowex-50 (NH4+) column. After washing the column twice with 10 ml glass-distilled water, the normetanephrine-H3 was eluted with 10 ml of 3 N NH4OH. Radioactivity in the eluate was determined as described above.
Assay of tritiated deaminated products
The effluents from the Dowex-50 columns were collected, adjusted to pH 1, brought to constant volume, and aliquots were assayed for tritium. The remaining effluent was saturated with salt and extracted with 5 vol, ethyl acetate. Total 3-methoxy-4-hydroxymandelic acid (VMA)* plus 3-methoxy-4-hydroxyphenylglycoI (MHPG), about 85 per cent of which was extracted, was determined by placing 5 ml of the organic phase into 10 ml phosphor-ethanol (9:1) solution and assaying for tritium as described previously.
Assay of norepinephrine-H3 and metabolites
In those experiments in which norepinephrine-H3 was used, a modification of the method (by absorption on alumina) of Whitby et al,9 was used for its assay. Deaminated catechols, dihydroxymendelic acid (DHMA), and dihydroxyphenylglycol (DHPG) were also determined.10 Other metabolites were determined in the alumina effluent as described herein.
Paper chromatography
One hour after the injection of normetanephrine-H3 into the cisterna magna, rats were killed and the brains removed. Each brain was homogenized in 3 ml cold 0·4 N perchloric acid containing 5 μg each of authentic normetanephrine, VMA, and MHPG. The homogenate was centrifuged and the supernatant fluid evaporated in vacuo until about 0·5 ml remained. Aliquots of this extract as well as authentic normethanephrine, VMA, and MHPG (for identification) were spotted on Whatman No. 1 filter paper. Chromatograms were developed in two different solvent systems: butanol-acetic acid-water (4:1:1), and butanol-ethanol-water (4:1:1). Authentic normetanephrine, VMA, and MHPG were located by spraying the chromatogram with a solution of diazotized p-nitroaniline. Strips were cut into 1-cm widths, placed into counting vials containing 10 ml phosphor-ethanol solution (9:1), and tritium was assayed. There was virtually no quenching due to the paper or the color obtained after spraying.
RESULTS
Disappearance of normetanephrine-H3 from brain
After the intracisternal administration of normetanephrine-H3, the half-life of this amine was approximately 0·2 hr during the first hour. Normetanephrine-H3 represented only 21 and 13 per cent of the total radioactivity in the brain at 1 and 2 hr, respectively.
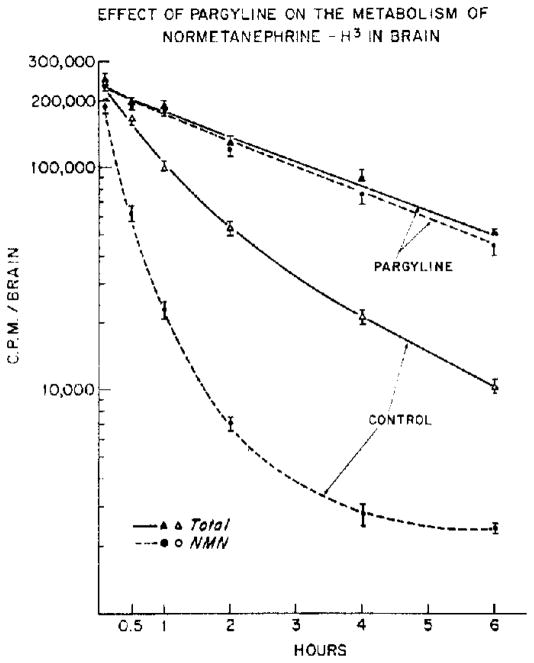
Prior treatment with pargyline almost completely prevented the conversion of normetanephrine-H3 to other metabolites and markedly diminished the rate of disappearance of the unaltered amine in brain. Throughout the entire time course of these experiments, normetanephrine-H3 accounted for over 98 per cent of the total radioactivity and had a half-life of 2·4 hr (Fig. 1). This radioactivity was also shown by paper chromatography to have an Rf value identical to that of authentic normetanephrine.
Fig. 1.
Parglyine (75 mg/kg, i.p.) or saline (control) was injected 45 min prior to the intracisternal injection or normetanephrine-H3. Total radioactivity and normetanephrine-H3 were assayed at various times after the intracisternal injection. Each point represents the mean ± S.E.M. of 8 determinations.
Metabolites of normetanephrine-H3 in brain
Six minutes after the intracisternal injection, about 89 per cent of the total radioactivity in brain was present as either unchanged normetanephrine-H3 or its tritiated deaminated metabolites, VMA or MHPG (Table 1). At 1 hr, however, normetanephrine-H3 and its metabolites, VMA-H3 and MHPG-H3, accounted for only 35 per cent of the total tritium in brain. Sixty-five per cent of the total radioactivity was not adsorbed onto the Dowex-50 column and was not extracted into ethyl acetate over a wide pH range (pH 1–9).
Table 1.
Normetanephrine-H3 and its metabolites in rat brain*
| Time(min) | Total radioactivity in brain ± S.E.M. | Per cent total radioactivity ± S.E.M. |
||
|---|---|---|---|---|
| NMN | VMA plus MHPG | Unknown in effluent | ||
| 3 | 238,000 ±13,700 | 80 ± 6 | 9 ± 1 | 12 ± 2 |
| 60 | 93,500 ±7400 | 22 ± 2 | 13 ± 1 | 65 ± 8 |
Normetanephrine-H3 was injected intracisternally. Normetanephrine and metabolites were analyzed in brain at 6 or 60 min after the injection. Each value represents the mean ± S.E.M. of 8 determinations.
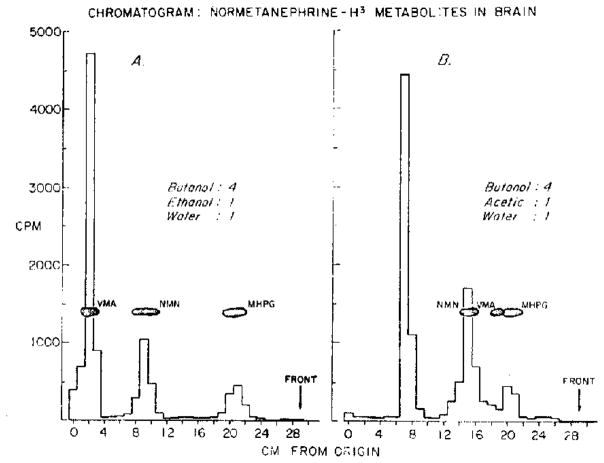
Extracts of brain from animals killed 1 hr after the intracisternal injection of normetanephrine-H3 were chromatographed in two different solvent systems. After chromatography in butanol-ethanol-water (4:1:1), radioactive peaks having mobilities identical to authentic normetanephrine, VMA, and MHPG were found. The largest peak moved only a small distance from the origin and did not separate from VMA (Fig. 2A). When butanol-acetic acid-water (4:1:1) was used as the solvent system, however, the largest peak again had the lowest Rf but in this system was sharply separated from normetanephrine, MHPG, and VMA (Fig. 2B). Over 90 per cent of the radioactivity present in brain could be accounted for by these metabolites.
Fig. 2.
Normetanephrine-H3 was injected intracisternally. Animals were killed 1 hr after the injection and brains were homogenized in 0·4 N perchloric acid. Aliquots of supernatant fluid were chromatographed as described in Methods.
Enzymatic hydrolysis
The large radioactive peak shown in Fig. 2B, which was not normetanephrine, VMA, or MHPG, was eluted with water. Aliquots of this eluate with 1/10 vol. acetate buffer (0·5 N, pH 6·5) were treated with one of three enzyme preparations (Table 2). It was found that only the preparation which contained sulfatase (“glusulase”) effected hydrolysis. β-Glucuronidase and acid phosphatase were ineffective. When p-nitrocatechol sulfate was added to the incubation mixture, hydrolysis of the radioactive metabolite by “glusulase” was almost completely inhibited. Addition of p-nitrocatechol phosphate had no significant effect. After hydrolysis by incubation with 0·04 vol. glusulase at 37° for 20 hr,10 over 85 per cent of the radioactivity in the effluent could be extracted into ethyl acetate at neutral pH, indicating almost complete hydrolysis of the conjugate.
Table 2.
Enzymatic hydrolysis of 3-methoxy-4-hydroxyphenylglycol conjugate isolated from rat brain*
| Enzyme treatment | Ethyl acetate extractable radioactivity(cpm) |
|---|---|
| None (control) | 6200 |
| β-Glucuronidase and sulfatase(glusulase) | 54,200 |
| plus p-nitrocatechol sulfate | 7300 |
| plus p-nitrocatechol phosphate | 51,000 |
| β-Glucuronidase | 5700 |
| Acid phosphatase | 6200 |
The radioactive conjugate found in rat brain after the intracisternal injection of normetanephrine-H3 was isolated (see text). This conjugate was incubated under various conditions (control, enzyme, and enzyme plus inhibitors). Hydrolysis was estimated by measuring the radioactivity extracted from the incubate into ethyl acetate at pH 7. Each value represents the mean of 4 determinations. p-Nitrocatechols were used at a concentration of 1 × 10−2M.
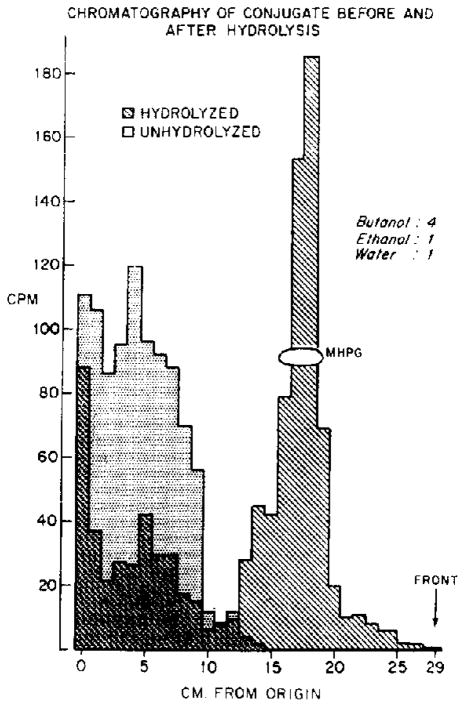
In order to identify the conjugated metabolite, two aliquots of the eluate containing the radioactive conjugate were incubated with glusulase for 1 min (unhydrolyzed) or for 20 hr (hydrolyzed), respectively. After chromatography in butanol-ethanol-water (4:1:1), radioactivity in the control sample remained near the origin, but the radioactivity from the sample hydrolyzed for 20 hr appeared at a position identical to authentic MHPG (Fig. 3). The radioactive product of hydrolysis, like MHPG, but unlike VMA or normetanephrine, was equally well extracted into ethyl acetate over a wide pH range (pH 0·6–8·7).
Fig. 3.
The radioactive conjugate formed in rat brain after the intracisternal injection of normetanephrine-H3, was isolated (see text). This conjugate was incubated with “glusulase” for 1 min (un- hydrolyzed) or for 20 hr (hydrolyzed). The incubates were chromatographed and assayed for radioactivity; histograms were constructed for hydrolyzed and unhydrolyzed samples. The two resulting histograms are superimposed in this figure.
After the intracisternal administration of norepinephrine-H3, an identical conjugated metabolite, presumably the sulfate conjugate of MHPG-H3 (as characterized by paper chromatography, partition coefficient, and enzymatic hydrolysis), was isolated from extracts of brain. At various times after the intracisternal injection of norepinephrine-H3, tritiated MHPG-SO4 appeared to be the major metabolite present in brain, representing over 30 per cent of the total radioactivity. All other metabolites accounted for a total of less than 10 per cent of the radioactvity, while the remaining 50–60 per cent of the radioactivity was present as unchanged norepinephrine-H3 Table 3).
Table 3.
Norepinephrine-H3 and its metabolites in rat brain*
| Radioactive substance | Per cent total radioactivity ± S.E.M. |
||
|---|---|---|---|
| (33 min) | (90 min) | (150 min) | |
| Norepinephrine | 49·8 ± 1·4 | 56·0 ± 1·8 | 58·1 ± 5·2 |
| DHPG plus DHMA | 1·96 ± 0·07 | 1·18 ± 0·07 | 0·76 ± 0·04 |
| Normetanephrine | 7·03 ± 0·22 | 3·78 ± 0·18 | 4·08 ± 0·23 |
| VMA plus MHPG | 3·56 ± 0·15 | 3·39 ± 0·16 | 1·26 ± 0·08 |
| MHPG-SO4 | 31·9 ± 0·8 | 35·9 ± 1·4 | 33·1 ± 2·1 |
Norepinephrine-H3 (5·8 μc) was injected intracisternally, Norepinephrine-H3 and metabolites were analyzed at various times after the injection. Each value represents the mean ± S.E.M. of 8 determinations.
Metabolism of normetanephrine-H3 in brain
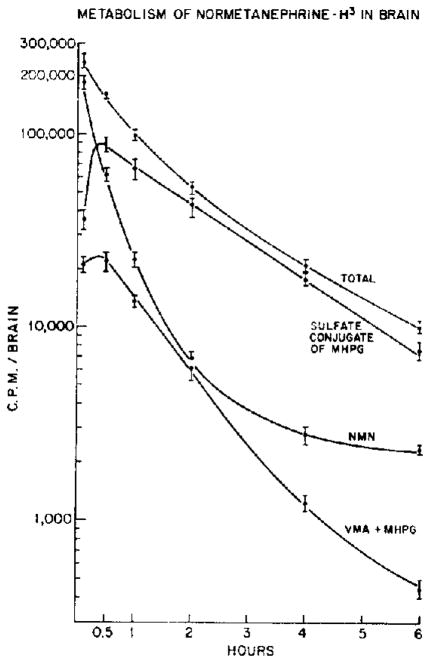
The course of disappearance of tritiated metabolites of normetanephrine-H3 was examined. MHPG-SO4 was the major metabolite present in brain throughout the time course studied. At 15 min, it represented over 50 per cent of the total radioactivity, and the proportion increased to 80 per cent at 2 hr (Fig. 4).
Fig. 4.
Normetanephrine-H3 was injected intracisternally. Normetanephrine-H3 and metabolites were analyzed in brain at various times after injection. Each point represents the mean ± S.E.M. of 8 determinations.
In contrast to its metabolites, the disappearance of normetanephrine-H3 from the brain appears to be multiphasic. During the first hour, its half-life was about 12 min. After 4 hr, the rate of disappearance was markedly decreased ( ).
DISCUSSION
Normetanephrine is formed from endogenous norepinephrine in brain and is normally present in low concentration; levels of normetanephrine, however, are markedly increased after inhibition of monoamine oxidase.8,11 These earlier observations are consistent with the present findings of a rapid metabolism and disappearance of intracisternally administered normetanephrine-H3 from the brain, which is prevented by prior treatment with monoamine oxidase inhibitors. The very slow rate of disappearance of normetanephrine-H3 from the brain after inhibition of monoamine oxidase shows that unaltered normetanephrine does not readily pass out of the brain. Indirect evidence has been reported which also supports this conclusion.12–13 In contrast to the initially rapid deamination of normetanephrine-H3, a small fraction of the injected amine appears to be metabolized very slowly. On the basis of these findings, however, it cannot be determined whether this represents a functionally significant binding of normetanephrine.
In the present study, the sulfate conjugate of 3-methoxy-4-hydroxyphenylglycol was identified as the major metabolite of normetanephrine-H3 in rat brain. After intracisternal administration of norepinephrine-H3, MHPG-SO4 was also found to be the major metabolite present in brain. These findings may account for the unidentified radioactive metabolite observed by Milhaud et al.14 in rat brain after the intraventricular injection of norepinephrine-H3. We have also found that the sulfate conjugate of MHPG is formed by brain slices incubated with radioactive norepinephrine (unpublished observations). The present findings suggest that deamination, reduction, and subsequent conjugation with sulfate is the major route of metabolism of normetanephrine in rat brain and that norepinephrine may also be metabolized to a similar sulfate conjugate.
Conjugates of radioactive phenolic alcohols (dihydroxyphenylethanol and 3-methoxy-4-hydroxyphenylethanol) have also been reported by Goldstein et al.15,16 to be present in rat brain after the intravenous administration of Dopa-C14. Mannarino et al.,17 however, found no evidence of a glycol conjugate in cat brain after intraventricular injection of norepinephrine-C14, although significant levels of free MHPG were demonstrated. These apparently conflicting results may be the consequence of species differences.
We have been able to identify endogenous MHPG-SO4 in rat brain, but free MHPG has not been detected (unpublished observations). These findings may explain the failure to detect significant levels of norepinephrine metabolites in human brain or spinal fluid. Studies are currently in progress to investigate endogenous levels of MHPG-SO4 in brain and spinal fluid of animals and man.
Footnotes
Preliminary results presented to the Fifty-first Annual Meeting of the Federation of American Societies for Experimental Biology, Chicago, III., April 16-21, 1967 (Fed. Proc. 26, 464, 1967).
The following abbreviations are used throughout the text and figures: VMA, 3-methoxy-4-hydroxymandelic acid; MHPG, 3-methoxy-4-hydroxyphenylglycol; MHPG-SO4, 3-methoxy-4-hydroxyphenylglycol sulfate; DHMA, dihydroxymandelic acid; DHPG, dihydroxyphenylglycol; NMN, normetanephrine; MAO, monoamine oxidase.
References
- 1.Axelrod J. Science. 1957;126:400. doi: 10.1126/science.126.3270.400. [DOI] [PubMed] [Google Scholar]
- 2.Kopin IJ. Pharmac Ret. 1966;18:513. [PubMed] [Google Scholar]
- 3.Schanbero SM, Schildkraut JJ, Kopin IJ. Biochem Pharmac. 1967;16:393. doi: 10.1016/0006-2952(67)90040-8. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson A, Lindqvist M. Acta pharmac tox. 1963;20:140. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 5.Glowinski J, Axelrod J. J Pharmac exp Ther. 1965;149:43. [PubMed] [Google Scholar]
- 6.Hagqendal J. Acta pkysiol scand. 1963;59:261. [Google Scholar]
- 7.Schanbero SM, Schildkraut JJ, Kopin IJ. J Pharmac exp Ther. in press. [PubMed] [Google Scholar]
- 8.Zeigler CA, Chleck DJ, Brinkerhoff J. In: Liquid Scintillation Counting. Bell CG Jr, Hayes FN, editors. Pergamon Press; Oxford: 1958. p. 186. [Google Scholar]
- 9.Whitby G, Axelrod J, Well-Malherbe H. J Pharmac exp Ther. 1961;132:193. [PubMed] [Google Scholar]
- 10.Kopin IJ, Axelrod J, Gordon E. J biol Cftem. 1961;236:2109. [PubMed] [Google Scholar]
- 11.Carlsson A, Lindqvist M, Maonusson T. Adrenergic Mechanisms. In: Wolstenholme GEW, O’Connor M, editors. Ciba Found Symp. Churchill; London: 1960. p. 432. [Google Scholar]
- 12.Glowinski J, Kopin IJ, Axelrod J. J Neurochem. 1965;12:25. doi: 10.1111/j.1471-4159.1965.tb10247.x. [DOI] [PubMed] [Google Scholar]
- 13.Maas J, Landb DH. Fedn Proc. 1967;56:463. [Google Scholar]
- 14.Milhaud H, Glowinski J. Cr hebd Sianc Acad Sci, Paris. 1963;256:1033. [Google Scholar]
- 15.Goldstein M. Int J Pharmac. 1964;3:37. doi: 10.1016/0028-3908(64)90043-7. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein M, Gerber H. Life Sci. 1963;2:97. doi: 10.1016/0024-3205(63)90015-8. [DOI] [PubMed] [Google Scholar]
- 17.Mannarino E, Kirshner N, Nashold BS., Jr J Neurochem. 1963;10:373. [Google Scholar]