Abstract
Objectives
To identify abnormal function of the limbic cortex (LC) in response to urinary urgency among patients with Overactive Bladder (OAB) using brain functional MRI (fMRI)
Methods
5 OAB subjects and 5 Controls underwent bladder filling and rated urgency sensations while fMRI measured activation in discrete volumes (voxels) within the brain. Changes in brain activation were related to bladder distension and individual subject’s rating of urgency via multiple regression analysis. Beta weights from regression equations were converted into percent signal change (PSC) for each voxel and PSC compared to the null hypothesis using T-tests. Significance threshold of P<.05 was applied along with a cluster size threshold of.32 ml (5 voxels).
Results
OAB patients showed increased brain activation in LC, specifically the insula (IN) and Anterior Cingulate Gyrus (ACG), associated with increased urgency. Urgency sensations during low volumes were associated with bilateral IN activation in OAB subjects (7,621 voxels right IN, 4,453 voxels left IN, mean beta weights .018 +/− .014 and .014 +/− .011) Minimal activation was present in Controls (790 voxels right IN, beta weight =.010 +/− .007). Urgency sensations during high volumes were associated with bilateral ACG activation in OAB subjects (2,304 voxels right IN, 5,005 voxels left IN, mean beta weights of 005 +/− .003 and 004+/−.003) without activation in Controls.
Conclusions
Urinary urgency in patients with OAB is associated with increased activation of the LC. This activation likely represents abnormal processing of sensory input in brain regions associated with emotional response to discomfort.
Keywords: OAB, fMRI, urinary urgency
Introduction
Overactive Bladder (OAB) Syndrome is a functional disorder of bladder storage characterized by urinary urgency with or without incontinence, usually associated with frequency and nocturia.(1) It is common, affecting 10–15% women, and costly, projected to consume 76.2 billion dollars by 2015.(2) Treatment of OAB, behavioral and pharmacologic, have uncertain durability. The former lacks data regarding long term follow-up and data for the latter indicates side-effects limit efficacy with one year continuation rates as low as 13 %.(3,4) Development of more long-lasting therapy has been hampered by our still incomplete understanding of OAB pathophysiology.
Patients with OAB do not have anatomic abnormalities of the urinary bladder, defining OAB as a functional rather than a structural disorder. Recent work suggests that similar functional disorders (such as irritable bowel syndrome) result from abnormal interoception, that is abnormal perception or interpretation of physiologic events.(5,6) This disorder of perception is more likely caused by abnormal function within the brain rather than abnormal function within the peripheral nervous system. (6,7)
Functional Magnetic Resonance Imaging (fMRI) provides a means for assessing the brain’s response to interoceptive stimuli and has advanced understanding of this and other functional disorders. (8,9) In these disorders fMRI has revealed abnormalities in the function of the limbic cortex, the portion of the brain associated with emotions.(10) Seminal studies by Griffiths and associates (11, 12,13) have used fMRI to show abnormal activation of the limbic cortex in patients OAB.
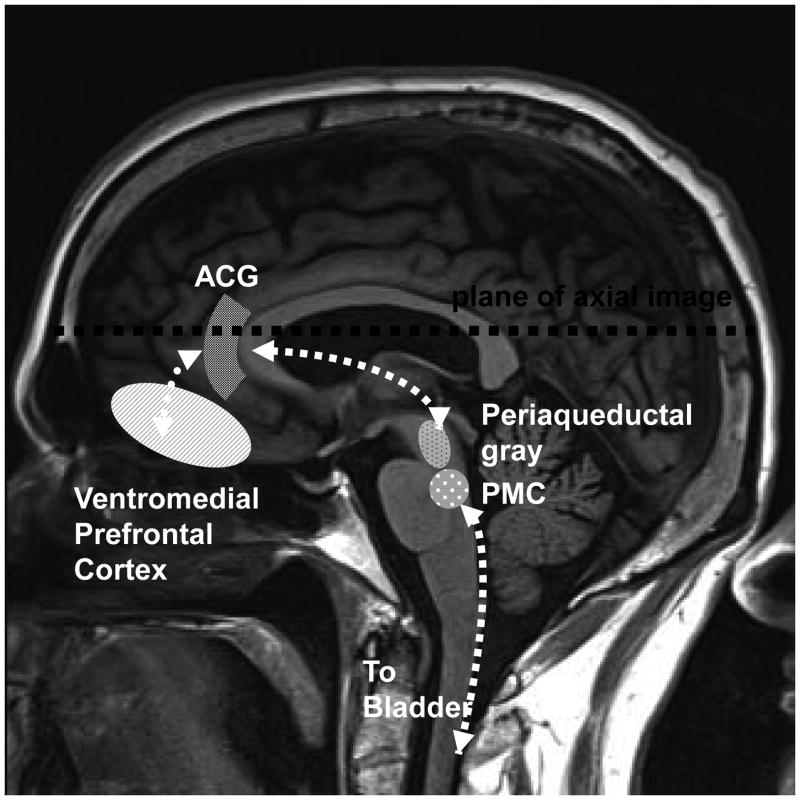
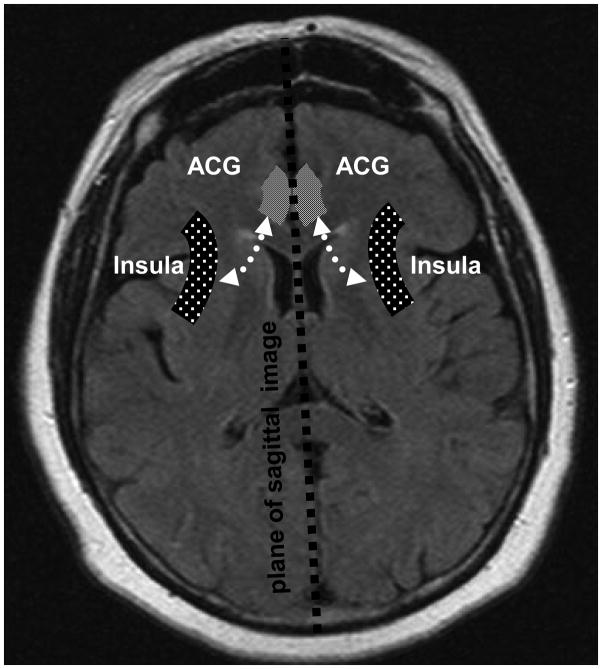
Griffiths et al. evaluated subjects with and without OAB during controlled filling and emptying of the bladder.(11) They found abnormal activation in the limbic region, which included the Anterior Cingulate Gyrus (ACG) and insula, and limbic associated (orbital frontal) cortex (Figures 1& 2). In general the ACG is responsible for the behavioral and autonomic responses to visceral stimuli, considered by some as the motor cortex for the autonomic nervous system. (14) The insula on the other hand, maps and processes visceral stimuli, and is considered by some to function as the sensory cortex for autonomic nervous system. Recent work has revealed the connectivity between these limbic cortical sites and the periaqueductal gray matter (PAG). Input from these cortical regions and afferent input from the bladder are integrated in the PAG which then mediates micturition or storage through the pontine micturition center (PMC) (Figures 1& 2).(8)
Figure 1.
Diagram of regions of brain involved in bladder control. Sagittal view.
Figure 2.
Diagram of regions of brain involved in bladder control. Axial view.
In this study we sought to determine whether abnormal activation in the limbic and limbic associated cortex is more closely related to structural distension of the bladder or to patient perception of urgency. To delineate this relationship, we sought to simultaneously assess both bladder distention and subjects’ rating of urinary urgency during performance of fMRI.
Materials and Methods
Subjects
Subjects were recruited from the University of New Mexico Health Sciences Center (UNMHSC) Urogynecology Clinic and underwent fMRI scanning at the Mind Research Network (MRN) Neurodiagnostic Research Facility. A convenience sample of non-pregnant women over 18, without dementia or psychosis and without contraindication to fMRI scanning were eligible to participate. This study was approved by the UNMHSC IRB (HRRC#09-314).
Women with and without OAB were recruited. OAB subjects were women who experienced ≥ 8 voids/24 hours with associated urgency, and who scored ≥ 8 on the OAB Awareness Questionnaire, a tool validated to screen for OAB in the general population.(15) All women had urine dipstick, pregnancy and post void residual testing performed prior to undergoing fMRI scanning. Women were excluded from study participation if they had a urinary tract infection or if their post void residual was greater than 100 cc. Women with vaginal descent past the hymen with Valsalva were also excluded to avoid inclusion of women with urinary obstruction. Written informed consent, demographic information, and medical history information were obtained.
Subjects were administered the OAB-q Short Form Questionnaire, a validated questionnaire which measures the effect of OAB on symptom bother and quality of life scales.(16) High symptom bother scale scores and low quality of life scale scores indicate worse OAB effects. Subjects were asked to keep a 2 day voiding diary and bedside cystometric testing was scheduled.
FMRI Overview
FMRI scanning evaluates change in brain activation by measuring local changes in blood oxygenation level dependent (BOLD) response resulting from vascular responses to post-synaptic neuronal activity and related metabolic demands. The changes in BOLD response are putatively correlated with the subject’s performance of actions or perception of changes in the environment while in the MRI scanner. The cognitive and/or motor-related functional activity evoked by tasks performed by subjects while in the scanner may be evaluated via recording of subjects’ behavioral responses during the task, and subsequent analyses to evaluate correlations between these behavioral responses, task-design parameters, and concurrent fluctuations of BOLD signal measured throughout the task. Specifically, in this study the task consisted of controlled filling and emptying of the bladder using volumes derived from bedside urodynamics. The task was modeled on the work by Griffiths and associates,(11) modified to allow for the continuous recording of each subject’s subjective appraisal of urinary urgency. Temporal changes in bladder distension and temporal changes in subject perception of urinary urgency were used to model three fixed task design-related regressors (see statistical analysis section below) and two subject-specific regressors (see statistical analysis section below). These regressors were then entered into a multiple regression analysis to evaluate the contribution of each regressor to variance of BOLD signal-related activity within the brain.
Bedside cystometrics and the fMRI task performance
In the week prior to undergoing fMRI, bedside cystometrics were performed. After the subjects voided a catheter was placed in the bladder and post void residuals measured. The catheter was then attached to a 60cc syringe placed at the level of the bladder and the bladder filled via gravity in 60cc increments with room temperature normal saline. Subjects’ first sensation, normal and strong urge to void and maximal cystometric capacities, as defined by the International Continence Society,(1) were recorded. In order to minimize subject anxiety during later performance of the fMRI, abbreviated simulations of the bladder filling and emptying sequences (see below) were performed following cystometric testing. Similar to the fMRI task, subjects were also asked to rate their perception of degree of urinary urgency on a 0–10 Likert scale during this simulation.
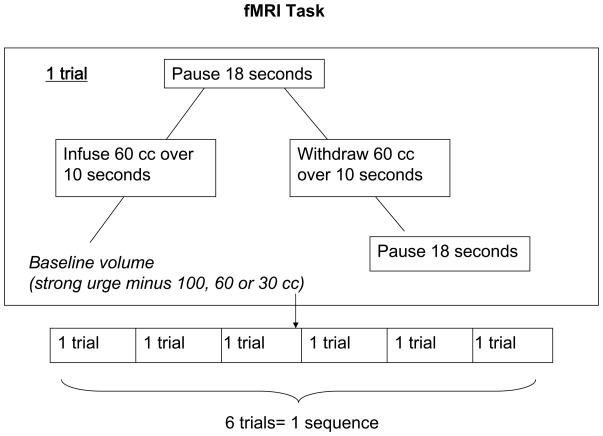
The fMRI exam was performed within one week of bedside urodynamic testing. During the fMRI subjects underwent bladder filling and emptying with 60 cc saline at 3 different volumes determined by the subjects’ strong urge volumes. The lowest volume equaled the strong urge to void volume minus 100 cc, medium volume the strong urge to void minus 60cc and high volume the strong urge to void minus 30cc (Figure 1). Each infusion/withdrawal cycle consisted of the following: 1) 60 cc of saline fluid infused over 10 seconds 2) bladder volume held unchanged for 18 seconds 3) 60cc of fluid withdrawn over 10 seconds 4) bladder volume held unchanged for 16–20 seconds (i.e. baseline state). Six trials were performed at each bladder volume, constituting a low volume sequence, medium volume sequence and high volume sequence (Figure 3).
Figure 3.
fMRI Task
Subjects were asked to rate their perception of urinary urgency during these three sequences using a 0–10 scale that was updated at a 10 Hz frequency. A score of 0 represented no urgency and 10 represented severe urgency. Subjects continuously rated their urgency on a 0–10 scale by pressing buttons on a finger operated device placed within the scanner and could observe the scale and their current rating projected on a screen via a mirror mounted at eye level. During fMRI tasks, subjects were not told if fluid was being infused, withdrawn or whether they were in the pause portion of the sequence.
This perception rating system generated two unique perception-related regressors, one modeled on scalar values of subject’s increasing or decreasing urgency to void (ratings regressor), and the other modeled on button-press related activity (motor regressor). These regressors were used to model mathematical functions that allowed for correlation of subject urge perception with brain activation during fMRI.
FMRI Data Acquisition & Image Processing
High resolution T1-weighted anatomic images were acquired with a 5-echo multiecho MPRAGE sequence [TE(echo time)=1.64ms, TR (repetition time)=2.53 s, TI=a.2s,7°flip angle, number of excitations (NEX)=1, slice thickness=1mm, FOV (field of view) =256 mm, resolution=256 × 256] on a 3 Tesla Siemens Trio scanner before the bladder-filling fMRI tasks. For each of the FMRI tasks, echo-planar images were collected using a single-shot, gradient-echo echo planar pulse sequence [TR = 2000 ms; TE = 29 ms; flip angle = 75°;FOV = 240 mm; matrix size = 64 × 64]. Thirty-three contiguous sagittal 3.5-mm thick slices with a gap factor of 1.05 mm were selected to provide whole-brain coverage (voxel size: 3.75 × 3.75 × 4.55 mm). The first image of each run was eliminated to account for T1 equilibrium effects in addition to two dummy scans.
Functional images were generated using Analysis of Functional NeuroImages (AFNI) software package.(17) Time series images were spatially registered in both 2 and 3 dimensional space to the second EPI image of the first run to minimize the effects of head motion, temporally interpolated to correct for slice-time acquisition differences and de-spiked.
Statistical Analysis
A voxel-wise multiple regression analysis was used to estimate regression coefficients, termed beta weights, corresponding to bladder distension conditions and the individual subject’s urgency ratings. These beta weights reflect the contribution to variance in brain activation resulting from each of the separately modeled conditions, or regressors. Separate regressors for fluid infusion, static distended volume and fluid withdrawal conditions were created by convolving the experimental time-course for each condition with a gamma variate function derived from known parameters of the hemodynamic response.(18) Given the fixed experimental design, these three regressors were identical for all subjects. The ratings regressor was tailored for each participant based on their individualized continuous urgency ratings during all three runs. The urgency ratings regressor resembled a step function, with each button press signaling the onset of either increasing or decreasing subjective levels of urinary urgency. The resulting step function was then convolved with a gamma variate function derived from known patterns of hemodynamic responses in the brain. A single-event model was used to account for concurrent changes in the motor and visual system activation associated with button presses, updating of the Likert scale and other cognitive activity.
Beta weights from the regression equations were divided by the baseline coefficients to produce percent signal change maps normalized to stereotaxic space. T-tests were then performed for the OAB and Control groups separately to evaluate the null hypothesis. A significance threshold corresponding to P<.05 (uncorrected for multiple comparisons) was applied in combination with a minimum cluster size threshold of .32 ml (5 native voxels) to all of the data to minimize the likelihood of false positives. A subsequent region of interest (ROI) analysis was performed to extract mean beta weights within significant clusters of activation in the OAB group analysis. Based on the work by Griffiths and colleagues (11,12,13) regions of interest included the ACG and insula.
Subject characteristics, subject grading of urgency sensations prior to fMRI, and questionnaire results from OAB and Control groups were compared using t-tests assuming unequal variance and Fischer’s exact tests where appropriate with significance set at P<.05.
Results
Ten subjects were evaluated using the fMRI protocol described previously; 5 non-OAB subjects (Controls) and 5 OAB subjects. There were no differences between Controls and OAB subjects’ age (51 versus 54 years, P=.35) nor were there differences between the two group’s other descriptive characteristics (Table 1). None of the subjects had a history of neurologic diseases. One OAB subject had undergone prior OAB treatment and continued to take anticholinergic medications whereas the remainder of subjects did not.
Table 1.
Subject Characteristics
| Controls (+/−Standard Deviation) or (%)N=5 | OAB subjects (+/−Standard Deviation) or (%)N=5 | P | |
|---|---|---|---|
| Age | 51 (4.3) | 54 (5.8) | .35* |
| Parity | 2.0 (0) | 1.8 (1.8) | .62* |
| Body Mass Index kg/m2 | 24.2 (3.1) | 29.1 (6.9) | .20* |
| Ethnicity | |||
| Non-Hispanic White | 3 | 5 | .44† |
| Hispanic White | 1 | 0 | |
| Asian | 1 | 0 | |
| History Stress Incontinence Surgery | 2/5 (40%) | 1/5 (20%) | 1.0† |
| History Prolapse Surgery | 2/5 (40%) | 0/5 (0%) | .44† |
| History Hysterectomy | 2/5 (40%) | 3/5 (60%) | 1.0† |
| Cigaretter Smoker | 0 (0%) | 1/5 (20%) | 1.0† |
| Taking anticholinergic medication | 0 (0%) | 1/5 (20%) | 1.0† |
| Handedness | |||
| Right | 5 (100%) | 4/5 (80%) | 1.0† |
| Left | 0 | 0 | |
| Both | 0 | 1/5 (20%) | |
| POP-Q Stage | |||
| 0 | 0 | 0 | 1.0† |
| 1 | 0 | 1/5 (20%) | |
| 2 | 5/5 (100%) | 4/5 (80%) | |
Satterthwaite t-test
Fisher’s exact test
There were significant differences between the OAB and Control groups’ strong urge to void volumes and maximal cystometric capacity volumes; the OAB group had smaller mean volumes compared to controls (Table 2). There were also significant differences between the two group’s OAB-q Short Form Symptom Severity and Quality of Life Scores (Table 2). The OAB group had far greater symptom severity and far worse quality of life scores than controls.
Table 2.
OABq Short form scores and Cystometric findings
| Controls (+/Standard Deviation) N=5 | OAB Subjects (+/−Standard Deviation) N=5 | P value* | |
|---|---|---|---|
| Mean OABq Short Form Symptom Severity Score | 1.3 (1.8) | 58.7 (21.2) | .004 |
| Mean OABq Short Form Quality of Life Score | 99.1 (2.1) | 55.7 (17.6) | .005 |
| Average voids/day | 6.0 (1.4) | 10.7 (2.5) | .06 |
| Average volume per void in cc | 421 (183.4) | 189.3 (53.3) | .08 |
| 1st sensation on cystometry in milliliters | 9.0 (4.1) | 32.4 (21.3) | .15 |
| Strong urge to void on cystometry in milliliters | 361.0 (81.3) | 147 (54.0) | .002 |
| Maximal Capacity on cystometry in milliliters | 396.3 (66.0) | 173 (59.8) | .002 |
Satterthwaite t- test
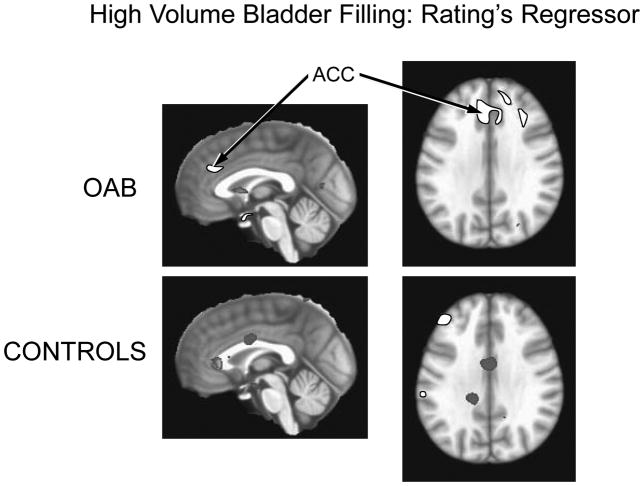
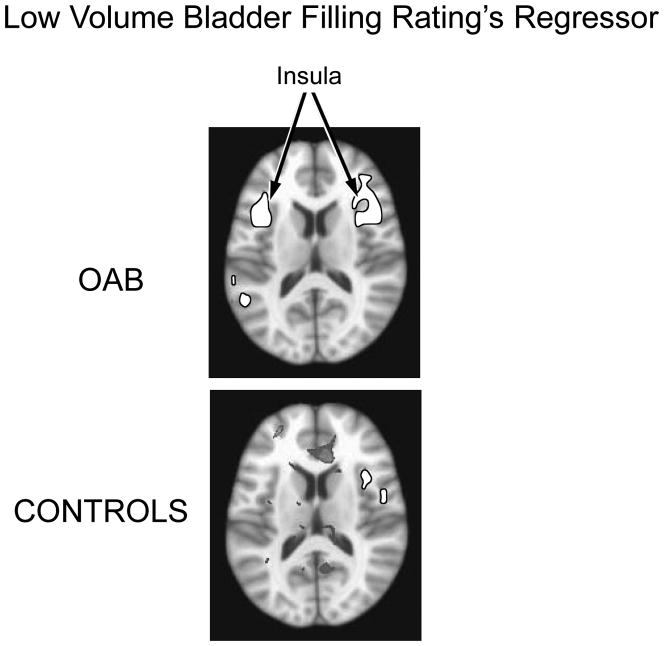
Analysis revealed that the urge regressor during the low bladder volume sequence was associated with activation of the insula bilaterally in OAB subjects. A volume of 7,621 voxels (1 × 1 × 1 mm3) were activated in the right insula and 4,453 voxels in the left insula with mean beta weights of .018 +/− .014 and .014 +/− .011, respectively. In controls the urge regressor was associated with activation of only a small volume of the right insula (790 voxels) with an average beta weight of (.010 +/−.007) (See Figure 4). In OAB patients the urge regressor during the high bladder volume sequence was associated with activation of the right (2,304 voxels) and left ACG (5,005 voxels) with mean beta weights of 005 +/− .003 and 004+/−.003 respectively. No activated voxels were observed within the ACG in Control subjects (See Figure 5). Urgency ratings did not differ at low bladder filling between OAB and Control subjects (5.8 ± 2.6 and 5.2 ± 2.5, respectively, P=.72), nor did they differ significantly during the high volume bladder filling (8.9 ± 1.3 and 7.0 ± 1.8, respectively, P=.26) although there was a trend towards higher ratings in OAB subjects.
Figure 4.
Activation of the insula associated with the ratings regressor during low volume bladder filling. Bilateral insula activation is present in OAB subjects; activation confined to small area of left insula in Controls
Figure 5.
Activation of the ACG associated with the ratings regressor during high volume bladder filling. Bilateral ACG activation is present in OAB subjects; no voxel cluster activation is observed within the insula in Controls.
Discussion
This study provides preliminary data on the relationship between urinary urgency and abnormal brain activity among women with OAB. In this work, activation of the ACG in OAB subjects correlated with sensations of increasing urinary urgency. Despite similar perceived levels of urinary urgency, Control subjects did not show similar ACG activation. This is in keeping with recent studies of interoceptive stress that have demonstrated ACG activation to be a determinant of behavioral and autonomic response to visceral stimuli, including that from bladder and bowel distension. When stressed by bladder distension ACG activation in patients with OAB may be related to recruitment of accessory pathways to prevent loss of bladder control.(19)
During low volume bladder filling the urgency regressor in OAB subjects correlated with activation of both the right and left insula, while Control subjects showed a lesser extent of activation (right insula only). This parallels prior work that has shown more widespread activation of the insula among patients with poor bladder control.(11,12)Activation of the insula is indicative of its role in processing afferent input from the autonomic nervous system in general and sensation from bladder distension specifically.
Other work has shown increased insular activation in response to greater degrees of bladder distension.(11,12,13) However, we did not observe this phenomenon in our subjects. Several factors could be responsible for the observed lack of correlation between the urge regressor and insula activation at higher bladder volumes in our subjects. Both our control and OAB subject groups were homogenous in age and did not include the young adult women that would be expected to have the highest insular activation with bladder distension.(12) Additionally, we chose to center bladder filling and emptying around the volume that patients felt a strong urge to void, rather than maximal bladder capacity (MCC) as used by Griffiths.(11) This was based on concerns regarding subjects’ acceptance of bladder discomfort at the higher MCC volumes during fMRI scanning as well as the rationale that the strong urge volumes would more accurately reflect the subjects’ routine voiding volumes.
Alternatively, the lack of insular activation during high bladder volume runs performed towards the end of the fMRI task may represent habituation caused by repetitive stimuli to the bladder. Habituation to the repeated filling and emptying of the bladder during the fMRI task may have blunted the efferent output from the bladder, diminishing the brain’s response to bladder filling. Finally, given our small sample size we may have not had the necessary statistical power to detect this effect.
Regardless of the cause, our data raise the possibility that patient rated urgency scores may correlate better with ACG activation than with activation of the insula. This would suggest that perception of urinary urgency is more dependent on the autonomic and behavioral response to bladder distension (mediated through ACG) than upon direct sensory input (mediated via the insula).
The correspondence between ACG activation and perception of urinary urgency may have important therapeutic implications for the treatment of OAB, particularly for the use of behavioral therapy. Activation of the ACG and other portions of the limbic cortex are modulated by cortical areas involved with executive function including decision making in an emotional context or conflict resolution. Cortical areas, such as the prefrontal cortex, have the ability to inhibit limbic system activation and have been implicated in the therapeutic effects of distraction techniques, hypnosis treatment and the use of placebo. (20)
The role of the ACG and its associated cortex, however, is extremely complex. Clinically applicable understanding will require not only knowledge of the magnitude of neural activation at these sites, but fMRI analysis of the neural connections between limbic cortex, prefrontal cortex, midbrain (PAG) and brainstem structures (PMC). This study’s demonstration of temporal correlation between ACG signal and subjects’ urgency (urge regressor) may add to the understanding of the relationship between brain activation and symptoms in OAB patients. Over the last decade the urologic and neuroscience literature have made significant advances in understanding the etiology and control of urinary incontinence by the use of functional brain imaging. We expect that our work is only the beginning of wider contribution by the obstetrics and gynecology community.
Acknowledgments
This study was supported by The Dept. of Health and Human Services/National Institutes of Health/Graduate Clinical Research Center University of New Mexico Grant #5M01 RR00997
Footnotes
Presented at the 31st Annual Scientific Meeting of the American Urogynecologic Society, October 1, 2010 in Long Beach, California.
References
- 1.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4–20. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- 2.Ganz ML, Smarlarz AM, Krupski TL, et al. Economic Costs of Overactive Bladder in the United States. Urology. 2010;75:526–32.e18. doi: 10.1016/j.urology.2009.06.096. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann KE, McPheters Ml, Biller DH, et al. AHRQ Publication No. 09-E017. Rockville, MD: Agency for Healthcare Research and Quality; Aug, 2009. Treatment of Overactive Bladder in Women. Evidence Report/Technology Assessment No. 187 (Prepared by the Vanderbilt Evidence-based Practice Center under Contract No. 290-2007-10065-I) [Google Scholar]
- 4.D’Souza AO, Smith MJ, Miller LA, et al. Persistence, adherence, and switch rates among extended-release and immediate-release overactive bladder medications in regional managed care plan. J Manag Care Pharm. 2008;14(3):291–301. doi: 10.18553/jmcp.2008.14.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang L. Brain Responses to Visceral and Somatic Stimuli in Irritable Bowel Syndrome: a Central Nervous System Disorder? Gastroenterol Clin N Am. 2005;34:271–279. doi: 10.1016/j.gtc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 6.van Marle HJ, Hermans EJ, Qin S, Fernandez G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry. 2009;66(7):649–55. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Labus JS, Naliboff BD, Berman SM, et al. Brain networks underlying perceptual habituation to repeated aversive visceral stimuli in patients with irritable bowel syndrome. Neuroimage. 2009;47(3):952–60. doi: 10.1016/j.neuroimage.2009.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler CJ, Griffiths D. A Decade of Functional Brain Imaging Applied to Bladder Control. Neurourology and Urodynamics. 2010;29:49–55. doi: 10.1002/nau.20740. [DOI] [PubMed] [Google Scholar]
- 9.Berman SM, Naliboff BD, Suyenobu B, et al. Reduced brainstem inihibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28(2):349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holstege G. The Emotional Motor System and Micturition Control. Neurourol and Urodynamics. 2010;29:42–48. doi: 10.1002/nau.20789. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths D, Derbyshire S, Stenger A, et al. Brain control of normal and overactive bladder. J of Urol. 2005;174:1862–67. doi: 10.1097/01.ju.0000177450.34451.97. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths D, Tadic SD, Schaefer W, et al. Cerebral control of the bladder in normal and urge-incontinent women. NeuroImage. 2007;37:1–7. doi: 10.1016/j.neuroimage.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tadic SD, Griffiths D, Schaefer W, et al. Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. NeuroImage. 2008;39:1647–53. doi: 10.1016/j.neuroimage.2007.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DasGupta R, Kavia RB, Fowler CJ. Cerebral mechanisms and voiding function. BJU Int. 2007;99:731–4. doi: 10.1111/j.1464-410X.2007.06749.x. [DOI] [PubMed] [Google Scholar]
- 15.Coyne KS, Zyczynski T, Margolis MK, et al. Validation of an overactive bladder awareness tool for use in primary care settings. Advances in Therapy. 2005;22(4):381–94. doi: 10.1007/BF02850085. [DOI] [PubMed] [Google Scholar]
- 16.Coyne K, Revicki D, Hunt T, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: The OAB-q. Qual Life Res. 2002;11:563–74. doi: 10.1023/a:1016370925601. [DOI] [PubMed] [Google Scholar]
- 17.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 18.Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 19.Drake MJ, Fowler CJ, Griffiths D, et al. Neural Control of the Lower Urinary and Gastrointestinal Tracts: Supraspinal CNS Mechanisms. Neurourol and Urodynamics. 2010;29:119–27. doi: 10.1002/nau.20841. [DOI] [PubMed] [Google Scholar]
- 20.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]