Abstract
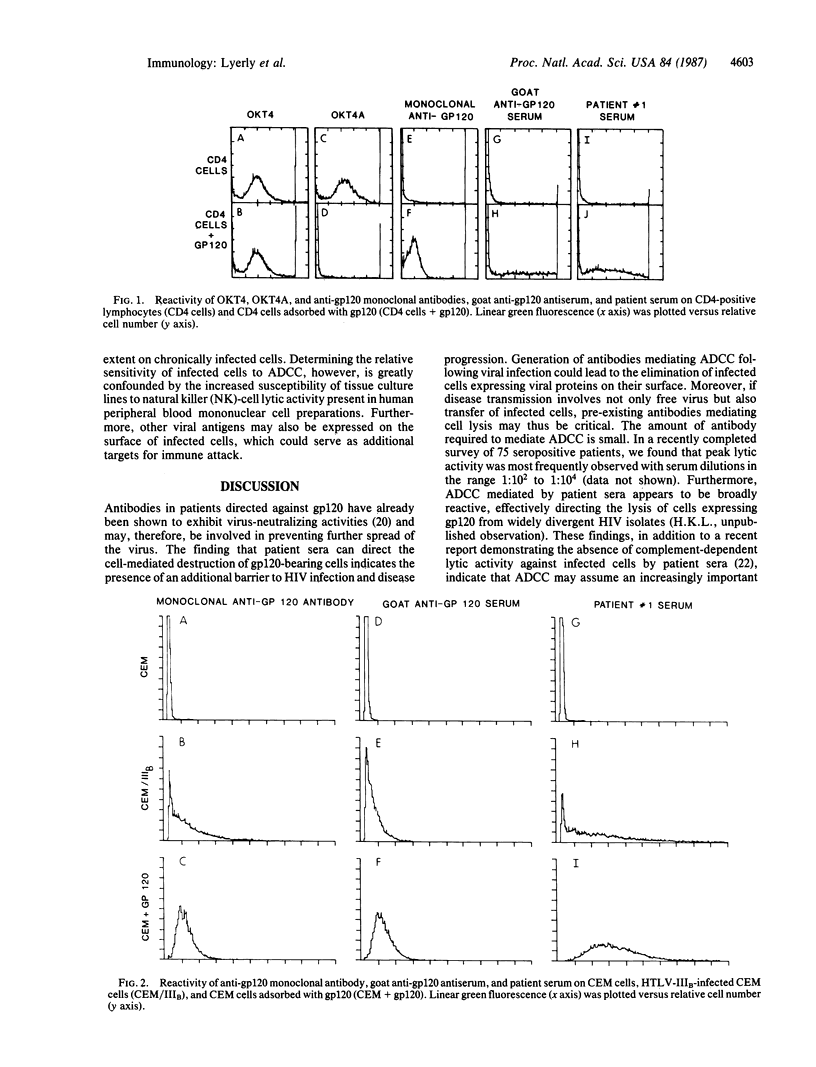
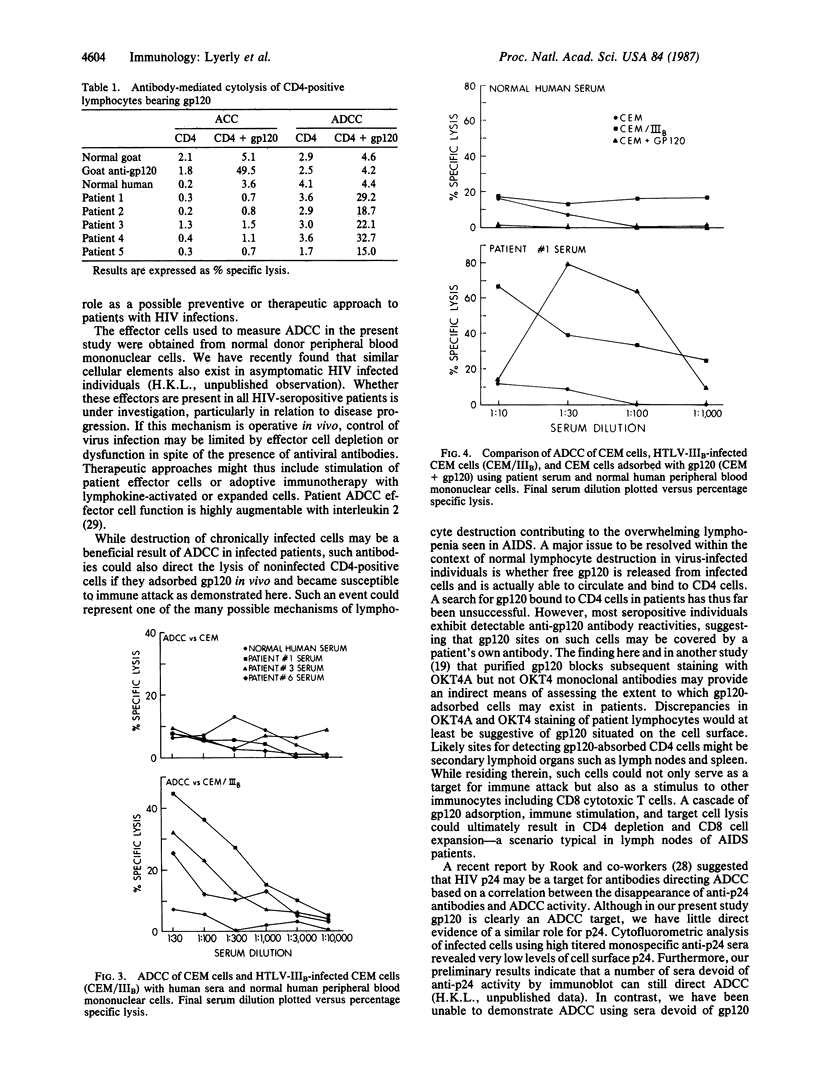
The lymphocyte differentiation antigen CD4 serves as a receptor for human retroviruses associated with acquired immunodeficiency syndrome (AIDS) through its interaction with the major envelope virion glycoprotein, gp120, which is also expressed on the surface of infected cells. In these experiments, purified gp120 was shown to bind to normal human T-lymphocyte populations. The gp120-CD4 complex served as a target antigen for antibody-dependent complement-mediated cytolysis by a goat serum raised against native gp120. However, patient sera that bound to gp120-adsorbed cells failed to direct their destruction in the presence of complement. In contrast, these sera were potent mediators of antibody-dependent cellular cytotoxicity. These studies demonstrate that gp120 situated on the cell surface can serve as an effective target for immune destruction by patient antibodies and effector lymphocytes. The possible contribution of this type of immunity to control of disease progression, on the one hand, and to lymphocyte destruction and immunopathology observed in AIDS, on the other, is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammann A. J., Abrams D., Conant M., Chudwin D., Cowan M., Volberding P., Lewis B., Casavant C. Acquired immune dysfunction in homosexual men: immunologic profiles. Clin Immunol Immunopathol. 1983 Jun;27(3):315–325. doi: 10.1016/0090-1229(83)90084-3. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Broder S., Gallo R. C. A pathogenic retrovirus (HTLV-III) linked to AIDS. N Engl J Med. 1984 Nov 15;311(20):1292–1297. doi: 10.1056/NEJM198411153112006. [DOI] [PubMed] [Google Scholar]
- Fahey J. L., Prince H., Weaver M., Groopman J., Visscher B., Schwartz K., Detels R. Quantitative changes in T helper or T suppressor/cytotoxic lymphocyte subsets that distinguish acquired immune deficiency syndrome from other immune subset disorders. Am J Med. 1984 Jan;76(1):95–100. doi: 10.1016/0002-9343(84)90756-3. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Fauci A. S. Immunologic abnormalities in the acquired immunodeficiency syndrome. Annu Rev Immunol. 1985;3:477–500. doi: 10.1146/annurev.iy.03.040185.002401. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Masur H., Gelmann E. P., Longo D. L., Steis R. G., Chused T., Whalen G., Edgar L. C., Fauci A. S. Correlation between immunologic function and clinical subpopulations of patients with the acquired immune deficiency syndrome. Am J Med. 1985 Mar;78(3):417–422. doi: 10.1016/0002-9343(85)90332-8. [DOI] [PubMed] [Google Scholar]
- Langlois A. J., Matthews T. J., Weinhold K. J., Bolognesi D. P. Immunologic control of a retrovirus-associated murine adenocarcinoma. VII. Tumor cell destruction by macrophages and IgG2A. J Natl Cancer Inst. 1985 Oct;75(4):709–715. [PubMed] [Google Scholar]
- Langlois A. J., Matthews T., Roloson G. J., Thiel H. J., Collins J. J., Bolognesi D. P. Immunologic control of the ascites form of murine adenocarcinoma 755. V. Antibody-directed macrophages mediate tumor cell destruction. J Immunol. 1981 Jun;126(6):2337–2341. [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Reyes G. R., McGrath M. S., Stein B. S., Engleman E. G. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science. 1986 May 30;232(4754):1123–1127. doi: 10.1126/science.3010463. [DOI] [PubMed] [Google Scholar]
- Matthews T. J., Collins J. J., Roloson G. J., Thiel H. J., Bolognesi D. P. Immunologic control of the ascites form of murine adenocarcinoma 755. IV. Characterization of the protective antibody in hyperimmune serum. J Immunol. 1981 Jun;126(6):2332–2336. [PubMed] [Google Scholar]
- Matthews T. J., Weinhold K. J., Langlois A. J., Bolognesi D. P. Immunologic control of a retrovirus-associated murine adenocarcinoma. VI. Augmentation of antibody-dependent killing following quantitative and qualitative changes in host peritoneal cells. J Natl Cancer Inst. 1985 Oct;75(4):703–708. [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- Nara P. L., Robey W. G., Gonda M. A., Carter S. G., Fischinger P. J. Absence of cytotoxic antibody to human immunodeficiency virus-infected cells in humans and its induction in animals after infection or immunization with purified envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3797–3801. doi: 10.1073/pnas.84.11.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Read-Connole E., Gallo R. C. T4 positive human neoplastic cell lines susceptible to and permissive for HTLV-III. Lancet. 1984 Dec 22;2(8417-8418):1472–1473. doi: 10.1016/s0140-6736(84)91666-0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Matthews T. J., Robey W. G., Lynn D. L., Robert-Guroff M., Mueller W. T., Langlois A. J., Ghrayeb J., Petteway S. R., Jr, Weinhold K. J. HTLV-III/LAV-neutralizing antibodies to an E. coli-produced fragment of the virus envelope. Science. 1986 Dec 12;234(4782):1392–1395. doi: 10.1126/science.2431482. [DOI] [PubMed] [Google Scholar]
- Robey W. G., Arthur L. O., Matthews T. J., Langlois A., Copeland T. D., Lerche N. W., Oroszlan S., Bolognesi D. P., Gilden R. V., Fischinger P. J. Prospect for prevention of human immunodeficiency virus infection: purified 120-kDa envelope glycoprotein induces neutralizing antibody. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7023–7027. doi: 10.1073/pnas.83.18.7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook A. H., Lane H. C., Folks T., McCoy S., Alter H., Fauci A. S. Sera from HTLV-III/LAV antibody-positive individuals mediate antibody-dependent cellular cytotoxicity against HTLV-III/LAV-infected T cells. J Immunol. 1987 Feb 15;138(4):1064–1067. [PubMed] [Google Scholar]
- Sarngadharan M. G., Popovic M., Bruch L., Schüpbach J., Gallo R. C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984 May 4;224(4648):506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Stahl R. E., Friedman-Kien A., Dubin R., Marmor M., Zolla-Pazner S. Immunologic abnormalities in homosexual men. Relationship to Kaposi's sarcoma. Am J Med. 1982 Aug;73(2):171–178. doi: 10.1016/0002-9343(82)90174-7. [DOI] [PubMed] [Google Scholar]
- Weinhold K. J., Bolognesi D. P., Matthews T. J. Immunologic control of a retrovirus-associated murine adenocarcinoma. VIII. Corynebacterium parvum-activated natural killer cells as potent antibody-dependent cell-mediated cytotoxicity effectors. J Natl Cancer Inst. 1985 Oct;75(4):717–724. [PubMed] [Google Scholar]



