Abstract
BACKGROUND AND PURPOSE
Lysophosphatidylcholines (lysoPCs) with polyunsaturated acyl chains are known to exert anti-inflammatory actions. 15-Lipoxygeanation is crucial for anti-inflammatory action of polyunsaturated acylated lysoPCs. Here, the anti-inflammatory actions of 1-(15-hydroxyeicosapentaenoyl)-lysoPC (15-HEPE-lysoPC) and its derivatives were examined in a mechanistic analysis.
EXPERIMENTAL APPROACH
Anti-inflammatory actions of 15-HEPE-lysoPC in zymosan A-induced peritonitis of mice were examined by measuring plasma leakage and leucocyte infiltration, and determining levels of lipid mediators or cytokines.
KEY RESULTS
When each lysoPC, administered i.v., was assessed for its ability to suppress zymosan A-induced plasma leakage, 15-HEPE-lysoPC was found to be more potent than 1-(15-hydroperoxyeicosapentaenoyl)-lysoPC or 1-eicosapentaenoyl-lysoPC. Separately, i.p. administration of 15-HEPE-lysoPC markedly inhibited plasma leakage, in contrast to 15-HEPE, which had only a small effect. 15-HEPE-lysoPC also decreased leucocyte infiltration. Moreover, it reduced the formation of LTC4 and LTB4, 5-lipoxygenation products, as well as the levels of pro-inflammatory cytokines. The time-course study indicated that 15-HEPE-lysoPC might participate in both the early inflammatory phase and resolution phase. Additionally, 15-HEPE-lysoPC administration caused a partial suppression of LTC4-induced plasma leakage and LTB4-induced leucocyte infiltration. In the metabolism study, peritoneal exudate was shown to contain lysoPC-hydrolysing activity, crucial for anti-inflammatory activity, and a system capable of generating lipoxin A from 15-hydroxy eicosanoid precursor.
CONCLUSIONS AND IMPLICATIONS
15-HEPE-lysoPC, a precursor for 15-HEPE in target cells, induced anti-inflammatory actions by inhibiting the formation of pro-inflammatory leukotrienes and cytokines, and by enhancing the formation of lipoxin A. 15-HEPE-lysoPC might be one of many potent anti-inflammatory lipids in vivo.
Keywords: anti-inflammatory, 1-[15(S)-hydroxypentaenoyl]-lysoPC, zymosan A, LTB4, LTC4, PGE2, TNF-α, IL-6
Introduction
A large body of new evidences indicate that endogenous lipid mediators actively engage in the host response to inflammation (Serhan and Savill, 2005). During the time course of inflammation, the switching of lipid mediator class from pro-inflammatory prostaglandins and leukotrienes to the biosynthesis of anti-inflammatory or pro-resolving lipid mediators, such as lipoxins (Levy et al., 2001; Serhan, 2005a; Serhan and Savill, 2005; Maderna and Godson, 2009), lipoxenes (Wong et al., 1985; Lam et al., 1987; Lam and Wong, 1988; Stahl et al., 1989), resolvins and protectin D (Serhan, 2005b; Serhan and Savill, 2005; Serhan et al., 2008) has been well established. By the identification of endogenous anti-inflammatory and pro-resolving lipid mediators, it becomes apparent that the resolution phase of inflammation may involve an active biochemical process (Serhan and Savill, 2005), responsible for the generation of lipid mediators. Previously, lysophosphatidylcholine (lysoPC) had been reported to be pro-inflammatory (Fuentes et al., 2002; Muralikrishna Adibhatla and Hatcher, 2006; Shi et al., 2007). The pro-inflammatory action of lysoPCs, saturated or monounsaturated, may be largely due to the generation of reactive oxygen species or nitric oxide in various types of cells (Colles and Chisolm, 2000; Takeshita et al., 2000; Guzik et al., 2003; Matsubara and Hasegawa, 2005; Park et al., 2009). In contrast, our recent studies (Huang et al., 2010) have reported that 1-arachidonoyl-lysophosphatidylcholine (1-arachidonoyl-lysoPC) and 1-docosahexaenoyl-lysophosphatidylcholine (1-docosahexaenoyl-lysoPC) exhibit an anti-inflammatory action, whereas no significant anti-inflammatory effects were observed with 1-linoleoyl-lysoPC. Moreover, 1-(15-hydroperoxyeicosatetraenoyl)-lysoPC and 1-(17-hydroperoxydocosahexaenoyl)-lysoPC were more potent as anti-inflammatory lipids than 1-arachidonoyl-lysoPC and 1-docosahexaenoyl-lysoPC respectively. This led to the assumption that the prior oxygenation of 1-arachidonoyl-lysoPC and 1-docosahexaenoyl-lysoPC by 15-lipoxygenase (15-LOX) may be crucial for the expression of its anti-inflammatory action. In support of this, 4-methyl-2-(4-methylpiperazinyl)pyrimido[4,5-b] benzothiazine, an inhibitor of 15-LOX, was found to prevent the anti-inflammatory action of 1-arachidonoyl-lysoPC (Hung et al., 2009). With regard to the mechanisms of the anti-inflammatory action of 15-hydroperoxyeicosatetraenoyl-lysoPC, the inhibition of lipoxin formation has been proposed to be the main mechanism involved and the inhibition of 5-LOX activity to have minor role. Thus, the anti-inflammatory action of 15-hydroperoxyeicosatetraenoyl-lysoPC appears to be expressed mainly through its metabolism to the epoxide intermediate (Pettitt et al., 1991; Rowley et al., 1994). However, whether or not the anti-inflammatory action of 15-hydroperoxyeicosapentaenoyl-lysoPC is expressed after its reduction to 15-hydroxyeicosapentaenoyl-lysoPC has not been clarified. Earlier studies have shown that 15-hydroxyeicosatetraenoic acid (15-HETE), 15-hydroxyeicosapentaenoic acid (15-HEPE) and 17-hydroxydocosahexaenoic acid inhibit 5-LOX activity and prostaglandin synthetase (Miller and Ziboh, 1988; Miller et al., 1989; Gonzalez-Periz et al., 2006). Additionally, it was reported that 15-HETE and 15-HEPE are converted to lipoxins via 5-hydroperoxy, a 15-hydroxy acid derivative (Chiang et al., 2005; Serhan, 2005a). In this regard, it is hypothesized that 15-lipoxygenation products of polyunsaturated acylated lysoPC exert their anti-inflammatory actions after hydrolytic metabolism in vivo. Further, cellular glutathione peroxidase (GSH-peroxidase) activity readily converts hydroperoxy derivatives to hydroxyl derivatives in vivo (Huang et al., 2009). Nonetheless, there has been no extensive study on the anti-inflammatory actions of hydroxyl derivatives derived from 15-LOX-catalysed oxygenation of polyunsaturated fatty acylated lysoPCs.
In this study, we examined the anti-inflammatory actions of 1-eicosapentaenoyl-lysophosphatidylcholine (1-eicosapentaenoyl-lysoPC) and its oxygenation product, 1-(15-HEPE)-lysoPC. Further, the anti-inflammatory action of 1-(15-HETE)-lysoPC was extensively studied so as to elucidate the mechanisms responsible for its anti-inflammatory effects in vivo.
Methods
Materials
Dieicosapentaenoyl phosphatidylcholine (dieicosapentaenoyl-PC) and didocosahexaenoyl phosphatidylcholine were obtained from NOF corporation (Shibuya-ku, Tokyo, Japan). Diarachidonoyl phosphatidylcholine, eicosapentaenoic acid and docosahexaenoic acid (purity, 99%) were procured from Avanti Polar Lipid (Alabaster, AL, USA). Soybean LOX-1 (Type I-B), phospholipase A2 (PLA2) (honey bee venom), zymosan A (Saccharomyces cerevisiae) were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). 12/15-LOX (porcine leucocyte, 135.6 U·mL−1), 5-HETE, 15-HETE, 15-HEPE, 15-LOX-2 (human recombinant, 250 U·mL−1), 15-LOX inhibitor (PD146176), enzyme immunoassay (EIA) kits for prostaglandin E2 (PGE2), leukotriene B4 (LTB4) and leukotriene C4 (LTC4) were from Cayman Chemical (Ann Arbor, MI, USA). Lipoxin A4 (LXA4) and 12-HETE were from Oxford Biochemical Research Corp. (Oxford, MI, USA) and Assay Designs Inc. (Ann Arbor, MI, USA) respectively. elisa assay kits for cytokines [tumor necrosis factor alpha (TNF-α), interleukin 1-beta (IL-1β), interleukin-2 (IL-2), interleukin-6 (IL-6) and interferon gamma (IFN-γ)] were obtained from eBioscience, Inc. (Science Center Drive, San Diego, CA, USA). Boc2 (N-t-butyloxycarbony-Phe-Leu-Phe-Leu-Phe), a lipoxin receptor antagonist, was from Phoenix Phamarceuticals Inc. (Burlingame, CA, USA). 1-Eicosapentaenoyl-lysoPC, 1-docosahexaenoyl-lysoPC and 1-arachidonoyl-lysoPC were prepared from PLA2-catalysed hydrolysis of correlated didocosahexaenoyl phosphatidylcholine (PC), as described previously with slight modifications (Huang et al., 2007; 2008b; 2009;). In brief, each PC (2.5 mg), dissolved in chloroform, was dried under N2, and then rapidly dispersed in 10 mL of 50 mM borax buffer (pH 9.0) containing 10 mM CaCl2. The hydrolysis was started by adding PLA2 (100 U), and allowed to continue under N2 with constant stirring for 2 h at 25°C. The reaction mixture was partially purified by Sep-pack column (2 × 1 cm) and the lysophospholipid product was further purified by silica gel TLC in the solvent system (chloroform : methanol : water; 65:25:4). Finally, the spot containing lysoPC was scraped off, extracted with methanol, dried under N2 and kept at −80°C until used.
Oxygenation of 1-eicosapentaenoyl-lysoPC by LOXs
Oxygenation of 1-eicosapentaenoyl-lysoPC by LOXs was monitored via the increase in absorbance at 234 nm according to the formation of conjugated diene. Briefly, 1-eicosapentaenoyl-lysoPC (100 µM) was in turn incubated with soybean LOX-1 (2.5 U·mL−1), porcine leucocyte 12/15-LOX (1 U·mL−1), or human 15-LOX-2 (1 U·mL−1) in 50 mM borax buffer (pH 9.0), 50 mM phosphate buffer (pH 7.4) containing 5 mM EDTA and 0.03% Tween 20, or 50 mM Tris-HCl buffer (pH 7.2) containing 0.003 % Tween 20 respectively. One unit of LOX was defined as the amount of LOX that can produce one nanomol of conjugated diene min−1 (Huang et al., 2007; 2008a;).
Determination of kinetic values in LOXs-catalysed oxygenation of eicosapentaenoic acid, 1-eicosapentaenoyl-lysoPC or dieicosapentaenoyl-PC
Human recombinant 15-LOX-2 (1 U·mL−1), leucocyte 12-LOX-1 (1.5 U·mL−1) or soybean LOX-1 (10 U·mL−1) was incubated with lipids of various concentrations in selective buffer. The kinetic parameter values, Km and Vm, were calculated from Lineweaver–Burke plot analysis as described previously (Huang et al., 2006; 2008b;).
Preparation of 1-(15HEPE)-lysoPC
1-Eicosapentaenoyl-lysoPC (400 µM) was oxidized by soybean LOX-1B (4 KU·mL−1) in 5 mL of borax buffer (50 mM, pH 9.0). Then, hydroperoxide was further reduced to correlated hydroxide by 1 mM SnCl2 for 10 min at room temperature. The hydroxide derivative product was extracted with a mixture of chloroform and methanol (2:1) (Maskrey and O'Donnell, 2008; Morgan et al., 2009; Thomas et al., 2010). Briefly, to the above mixture, 5 mL of chloroform and 10 mL methanol was added, and the mixture was subjected to vortex for 5 min. Subsequently, after centrifugation at 3400×g for 3 min, the lower phase was collected and further purified by RP-HPLC, using Zorbrax eclipse XDB C18 column (5 µm, 50 × 4.6 mm, Agilent Technologies, Santa Clara, CA, USA) with an isocratic solvent system (methanol : water : acetic acid; 70:30:0.1). The amount of 1-(15-HEPE)-lysoPC was determined by absorbance of purified lipid at 234 nm by using E1m,1cm= 25 000, and stored at −80°C until used (Morgan et al., 2009).
SP-HPLC analysis for identification of 1-(15-HEPE)-lysoPC
1-(15-HEPE)-lysoPC from the above preparation was hydrolysed in 1 N NaOH and extracted with a mixture of chloroform and methanol (2:1). The final product was analysed by SP-HPLC system equipped with a silicagel column (10 µM, 300 × 7.8 mm, Phenomenex, Torrance, CA, USA), eluted (1.0 mL·min−1) with an isocratic mobile solvent system (hexane : isopropanol : acetic acid; 100:3:0.1), and the effluent was monitored at 234 nm. The major product was co-injected with 15-HEPE standard (Huang et al., 2010).
Identification of 1-15(HEPE)-lysoPC using LC/ESI-MS
1-Eicosapentaenoyl-lysoPC was incubated with soybean 15-LOX to produce 1-(15-HPEPE)-lysoPC. After 10 min incubation, the lipoxygenation product was reduced to corresponding hydroxyl derivative by using 1 mM SnCl2. The reduction product, after further purification using RP-HPLC, was analysed by LC-ESI-MS, using a MSDI spectrometer (HP 1100 series LC/MSA, Hewlett Packard, Palo Alto, CA, USA) equipped with Zorbrax eclipse XDB C18 column (5 µm, 50 × 4.6 mm), which was eluted (0.5 mL·min−1) with an isocratic solvent system (methanol : water : acetic acid; 70:30:0.1), and the eluate was monitored at 234 nm. The full-scan mass spectra were obtained within the range of m/z 250–700, and the data acquisition was conducted in a positive mode.
Animal experiments
Male ICR mice (29–30 g weight; 6 weeks of age) were procured (Koatech Co., Pyung Taek, Korea), assigned to plastic cages (10 mice per cage), housed under a 12 h light–dark cycle, and accessed to unlimited amounts of filtered water and chows (Teklad Global 18% Protein Rodent Diet obtained from Harlan laboratories, Madison, WI, USA) for one day before being used. All animal experiments were conducted in accordance with the Guide for Care and Use of Laboratory Animals of the National Research Council (NRC, 1996), which was approved by Committee of Animal Care and Experiments of Chungnam National University, Korea.
Zymosan A-induced peritonitis
Peritonitis was induced by i.p. administration of zymosan A (100 mg·kg−1) as described previously (Doherty et al., 1985; Rao et al., 1994; Byrum et al., 1999). For the measurement of plasma leakage, mice were administered, i.v. or i.p., 100 µL of lipid, dissolved in phosphate-buffered saline (PBS), 30 min prior to i.p. administration (100 mg·kg−1) of zymosan A, and 100 µL of 0.5% Evans blue dye, dissolved in PBS, was injected i.v. just prior to zymosan A injection (Arita et al., 2005b; Kolaczkowska et al., 2008). Sixty minutes later, unless indicated otherwise, mice were killed by isoflurane inhalation, and peritoneal lavage was performed with 3 mL of ice-cold PBS. Then cells were centrifuged out of the lavage fluid. Finally, Evans blue dye extravasation was determined by measuring the absorbance of supernatants at 610 nm (Byrum et al., 1999; Leite et al., 2007; Sun et al., 2007)
To evaluate the effect of the 15-LOX inhibitor (PD146176) on the anti-inflammatory action of 1-(15-HPEPE)-lysoPC, 1-(15-HPEPE)-lysoPC and PD146176 (100 mg·kg−1), dissolved in PBS (Jeon et al., 2009), were simultaneously administered to mice 30 min prior to i.p. administration of zymosan A, and the plasma leakage was assessed as described above. Separately, in order to measure leucocyte infiltration, lipids were administered 30 min prior to i.p. administration of zymosan A, and 120 min after zymosan A injection, peritoneal lavage was taken, and the total number of cells in the lavage fluid was enumerated by using light microscopy employing tryphan blue staining (Byrum et al., 1999; Bannenberg et al., 2004). Separately, the presence of neutrophils in lavage was determined through the detection of myeloperoxidase (MPO) activity in the lysis buffer (Bradley et al., 1982), prepared from sonication of cell pellet suspensions (0.5 mL) at 4°C for 5 min, using an MPO chlorination assay kit (Cayman Chemical, Ann Arbor, MI, USA). One unit is defined as the amount of enzyme that will cause the formation of 1 pmol of fluorescent product min−1 at 25°C.
Determination of inflammatory lipid mediators or cytokine levels in peritoneal lavage fluid
To determine the level of pro-inflammatory lipid mediators in exudates, 1 mL of peritoneal lavage fluid was transferred to microcentrifuge tubes and centrifuged (7600×g, 5 min). Supernatants were used directly for EIA analysis of PGE2, LTB4, LTC4, 12-HETE, 15-HETE or LXA4 level as described previously (Rao et al., 2007; Yuhki et al., 2008). Levels of pro-inflammatory cytokines, TNF-α, IL-1β, IL-2, IL-6 or INF-γ, were determined by using elisa assay according to the manufacturers' instructions.
Effect of 1-(15-HEPE)-lysoPC on zymosan A-induced formation of LTC4 or LTB4 in resident peritoneal cells
Resident peritoneal cells were harvested as described previously with a slight modification (Arita et al., 2005a; Dimitrova and Ivanovska, 2008). Mice were killed by administration of diethyl ether, and peritoneum were lavaged with 3 mL ice-cold PBS-/-. Harvested cells, after being washed twice with PBS, were suspended in Dulbecco's modified Eagle medium (DMEM) supplemented with 5% Fetal bovine serum (FBS), penicillin G (10 000 U·mL−1) and streptomycin (10 mg·mL−1) and divided into a 96-well plate at a density of 5 × 104 cells·mL−1. After 2 h incubation, the supernatants were removed and adhered cells were washed again with PBS twice (70–80% harvested cells attached to plate), and incubated again in 5% FBS-containing DMEM media. Next, the harvested cells were exposed to zymosan A (100 µg·mL−1) for 1 min, and then 1-(15-HEPE)-lysoPC (0.75, 2.5 or 7.5 µM) was added. The further incubation was performed at 37°C in 5% CO2 humid air for 20 h. Finally, the level of LTC4 and LTB4 in cell-free supernatants were measured by using an EIA kit as described above.
Effect of Boc2, a lipoxin receptor antagonist, on the suppression of zymosan A-induced leucocyte infiltration by 15-HEPE-lysoPC
Mice were concomitantly i.p. treated with Boc2 (von der Weid et al., 2004) and 15-HEPE-lysoPC 30 min prior to i.p. administration of zymosan A. Four hours later, mice were killed by isoflurane inhalation, and the peritoneum was lavaged with 3 mL of sterile PBS buffer. Total number of infiltrated leucocytes in the lavage fluid was counted by using light microscopy after tryphan blue staining (Byrum et al., 1999).
Determination of 15-HETE released from peritoneal cells treated with 1-(15-HETE)-lysoPC
Peritoneal cells (5 × 104 cells·mL−1), harvested as described above, were incubated with 1-(15-HETE)-lysoPC (7.5 µM) and zymosan A (100 µg·mL−1) at 37°C in a 5% CO2 humid air. Then, at different time intervals (30, 60 or 120 min), the level of 15-HETE in the cell-free cultured supernatants was measured by using an EIA kit according to manufacturer's instructions.
Statistical analysis
Results are expressed as means ± SEM. Statistical significance for differences between groups was determined by Student's t-test, or a conservative one-way anova for multiple group comparisons after establishing that the data in the groups were normally and equally distributed. Differences between or among groups were considered statistically significant if P-values were lower than or equal to 0.05.
Results
Previously, 15-lipoxygenation products of 1-arachidonoyl-lysoPC and 1-docosahexaenoyl-lysoPC have been reported to show potent anti-inflammatory activities (Hung et al., 2009; 2010;). Here, we examined the anti-inflammatory action of 1-(15-HEPE)-lysoPC, a reduction product of 1-(15-hydroperoxyeicosapentaenoyl)-lysoPC, and attempted to elucidate the mechanisms responsible for its anti-inflammatory action.
Preparation and identification of 1-(15-HEPE)-lysoPC
First, 1-eicosapentaenoyl-lysoPC was incubated with soybean 15-LOX to produce 1-(15-HPEPE)-lysoPC. After 10 min incubation, the lipoxygenation product was reduced to corresponding hydroxyl derivative by using 1 mM SnCl2. The reduction product, after further purification using RP-HPLC, was analysed by LC/ESI-mass spectrometry by using a positive scan mode. As shown in Figure 1 (inset), the peak (RT, ∼7.8 min), which appeared as a predominant product, possessed mass ions characteristic of 1-(15-HEPE)-lysoPC; molecular ions at m/z 557 [(M + H+)], m/z 580 [(M + Na+)] and m/z 596[(M + K+)]. From this, it was confirmed that 1-eicosapentaenoyl-lysoPC was oxygenated by 15-LOX to produce 1-(15-HPEPE)-lysoPC, which was further reduced to 1-(15-HEPE)-lysoPC. In a further experiment, 1-(15-HEPE)-lysoPC was hydrolysed in 1 N NaOH, and then the hydrolysis product was found to comigrate with standard 15-HEPE in straight phase-HPLC (data not shown).
Figure 1.
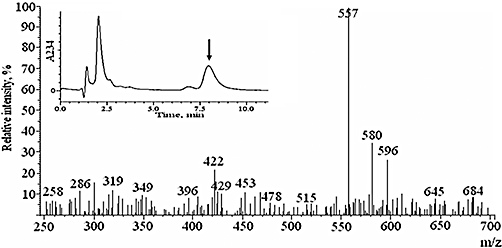
LC/ESI-MS analysis of 1-(15-HEPE)-lysoPC. The hydroxide derivative was analysed by LC-ESI-MS as described in Methods. The representative mass spectrum of the major peak with retention time of 7.8 min (inset) was obtained by ESI-MS system using a positive scan mode.
Determination of kinetic values in LOXs-catalysed oxygenation of eicosapentaenoic acid, 1-eicosapentaenoyl-lysoPC or dieicosapentaenoyl-PC
As 15-lipoxygenation of polyunsaturated acyl lysoPC was crucial for the expression of anti-inflammatory action (Hung et al., 2009; 2010;), the oxygenation of 1-eicosapenaenoyl-lysoPC by 15-LOXs was kinetically analysed. When 1-eicosapentaenoyl-lysoPC was incubated with soybean LOX-1, leucocyte 12/15-LOX or human 15-LOX-2, oxygenation of 1-eicosapentaenoyl-lysoPC by each LOX displayed a time-dependent increase in absorbance at 234 nm, indicative of the formation of a conjugated diene. In a kinetic study, where each LOX was incubated with 1-eicosapentaenoyl-lysoPC, the enzyme activity followed classical Michaelis–Menten kinetics. When the kinetic values for oxygenation of 1-eicosapentaenoyl-lysoPC by soybean 15-LOX were calculated (Huang et al., 2007) from Lineweaver–Burk plot analysis (Table 1), catalytic efficacy (Vm/Km) of 1-eicosapentaenoyl-lysoPC (49.5 U·mg−1·µM−1) was greater than that of eicosapentaenoic acid (20.1 U·mg−1·µM−1), or that of dieicosapentaenoyl-PC (0.002 U·mg−1·µM−1). Subsequently, when eicosapentaenoic acid or 1-eicosapentaenoyl-lysoPC was oxygenated by human 15-LOX-2, the Km and Vm values were estimated to be 3.7 µM and 47.7 U·mg−1 protein, respectively for eicosapentaenoyl-lysoPC, and 21.9 µM and 115.1 U·mg−1 protein, respectively for 1-eicosapentaenoic acid. Taken together, catalytic efficacy (Vm/Km, 12.8 U·mg−1·µM−1) of 1-eicosapentaenoyl-lysoPC is about twofold greater than that (5.9 U·mg−1·µM−1) of eicosapentaenoic acid, and much greater than that (0.002 U·mg−1·µM−1) of dieicosapentaenoyl-PC. Next, when lipoxygenation catalysed by leucocyte 12/15-LOX was analysed, catalytic efficacy (5.0 U·mg−1·µM−1) of 1-eicosapentaenoyl-lysoPC was higher than that (2.1 U·mg−1·µM−1) of eicosapentaenoic acid or that (0.002 U·mg−1·µM−1) of dieicosapentaenoyl-PC. From these, it is suggested that 1-eicosapentaenoyl-lysoPC may be utilized more efficiently than eicosapentaenoic acid in vivo.
Table 1.
Kinetic values in oxygenation of eicosapentaenoic acid (EPA), eicosapentaenoyl-lysoPC or dieicosapentaenoyl-PC by soybean LOX-1, leucocyte 12/15-LOX and human 15-LOX-2
| Enzyme | Substrate | Km (µM) | Vm (units mg−1) | Vm/Km (units mg−1·µM−1) |
|---|---|---|---|---|
| Soybean LOX | 1-eicosapentaenoyl-lysoPC | 4.5 ± 1.2a | 215.6 ± 24.6a | 49.5 ± 8.2a |
| Eicosapentaenoic acid | 10.7 ± 0.8b | 214.1 ± 33.4a | 20.1 ± 1.5b | |
| Dieicosapentaenoyl-PC | 34 064.1 ± 19 137.1c | 0.06 ± 0.02b | 0.002 ± 0.000c | |
| Leucocyte 12-LOX | 1-eicosapentaenoyl-lysoPC | 5.6 ± 0.2a | 22.9 ± 5.0a | 5.0 ± 1.9a |
| Eicosapentaenoic acid | 20.4 ± 6.2b | 41.8 ± 3.9b | 2.1 ± 0.5b | |
| Dieicosapentaenoyl-PC | 62 417.2 ± 508.2c | 0.11 ± 0.07c | 0.001 ± 0.000c | |
| Human 15-LOX-2 | 1-eicosapentaenoyl-lysoPC | 3.7 ± 1.6a | 47.7 ± 15.6a | 12.8 ± 1.2a |
| Eicosapentaenoic acid | 21.9 ± 11.1b | 115.1 ± 8.42b | 5.9 ± 0.61b | |
| Dieicosapentaenoyl-PC | 44 696.2 ± 106.6c | 0.09 ± 0.03c | 0.002 ± 0.000c |
Soybean LOX-1 (2.5 U·mL−1) was incubated with eicosapentaenoic acid, 1-eicosapentaenoyl-lysoPC or dieicosapentaenoyl-PC at various concentrations in 500 µL of 50 mM borax buffer, pH 9.0 at 25°C. Leucocyte 12/15-LOX (2.5 U·mL−1) was incubated with each substrate in 500 µL of 50 mM phosphate buffer (pH 7.4) containing 5 mM EDTA and 0.03% Tween 20. Human 15-LOX-2 (1 U·mL−1) was incubated with each substrate in 500 µL of 50 mM Tris-HCl buffer (pH 7.2) containing 0.003% Tween 20. One unit of LOX were defined as 1 nmol of oxygenation product formed min-1. Data are expressed as means ± SEM values of triplicates experiments. Means displayed with letter are not significantly different from each other.
Structure–activity relationship for anti-inflammatory actions of 1-eicosapentaenoyl-lysoPC derivatives administered i.v
Next, we examined the anti-inflammatory action of 1-eicosapentaenoyl-lysoPC and its oxygenation products in zymosan A-induced peritonitis. For this purpose, each lysoPC, administered i.v., was tested for its ability to attenuate zymosan A-induced plasma leakage into the peritoneum in mice, based on the extravasation of Evans blue dye as an indicator of vascular permeability. As demonstrated in Figure 2, when 1-eicosapentaenoyl-lysoPC was administered i.v. to mice 30 min prior to i.p. administration of zymosan A (100 mg·kg−1), it was found to show a dose-dependent inhibitory effect; 1-eicosapentaenoyl-lysoPC at 50 µg·kg−1 and 150 µg·kg−1 inhibited zymosan A-induced peritonitis by about 30 % and 55% respectively, and the 50% effective dose (ED50) value was estimated to be around 83 µg·kg−1. Subsequently, it was observed that i.v. 1-(15-HPEPE)-lysoPC (ED50, 53.7 µg·kg−1) or 1-(15-HEPE)-lysoPC (ED50, 35.7 µg·kg−1) were more potent than 1-eicosapentaenoyl-lysoPC in suppressing the zymosan A-induced peritonitis (Figure 2), indicating that 15-lipoxygenation of 1-eicosapentaenoyl-lysoPC may be crucial for its anti-inflammatory activity. In comparison, 1-(15-HEPE)-lysoPC seemed to be slightly more potent than 1-(15-HPEPE)-lysoPC at inducing a suppressive action. In contrast, 15-hydroperoxyeicosapentaenoic acid up to 150 µg·kg−1 (data not shown) did not suppress zymosan A-induced peritonitis, confirming the structural importance of lysoPC for anti-inflammatory action. Separately, in order to see whether 15-lipoxygenation products, endogenously generated in vivo, can affect the anti-inflammatory action of 1-(15-HEPE)-lysoPC, the effect of 1-(15-HPEPE)-lysoPC on zymosan A-induced plasma leakage was assessed in combination with PD146176, an inhibitor of 15-LOX. When 15-HPEPE-lysoPC and PD146176 were simultaneously administered i.v and i.p., respectively, to mice 30 min prior to i.p. administration of zymosan A, it was found that PD146176 did not significantly affect the suppressive effect of 1-(15-HPEPE)-lysoPC on zymosan A-induced plasma leakage (Figure 3), suggesting that 15-lipoxygenation products, endogenously generated, are not involved in the anti-inflammatory effects of 1-(15-HPEPE)-lysoPC.
Figure 2.
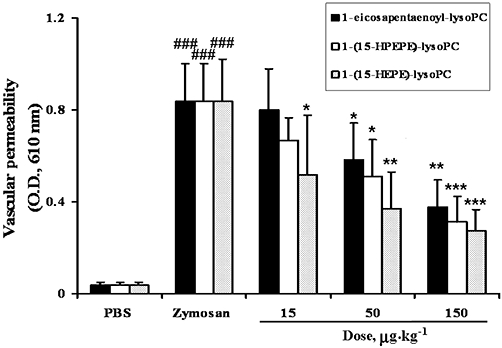
Effect of 1-eicosapentaenoyl-lysoPC derivatives, administered i.v., on zymosan A-induced plasma leakage in mice. 1-Eicosapentaenoyl-lysoPC, 1-(15-HPEPE)-lysoPC or 1-(15-HEPE)-lysoPC, dissolved in sterile phosphate-buffered saline (PBS) in a total volume of 100 µL, was given i.v. to mice (0–150 µg·kg−1), 30 min prior to i.v. administration of Evans blue dye (5%, 100 µL), followed by i.p. administration of zymosan A (100 mg·kg−1) to evoke peritonitis. After 60 min, the peritoneum was lavaged with 3 mL of sterile ice-cold PBS, and the plasma leakage was determined as described in Methods. Values are means ± SEM (n= 7–10). *P < 0.05; **P < 0.01; ***P < 0.001 versus zymosan A-treated group; ###P < 0.001 versus PBS-treated group (one-way anova followed by the Newman–Keuls-Student's test).
Figure 3.
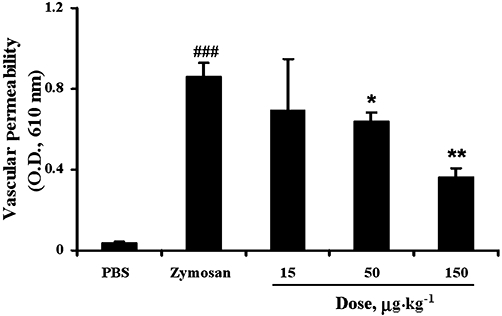
Effect of 1-(15-HPEPE)-lysoPC, in combination with 15-LOX inhibitor (PD146176), on zymosan A-induced plasma leakage in mice. 1-(15-HPEPE)-lysoPC (0–150 µg·kg−1) and PD146176 (100 mg·kg−1), dissolved in phosphate-buffered saline (PBS), were administered i.v. and i.p., respectively, to mice 30 min prior to i.v. administration of Evans blue dye, followed by i.p. administration of zymosan A (100 mg·kg−1). After 60 min, the peritoneum was lavaged with sterile cold PBS, and the plasma leakage was determined as described in Methods. Values are means ± SEM (n= 7–10). *P < 0.05; **P < 0.01, versus zymosan A-treated group; ###P < 0.001 versus PBS-treated group (one-way anova followed by the Newman–Keuls-Student's test).
Structural importance of 1-(15-HEPE)-lysoPC, administered i.p., for anti-inflammatory action
Subsequently, 1-(15-HEPE)-lysoPC was administered i.p. to mice, and then its anti-inflammatory action was extensively examined. As shown in Figure 4, i.p. 1-(15-HEPE)-lysoPC (ED50, 28.6 µg·kg−1) was more potent at suppressing plasma leakage than i.v. administration (ED50, 35.7 µg·kg−1). In a further study, it was observed that the suppressive effect of 1-(15-HEPE-lysoPC) on plasma leakage was comparable to that of 1-(17-hydroxydocosahexaenoic acid)-lysoPC (ED50, 32.03 µg·kg−1), but greater than that of 1-(15-HETE)-lysoPC (ED50, 43.1 µg·kg−1) (Figure 4). To further examine the anti-inflammatory action of i.p. 1-(15-HEPE)-lysoPC, the total number of leucocytes in the peritoneum were determined. As shown in Figure 5A, 1-(15-HEPE)-lysoPC attenuated zymosan A-induced infiltration of leucocytes into the peritoneum. Furthermore, the infiltration of neutrophils, measured as MPO activity in the lysate of infiltrated cells, was also diminished dramatically in groups treated with i.p. 1-(15-HEPE)-lysoPC (Figure 5B). In contrast, no significant suppression of plasma leakage and leucocyte infiltration was induced by 15-HEPE up to 150 µg·kg−1. Thus, 1-(15-HEPE)-lysoPC was much more potent than 15-HEPE at inducing an anti-inflammatory effect.
Figure 4.
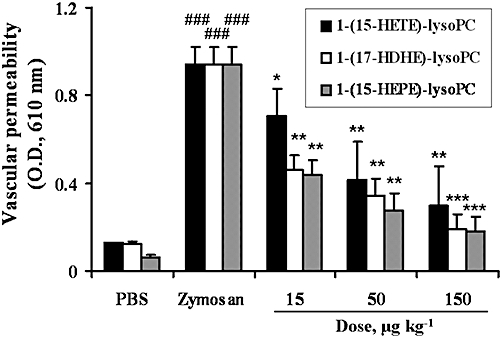
Effect of each polyunsaturated lysoPC hydroxide, administered i.p., on zymosan A-induced plasma leakage in mice. 1-(15-HETE)-lysoPC, 1-(17-HDHE)-lysoPC or 1-(15-HEPE)-lysoPC, dissolved in phosphate-buffered saline (PBS), was administered i.p. to mice (0–150 µg·kg−1). The effect on zymosan A-induced plasma leakage was assessed as described in Figure 2. Values are means ± SEM (n= 10) *P < 0.05; **P < 0.01; ***P < 0.001 versus zymosan A-treated group; ###P < 0.001 versus PBS-treated group (one-way anova followed by the Newman–Keuls-Student's test).
Figure 5.

Effect of 1-(15-HEPE)-lysoPC or 15-HEPE, administered i.p., on zymosan A-induced leucocyte infiltration in mice. (A) 15-HEPE or 1-(15-HEPE)-lysoPC, dissolved in phosphate-buffered saline (PBS), was administered i.p. 30 min prior to i.p. administration of zymosan A (100 mg·kg−1) to evoke peritonitis. Total cells were counted in lavage fluid collected at the 120 min time point by using light microscopy together with tryphan blue staining. (B) The number of peritoneal neutrophils, assessed by MPO activity in lysis buffer, determined by MPO chlorination assay kit. Values are means ± SEM (n= 10). *P < 0.05; **P < 0.01; ***P < 0.001 versus zymosan A-treated group; ###P < 0.001 versus PBS-treated group (one-way anova followed by the Newman–Keuls-Student's test).
Time course of zymosan A-induced plasma leakage and leucocyte infiltration in mice treated i.p. with 15-(HEPE)-lysoPC
In order to investigate the time-dependent effect of 15-(HEPE)-lysoPC on zymosan A-induced inflammation, the time course of plasma leakage and leucocyte infiltration were investigated. As shown in Figure 6A, plasma leakage sharply reached its maximal level at 2 h, and declined gradually to its control level within 10 h in mice treated with zymosan A alone. Meanwhile, the inclusion of 15-HEPE-lysoPC decreased zymosan A-induced plasma leakage by approximately 50% in the early phase as well as the late phase, but did not attenuate the time to peak of plasma leakage. In a related experiment, the leucocyte infiltration was gradually augmented to its maximal level (12 h), and then declined. Meanwhile, as displayed in Figure 6B, 15-HEPE-lysoPC treatment inhibited leucocyte infiltratrion by approximately 50% in the early phase and by 40% in the late phase. However, it had a negligible effect on the time to peak of leucocyte infiltration. These results indicate that 15-HEPE-lysoPC exerts a suppressive action during the inflammatory phase as well as resolution phase of the response, consistent with previous findings obtained after aspirin-triggered lipoxin administration (Schwab et al., 2007).
Figure 6.
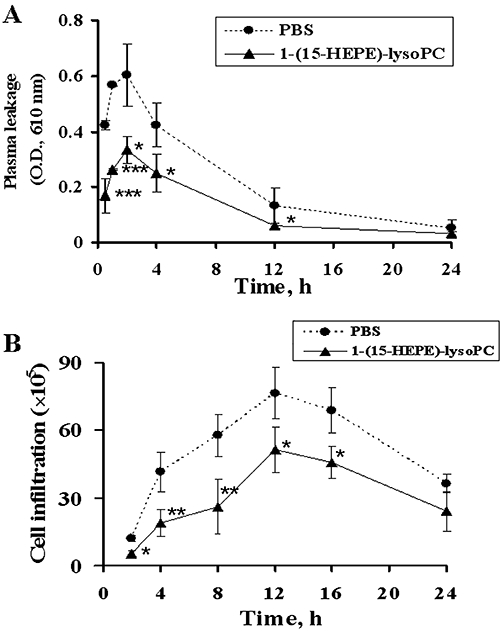
Time-dependent effect of 15-HEPE-lysoPC on zymosan A-induced plasma leakage and leucocyte infiltration. (A) Mice was administered either 15-HEPE-lysoPC (50 µg·kg−1) or phosphate-buffered saline (PBS) 30 min prior to i.v. administration of Evans blue dye, followed by zymosan A treatment. At indicated time points (0.5–24 h) after zymosan A challenge, lavage was collected and plasma leakage was determined as described in Figure 2. (B) Mice were administered i.p. either 15-HEPE-lysoPC (50 µg·kg−1) or PBS 30 min prior to zymosan A treatment. At different time points after zymosan A challenge, lavage was collected and leucocyte infiltration was assessed by using light microscopy and a Neubauer chamber. Values are means ± SEM (n= 5). *P < 0.05, **P < 0.01, ***P < 0.01 versus PBS treated group (one-way anova followed by the Newman–Keuls-Student's test).
Suppressive effect of i.p. 1-(15-HEPE)-lysoPC on the formation of pro-inflammatory lipid mediators in the peritoneum
The effect of 1-(15-HEPE)-lysoPC on the formation of pro-inflammatory lipid mediators, responsible for increase of vascular permeability, was examined. Firstly, the effect of 1-(15-HEPE)-lysoPC on the level of peritoneal LTC4 or PGE2, largely responsible for the increase in vascular permeability in zymosan A-induced peritonitis (Kolaczkowska et al., 2008), was investigated. Figure 7A shows that 1-(15-HEPE)-lysoPC markedly inhibited zymosan A-induced formation of LTC4 in peritoneum in a dose-dependent manner while 15-HEPE had only a small effect, indicating 1-(15-HEPE)-lysoPC is much more potent than 15-HEPE in reducing the level of LTC4. Noticeably, there seemed to be a parallel relationship between the suppression of LTC4 formation and the inhibition of plasma leakage. However, the suppressive effect of 1-(15-HEPE)-lysoPC on PGE2 formation was not significant (Figure 7B). In fact, administration of 15-HEPE seemed to enhance the level of PGE2, although no significant difference between the 15-HEPE-treated group (15–150 µg·kg−1) and the zymosan A alone-treated group was found.
Figure 7.
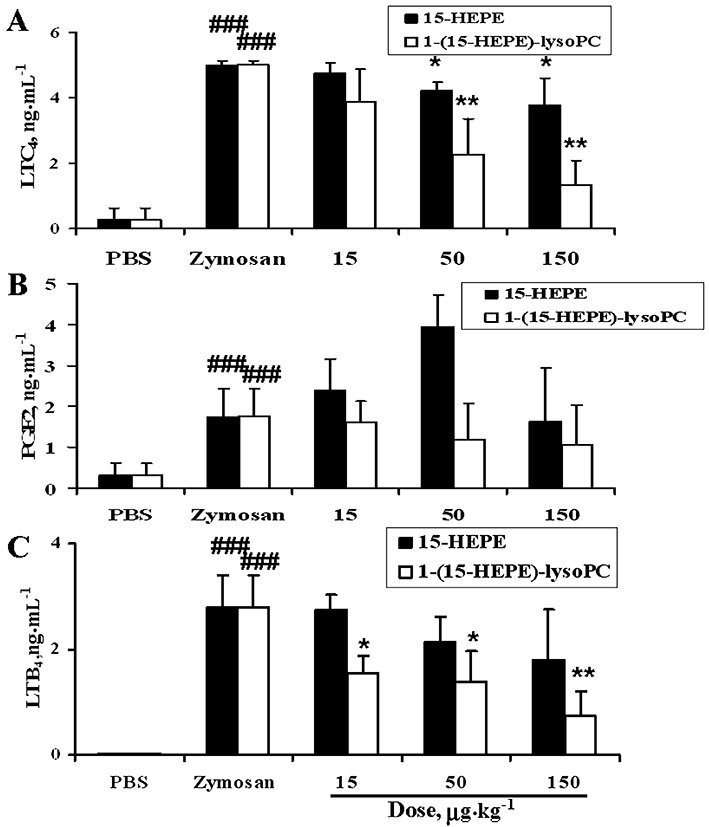
Effect of i.p. 15-HEPE or 1-(15-HEPE)-lysoPC on zymosan A-induced formation of pro-inflammatory lipid mediators in mice. 15-HEPE or 1-(15-HEPE)-lysoPC, dissolved in phosphate-buffered saline (PBS), was administered i.p. to mice (0–150 µg·kg−1) 30 min prior to i.p. administration of zymosan A. After 60 min, peritoneal lavages were collected, and levels of LTC4 (A), PGE2 (B) and LTB4 (C) were determined as described in Methods. Values are means ± SEM (n= 7). *P < 0.05; **P < 0.01 versus zymosan A-treated group; ###P < 0.001 versus PBS-treated group (one-way anova followed by the Newman–Keuls-Student's test).
In addition, the effect of 1-(15-HEPE)-lysoPC on the formation of LTB4, a chemo-attractant factor responsible for increased leucocyte infiltration, was examined. As shown in Figure 7C, i.p. 1-(15-HEPE)-lysoPC, markedly inhibited zymosan A-induced formation of LTB4 in the peritoneum, whereas 15-HEPE had only a slight effect, consistent with 1-(15-HEPE)-lysoPC being much more potent than 15-HEPE in reducing leucocyte infiltration. From the suppressive effect of 1-(15-HEPE)-lysoPC on both LTB4 and LTC4 formation, it was hypothesized that 1-(15-HEPE)-lysoPC or its metabolite inhibits peritoneal 5-LOX, responsible for the formation of leukotrienes from arachidonic acid (Funk, 2001). To test this possibility, peritoneal cells were collected from ICR mice, and the inhibitory effect of 1-(15-HEPE)-lysoPC or 15-HEPE on the formation of LTB4 and LTC4 in zymosan A-treated peritoneal cells in the presence of 10% serum was examined. As shown in Figure 8, 1-(15-HEPE)-lysoPC at 0.75 to 7.5 µM, close to the concentrations estimated from doses used in animal experiments, effectively reduced LTB4 and LTC4 formation in peritoneal cells, reaffirming that 1-(15-HEPE)-lysoPC or its metabolite inhibits cellular 5-LOX in the peritoneum. In contrast, 15-HEPE, up to 7.5 µM, had no inhibitory effect on zymosan A-induced formation of leukotrienes under our conditions. Hence, it is likely that 1-(15-HEPE)-lysoPC is much more efficient than 15-HEPE at inhibiting 5-LOX activity in peritoneal cells stimulated with zymosan A in the presence of 10% serum (Figure 8A).
Figure 8.
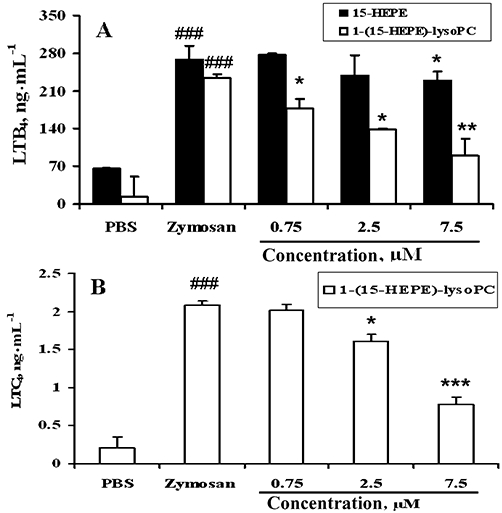
Effect of 15-HEPE and 1-(15-HEPE)-lysoPC on zymosan A-induced LTB4 (A) and LTC4 (B) production in resident peritoneal cells. Peritoneal cells (5 × 104 cells·mL−1), harvested and pretreated with 1-(15-HEPE)-lysoPC or 15-HEPE for 2 min, followed by addition of zymosan A (100 µg·mL−1) and incubated for 20 h at 37°C. Levels of LTB4 and LTC4 in the cell-free cultured supernatant were measured as described in Methods. Values are means ± SD of triplicate determinations. *P < 0.05; **P < 0.01; ***P < 0.001 versus zymosan A-treated group. ###P < 0.001 versus PBS-treated group (one-way anova followed by the Newman–Keuls-Student's test).
Separately, to clarify the structural requirements of 15-HETE derivatives for the inhibition of 5-LOX, we examined the inhibitory effect of 1-(15-HEPE)-lysoPC and 15-HETE on 5-LOX activity from potato. This potato 5-LOX activity was potently inhibited by 15-HEPE (IC50, 12 µM), but 1-(15-HEPE)-lysoPC had no significant effect up to 40 µM (data not shown). This result supports our hypothesis that the suppressive effect of 1-(15-HEPE)-lysoPC on the level of leukotrienes in peritoneal cells is due to the direct inhibition of 5-LOX by 15-HEPE, its hydrolysis product. In this respect, it is highly likely that 1-(15-HEPE)-lysoPC corresponds to a precursor for 15-HETE in vivo and ex vivo.
Suppressive effect of i.p. 1-(15-HEPE)-lysoPC on the formation of pro-inflammatory cytokines
In a separate experiment, the effect of 1-(15-HEPE)-lysoPC on the level of cytokines such as TNF-α, IL-1β, IL-2, IL-6 or IFN-γ was examined in peritoneum of mice treated with zymosan A. As demonstrated in Figure 9, 1-(15-HEPE)-lysoPC markedly inhibited zymosan A-induced formation of cytokines such as TNF-α, IL-2, IL-6 or IFN-γ, in contrast to 15-HEPE that had a negligible effect. Furthermore, this inhibitory effect was exhibited by 1-(15-HEPE)-lysoPC at low doses ranging from 15 to 150 µg·kg−1, providing further support for the anti-inflammatory actions of 1-(15-HEPE)-lysoPC. To elucidate the mechanism of this decrease in pro-inflammatory cytokines levels induced by 1-(15-HEPE)-lysoPC, the level of peritoneal 12-HETE, which has been reported to increase the level of pro-inflammatory cytokines such as TNF-α or IL-6 in cells treated with endotoxin (Wen et al., 2007; Chakrabarti et al., 2009; Moreno, 2009b), was determined. As shown in Figure 10, 1-(15-HEPE)-lysoPC dose-dependently inhibited zymosan A-induced 12-HETE formation in peritoneum in accordance with its suppressive effect on the level of pro-inflammatory cytokines. Thus, part of the anti-inflammatory effect of 1-(15-HEPE)-lysoPC may be ascribed to the inhibition of 12-LOX such as leucocyte 12/15-LOX (Wen et al., 2007). For the effective inhibition of 12-LOX, 15-HEPE may be more important than 1-(15-HEPE)-lysoPC, as 15-HEPE has been reported to potently inhibit 12/15-LOX (Tsunomori et al., 1996).
Figure 9.

Effect of 1-(15-HEPE)-lysoPC and 15-HEPE on zymosan A-induced formation of pro-inflammatory cytokines in mice. 15-HEPE or 1-(15-HEPE)-lysoPC, dissolved in phosphate-buffered saline (PBS), was administered i.p. to mice (0–150 µg·kg−1) 30 min prior to i.p. administration of zymosan A. After 60 min, peritoneal lavage was collected, and levels of TNF-α (A), IL-6 (B), IL-2(C) and IFN-γ (D) were determined. Values are means ± SEM (n= 7). *P < 0.05; **P < 0.01; ***P < 0.001 versus zymosan A-treated group; ###P < 0.001 versus PBS-treated group (one-way anova followed by the Newman–Keuls-Student's test).
Figure 10.

Effect of i.p. 1-(15-HEPE)-lysoPC on zymosan A-induced formation of 12-HETE in the peritoneum. 1-(15-HEPE)-lysoPC was administered i.p. to mice (0–150 µg·kg−1) 30 min prior to i.p. administration of zymosan A. After 60 min, peritoneal lavage was collected, and the level of 12-HETE was determined by EIA assay kit. Values are means ± SEM (n= 7). *P < 0.05; **P < 0.01 versus zymosan A-treated group; ###P < 0.001 versus PBS-treated group (one-way anova followed by the Newman–Keuls-Student's test).
Enzymatic release of acid from 1-acyl -lysoPC in vivo and ex vivo
Overall, it was observed that 1-(15-HEPE)-lysoPC was more potent than 15-HEPE at expressing anti-inflammatory actions in in vivo and ex vivo systems. Such a difference of activity between two lipids may be due to a different effectiveness in reaching target cells. It is quite possible that 1-(15-HEPE)-lysoPC readily passes through the cellular membrane, and then is hydrolysed by cellular hydrolytic activity to release free 15-HEPE. In this respect, it is possible that peritoneal cells contain a lipase that hydrolyses 1-acyl-lysoPC. To test this, 1-(15-HETE)-lysoPC, another lysoPC derivative, was administered i.p. into peritoneum of mice, and the formation of 15-HETE from 1-(15-HETE)-lysoPC in peritoneum was determined by using a 15-HETE EIA kit, commercially available. As shown in Figure 11A, the formation of 15-HETE in peritoneum was elevated in accordance with increasing dose of 1-(15-HETE)-lysoPC, showing that 1-(15-HETE)-lysoPC was hydrolysed by a lipase in the peritoneum. Furthermore, in a separate experiment, where 1-(15-HETE)-lysoPC was incubated with peritoneal cells collected from peritoneum of mice treated with zymosan A (Figure 11B), 15-HETE was found to be released from 1-(15-HETE)-lysoPC time-dependently, reaffirming the presence of lipase activity in peritoneal cells. These data support the notion that 1-(15-HETE)-lysoPC and 1-(15-HEPE)-lysoPC are hydrolysed by cellular lipase to generate 15-HETE and 15-HEPE respectively, which directly participate in the anti-inflammatory effect.
Figure 11.
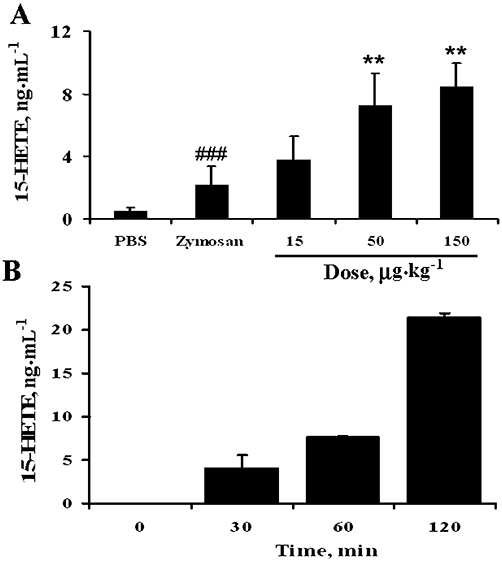
Conversion of 1-(15-HETE)-lysoPC to 15-HETE in vivo and ex vivo. (A) Effect of i.p. 1-(15-HETE)-lysoPC on zymosan A-induced formation of 15-HETE in the peritoneum. 1-(15-HETE)-lysoPC was administered i.p. to mice (0–150 µg·kg−1) 30 min prior to i.p. administration of zymosan A. After 60 min, peritoneal lavages were collected, and the levels of 15-HETE were determined by EIA assay kit. Values are means ± SEM (n= 7). *P < 0.05; **P < 0.01 versus zymosan A-treated group; ###P < 0.001 versus PBS-treated group (one-way anova followed by the Newman–Keuls-Student's test). (B) Time-dependent 15-HETE formation in peritoneal cells treated with 1-(15-HETE)-lysoPC. Peritoneal cells (5 × 104 cells·mL−1) were incubated with 1-(15-HETE)-lysoPC (7.5 µM) and zymosan A (100 µg·mL−1) at 37°C in a 5% CO2 humid air. Then, at different time intervals (30, 60 or 120 min), the levels of 15-HETE in the cell-free cultured supernatants were measured by EIA assay kit. Data are expressed as means ± SD values of triplicate determinations.
Effect of 1-(15-HEPE)-lysoPC on inflammation induced by LTB4 or LTC4
Previously, it was found that 15-HEPE not only inhibits LTB4 formation but also suppresses LTB4-induced chemotaxis (Ternowitz et al., 1988). Separately, the inflammatory action of LTB4 and LTD4 had been reported to be suppressed by LXA4 (Pouliot et al., 2000) and resolvin D1 (Spite et al., 2009) respectively. Accordingly, it is possible that the anti-inflammatory action of 1-(15-HEPE)-lysoPC could be partially related to the suppression of inflammation induced by LTB4 and/or LTD4. To prove this, the effect of 1-(15-HEPE)-lysoPC on either LTC4-induced increase in vascular permeability or LTB4-induced leucocyte infiltration was examined. As demonstrated in Figure 12A, administration of 1-(15-HEPE)-lysoPC at 15, 50 and 150 µg·kg−1 suppressed LTC4-induced increase in vascular permeability by 18 %, 33% and 55% respectively. Thus, 1-(15-HEPE)-lysoPC exhibited a limited but significant suppression of vascular permeability induced by LTC4. Similarly, LTB4-induced leucocyte infiltration was also suppressed by injection of 1-(15-HEPE)-lysoPC, although a complete suppression was not achieved (Figure 12B). Taken together, these data suggest that some part of the anti-inflammatory action of 1-(15-HEPE)-lysoPC may involve mechanisms other than the inhibition of 5-LOX activity. A possible mechanism for the above may be derived from the notion that 1-(15-HEPE)-lysoPC may be hydrolysed by a lipase to form 15-HEPE, which can be further converted to anti-inflammatory lipoxin A5, formerly called lipoxene (Pettitt et al., 1991), according to the pathway for the formation of LXA4 from 15-HETE via 5-hydroperoxy, 15-HETE (Pouliot et al., 2000). In an attempt to substantiate this notion, the conversion of 15-HETE-lysoPC to LXA4 in peritoneum of mice challenged with zymosan A was assessed by employing LXA4 EIA kit system, commercially available. Figure 13 demonstrates that the formation of LXA4 increased correspondingly with the dose of i.p.15-HETE-lysoPC, from 15 to 150 µg·kg−1. It is noteworthy that there appeared to be a good relationship between LXA4 formation and 15-HETE formation (Figure 11A) in peritoneum of mice administered 15-HETE-lysoPC, suggesting that the peritoneum contains enzyme systems necessary for the formation of a lipoxin type metabolite from 15-HETE-lysoPC. In a further experiment to examine whether lipoxin A5 is implicated in the anti-inflammatory action of 15-HEPE-lysoPC, 15-HEPE-lysoPC was concomitantly administered with Boc2, a lipoxin receptor antagonist (von der Weid et al., 2004). As shown in Figure 14, Boc2 (10 µg·kg−1) partially reverted the suppressive effect of 15-HEPE-lysoPC (50 and 150 µg·kg−1) on leucocyte infiltration, indicating that the anti-inflammatory action of 15-HEPE-lysoPC can at least partially be ascribed to lipoxin A5.
Figure 12.

Effect of i.p. 1-(15-HEPE)-lysoPC on LTC4-induced plasma leakage and LTB4-induced leucocytes infiltration in mice. (A) 1-(15-HEPE)-lysoPC was administered i.p. to mice (0–150 µg·kg−1) 30 min prior to i.v. administration of Evans blue dye (5%, 100 µL) followed by i.p. administration of LTC4 (10 mg·kg−1). Peritoneal lavages were collected at the 60 min time point and plasma leakage was determined as described in Figure 2. (B) 1-(15-HEPE)-lysoPC (0–150 µg·kg−1) was administered i.p. to mice 30 min prior to i.p administration of LTB4 (10 mg·kg−1). After 120 min, peritoneal lavage samples were collected and total cell counts performed. Values are means ± SEM (n= 10). *P < 0.05; **P < 0.01; ***P < 0.001 versus zymosan A-treated group; ###P < 0.001 versus PBS-treated group (one-way anova followed by the Newman–Keuls-Student's test).
Figure 13.

Effect of i.p. 1-(15-HETE)-lysoPC on zymosan A-induced formation of lipoxin A4 in mice. 1-(15-HETE)-lysoPC (0–150 µg·kg−1) was administered i.p. to mice 30 min prior to i.p. administration of zymosan A (100 mg·kg−1). After 60 min, peritoneal lavages were collected, and the level of lipoxin A4 was determined by EIA assay kit. Values are means ± SEM (n= 7). *P < 0.05; **P < 0.01; ***P < 0.001 versus zymosan A-treated group; ###P < 0.001 versus PBS-treated group (one-way anova followed by the Newman–Keuls-Student's test).
Figure 14.
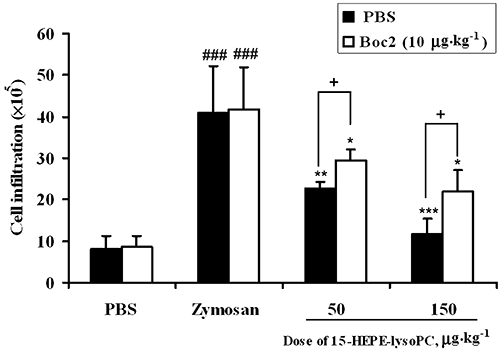
Effect of lipoxin receptor antagonist (Boc2) on 15-HEPE-lysoPC-induced suppression of zymosan A-induced leucocyte infiltration. Mice were treated i.p. with either only 15-HEPE-lysoPC (50 and 150 µg·kg−1) or 15-HEPE-lysoPC in combination with Boc2 of 10 µg·kg−1 30 min prior to i.p. administration of zymosan A. Four hours later, mice were killed by isoflurane inhalation and the peritoneum was lavaged with 3 mL of sterile phosphate-buffered saline (PBS). Total leucocyte infiltration was assessed by light microscopy and tryphan blue staining. Values are means ± SEM (n= 5).*P < 0.05, **P < 0.01, ***P < 0.001 versus zymosan A-treated group; ###P < 0.001 versus PBS-treated group;+P < 0.05 (significantly different among treatment groups) (one-way anova followed by the Newman–Keuls-Student's test).
Discussion
A new family of endogenous anti-inflammatory and pro-resolving lipid mediators derived from omega 3-polyunsaturated fatty acids have been discovered including resolvins, maresin and protectin D (Serhan and Savill, 2005; Bazan, 2009; Serhan et al., 2009). These lipid mediators, actively biosynthesized in the resolution phase of acute inflammation, control the duration and magnitude of inflammation (Serhan, 2005b; Serhan and Savill, 2005). Whereas the anti-inflammatory actions of lipid mediators derived from polyunsaturated fatty acids are well-established (Schwab and Serhan, 2006; Tjonahen et al., 2006; Sun et al., 2007; Hong et al., 2008), the anti-inflammatory effects of lysoPC derivatives are contentious. Recently, i.v and i.p. 1-arachidonoyl-lysoPC and 1-docosahexaenoyl-lysoPC have been shown to have an anti-inflammatory effect on zymosan A-induced peritonitis (Hung et al., 2009; 2010;); they demonstrated that the suppression of plasma leakage and leucocyte infiltration was accompanied by a reduction in the formation of LTC4 and some cytokines. Further, it was consistently observed that 15-lipoxygenation products were more potent than their corresponding polyunsaturated fatty acylated lysoPC in preventing the plasma leakage, suggesting that 15-LOX is involved in the metabolic activation of 1-arachidonoyl-lysoPC or 1-docosahexaenoyl-lysoPC. Likewise, in the present study, the greater potency of 1-(15-HPEPE)-lysoPC compared to 1-eicosapentaenoyl-lysoPC, indicates that oxygenation of 1-eicosapentaenoyl-lysoPC by 15-LOX may correspond to a metabolic activation of 1-eicosapentaenoyl-lysoPC. Thus, 15-lipoxygenation is required for metabolic activation of polyunsaturated fatty acylated lysoPC. Another metabolic pathway for polyunsaturated lysoPC involves a hydrolytic process. Previous studies have demonstrated that arachidonic acid has both pro-inflammatory and anti-inflammatory properties, whereas eicosapentaenoic acid is an anti-inflammatory (Wall et al., 2010). Therefore, the greater anti-inflammatory action of 1-eicosapentaenoyl-lysoPC, compared to 1-arachidonoyl-lysoPC, suggests that the hydrolytic product, rather than the lysoPC itself, might be important for the anti-inflammatory action of lysoPCs. In support of this, leukotriene B5 and leukotriene C5, derived from eicosapentaenoic acid, have been reported to be less pro-inflammatory than leukotrienes B4 and C4, respectively, derived form arachidonic acid (Moreno, 2009a). Additionally, 15-HEPE, produced from 15-lipoxygenation of eicosapentaenoic acid, was equipotent or more potent than 15-HETE, derived from arachidonic acid, in expressing an inhibitory effect on 5-LOX (Miller et al., 1989; Vang and Ziboh, 2005), necessary for the biosynthesis of leukotrienes. Meanwhile, the anti-inflammatory action of lipoxin A5, derived from eicosapentaenoic acid, was found to be similar to that of LXA4 from arachidonic acid (Wong et al., 1985; Lam and Wong, 1988; Stahl et al., 1989). Despite the different efficacy between 1-eicosapentaenoyl-lysoPC and 1-arachidonoyl-lysoPC, a similar anti-inflammatory potency between 1-(15-HPETE)-lysoPC and 1-(15-HPEPE)-lysoPC led to the assumption that the peroxide derivatives of lysoPC may exert their anti-inflammatory effects through the same mechanisms. Also, it is conceivable that 15-HPETE and 15-HPEPE, derived from the hydrolysis of 1-(15-HPETE)-lysoPC and 1-(15-HPEPE)-lysoPC, respectively, may go through the same metabolic pathway. In our present study, nonetheless, the anti-inflammatory effect of 1-(15-HEPE)-lysoPC was somewhat greater than that of 1-(15-HPEPE)-lysoPC. This led us to assume that the peroxide moiety is not important for maximal anti-inflammatory action. Moreover, in the in vivo system, 1-(15-HPEPE)-lysoPC is supposed to be readily reduced to a more stable hydroxyl form, 1-(15-HEPE)-lysoPC, by glutathione peroxidase (Huang et al., 2009). In this respect, 1-(15-HEPE)-lysoPC is highly likely to be the robust metabolite accountable for the anti-inflammatory effect of 1-eicosapentaenoyl-lysoPC in vivo. Further, 1-(15-HEPE)-lysoPC may be hydrolysed by cellular lipase to produce 15-HEPE, an inhibitor of 5-LOX and 12-LOX (Miller et al., 1989; Tsunomori et al., 1996), as well as a precursor for the formation of anti-inflammatory lipids such as lipoxin A5 (Lam and Wong, 1988).
However, i.p. 15-HEPE failed to inhibit zymosan A-induced plasma leakage, despite previous findings that 15-HEPE itself inhibited 5-LOX activity potently in vitro (Miller et al., 1989). This result might be because i.p. 15-HEPE binds to serum proteins before reaching its target cells (Thumser et al., 1994) or is easily incorporated into cellular lipids during cell activation (Engelmann et al., 1999). In this regard, it was suggested that lysoPC form might be an efficient precursor for the delivery of the 15-hydroxyeicosapentaenoyl group to target cells as well as tissues in vivo. It is of note that 1-(15-HEPE)-lysoPC was found to have an anti-inflammatory effect at relatively low doses (15 or 50 µg·kg−1), estimated to be equivalent to a few µM in the peritoneal cavity. If it is supposed that 1-(15-HEPE)-lysoPC is entrapped selectively into peritoneal cells, it is conceivable that the cellular level of 1-(15-HEPE)-lysoPC may be augmented several-fold, accumulating to a level sufficient to influence target enzymes.
Previously, it was reported that in zymosan A-induced peritonitis, early vascular permeability, followed by subsequent infiltration of neutrophils into peritoneum, depended largely on cysteinyl-leukotrienes released by resident peritoneal macrophages, and to a lesser extent, on histamine from mast cells and PGE2 of multiple cellular origins (Leite et al., 2007; Kolaczkowska et al., 2008). Consistent with the above, 1-(15-HEPE)-lysoPC reduced the formation of LTC4 in peritoneum exudate from mice treated with zymosan A. Previously, the infiltration of neutrophils into peritoneum had been reported to be ascribed mainly to LTB4 (Griswold et al., 1991). Along this line, the infiltration of leucocytes into peritoneum was also reduced in mice treated with 1-(15-HEPE)-lysoPC, further confirming the anti-inflammatory action of 1-(15-HEPE)-lysoPC. Taken together, these results strongly suggest that one mechanism for the anti-inflammatory action of 1-(15-HEPE)-lysoPC may be ascribed to its suppressive effect on the formation of pro-inflammatory lipid mediators, LTB4 or LTC4. As LTC4 and LTB4 are known to be generated form 5-LOX-catalysed oxygenation of arachidonic acid, it was supposed that the suppressive effect of 1-(15-HEPE)-lysoPC on LTC4 and LTB4 formation in peritoneum could be largely due to the inhibition of 5-LOX by 1-(15-HEPE)-lysoPC or 15-HEPE, its hydrolysis product. Although 1-(15-HEPE)-lysoPC suppressed LTB4 formation in peritoneal cells collected from peritoneum of mice challenged with zymosan A (IC50, ∼3.0 µM), it had no effect on LTB4 formation in mice not treated with zymosan A (Hung & Sok, unpubl. data). Moreover, 1-(15-HEPE)-lysoPC failed to significantly inhibit potato 5-LOX activity up to 40 µM. Meanwhile, 15-HEPE, which had previously been reported to potently inhibit 5-LOX activity in RBL-1 cells with an IC50 value of 10–20 µM (Miller et al., 1989), potently inhibited potato 5-LOX activity (IC50, 12 µM). From these findings, it is clear that the inhibition of 5-LOX activity by 15-HEPE, rather than 1-(15-HEPE)-lysoPC, may be implicated in the anti-inflammatory action of 1-(15-HEPE)-lysoPC. Further support for this hypothesis comes from the finding that 15-HEPE can be utilized as a substrate for 5-LOX, but not 1-(15-HEPE)-lysoPC (Hung & Sok, unpubl. data). Separately, it was supposed that 15-HEPE, a hydrolysis product of 15-HEPE-lysoPC, may inhibit the peritoneal 12-LOX activity responsible for the formation of 12-HETE, as suggested from the marked inhibition of leucocyte 12/15-LOX by 15-HETE (Tsunomori et al., 1996). Consistent with this, the results from the present study indicate that administration of 1-(15-HEPE)-lysoPC into the peritoneum partially reduced the total amount of 12-HETE in the peritoneum. In turn, the reduction in the level of peritoneal 12-HETE may lead to a decrease in the level of cytokines in the peritoneum, as suggested from a previous report that showed 12-HETE increased the cytokines level in cells treated with endotoxin (Wen et al., 2007; Chakrabarti et al., 2009; Moreno, 2009b). Furthermore, the pattern of 12-HETE reduction seems to correspond to that of cytokine reduction, suggesting that reduced formation of cytokines such as TNF-α and IL-6 may be at least partly be related to the decrease in 12-HETE. Additionally, it is possible that the decrease of 5-LOX activity may contribute to the reduction in the levels of IL-2 or TNF-α (Bertolini et al., 2001). Based on these findings, the suppressive effect of 1-(15-HEPE)-lysoPC on the level of cytokines was at least partially ascribed to the inhibition of 5-LOX and/or 12-LOX activity by 15-HEPE, a hydrolysis product of 1-(15-HETE)-lysoPC. Evidence for existence of lipase activity in peritoneum is well provided in our present studies; the administration of 1-(15-HETE)-lysoPC into the peritoneum resulted in an increase of 15-HETE in the peritoneal cavity. Moreover, the incubation of 1-(15-HETE)-lysoPC with peritoneal cells in the presence of zymosan A resulted in a time-dependent release of 15-HETE.
Nonetheless, the possibility that 15-HEPE-lysoPC and/or its metabolite may alter the availability of free arachidonic acid or the level of calcium ion, or the transfer of free arachidonic acid to 5-LOX activating protein cannot be excluded (Radmark and Samuelsson, 2010). Also, it is conceivable that the lipids may interfere with the activation of 5-LOX by mitogen-activated protein kinases (Radmark and Samuelsson, 2010) or extracellular signal-regulated kinase 1/2 (Burkert et al., 2003). Thus, the possible effect 15-HEPE-lysoPC or 15-HEPE on 5-LOX could affect the formation of pro-inflammatory mediators such as LTB4, LTC4 and 12-HETE in vivo.
Another possible mechanism for the anti-inflammatory action of 15-HEPE-lysoPC could be its conversion to an anti-inflammatory lipoxin-type derivative in vivo; 15-HEPE-lysoPC may be hydrolysed by lipases, and then oxygenated by 5-LOX to form 5-hydroperoxy, 15-HEPE, a precursor for the formation of lipoxin A5 (Wong et al., 1985; Lam and Wong, 1988; Ho and Wong, 1990), which is known to possess the same biological activity as LXA4 (Maderna and Godson, 2009). In this study, the formation of lipoxin A5 after administration of 1-(15-HEPE)-lysoPC in vivo was not examined, as a lipoxin A5 EIA kit was not available. However, it is quite possible that lipoxin A5 is generated from 1-(15-HEPE)-lysoPC in the peritoneum, which contains a lipoxin-generating enzyme system, as the formation of lipoxin A5 from 15-HEPE follows the same pathway as the production of LXA4 from 15-HETE via 5-hydroperoxy, 15-HETE. Consistent with the above, in the present study, LXA4 was produced dose-dependently in the peritoneum following the administration of 1-(15-HETE)-lysoPC, adding to the advantages of 1-(15-HETE)-lysoPC as an anti-inflammatory lipid. From this, it is supposed that the anti-inflammatory action of 1-(15-HEPE)-lysoPC in vivo may be explained partly by the metabolic conversion of 1-(15-HEPE)-lysoPC to lipoxin A5. Consistent with this hypothesis, Boc2, a lipoxin receptor antagonist, partially reverted the suppressive effect of 15-HEPE-lysoPC on leucocyte infiltration, indicating that the anti-inflammatory action of 15-HEPE-lysoPC might be partially ascribed to lipoxin A5 formation. However, the contributory role of lipoxin A5 is probably less than expected, as the suppressive effect of 15-HEPE-lysoPC was relatively small after 12 h; this presumably corresponds to the resolution phase. Nonetheless, the time course for the suppressive effect of 15-HEPE-lysoPC was comparable to that of aspirin-triggered lipoxin (Schwab et al., 2007). Therefore, the anti-inflammatory effect of 15-HEPE-lysoPC may contain anti-inflammatory as well as pro-resolving actions. This might partly explain why 1-(15-HEPE)-lysoPC injection prevented LTB4- or LTC4-induced inflammation in the present study, consistent with previous reports that the inflammatory action of LTB4 and LTD4 is suppressed by LXA4 (Pouliot et al., 2000) or resolvin D1 (Spite et al., 2009).
An additional plausible mechanism is that during the inflammation process, some part of 1-(15-HEPE)-lysoPC is re-acylated by acyl transferase to form phosphatidylcholine containing 15-hydroxyeicosapentaenoyl group at C-2 (Jackson et al., 2008; Perez-Chacon et al., 2009); this process may also lead to a reduction in the cytokine levels as 1-stearoyl, 2-(15-hydroxyeicosapentaenoyl)-PC has been shown to have a negative effect on cytokine levels in LPS-stimulated cells (Morgan et al., 2009). Together, these findings indicate that the anti-inflammatory effects of 1-(15-HEPE)-lysoPC could result from effects of 1-(15-HEPE)-lysoPC as well as its metabolites such as 15-HEPE and lipoxin A5. Thus, the anti-inflammatory action of 1-(15-HPEPE)-lysoPC could be exerted through multiple mechanisms.
Previously it was shown that 1-arachidonoyl-lysoPC and 15-HPETE-lysoPC are more efficient than arachidonic acid and 15-HPETE, respectively, at expressing anti-inflammatory effects (Hung et al., 2009; 2010;). The present data corroborate the greater efficacy of 1-(15-HEPE)-lysoPC compared to 15-HEPE. Thus, it is proposed that lysoPC form may be more efficient than its acid form in preventing the inflammatory process in vivo. There may be at least two advantages of lysoPC type lipids as acyl carriers: (i) 1-(15-HEPE)-lysoPC is an efficient carrier of the acyl group for cellular uptake, whereas 15-HEPE is readily bound to proteins or directly incorporated into the lipid membrane during cell activation; and (ii) 1-(15-HEPE)-lysoPC is resistant to metabolism other than by hydrolysis, whereas 15-HEPE is easily subjected to 5- or 12-lipoxygenation (Ho and Wong, 1990), oxidation by 15-HETE dehydrogenase (Sok et al., 1988) or re-acylation to phospholipids (Ho and Wong, 1990; Jackson et al., 2008). On the other hand, it is also possible that the anti-inflammatory action of 1-eicosapentaenoyl-lysoPC is derived from the effect of resolvin E or maresin E produced from eicosapentaenoic acid (Tjonahen et al., 2006; Hong et al., 2008). However, the greater efficacy of 1-(15-HEPE)-lysoPC, compared to 1-eicosapentaenoyl-lysoPC, supports the notion that the formation of lipoxin A5 via a 5-HEPE intermediate is more important for the anti-inflammatory action of 1-eicosapentaenoyl-lysoPC than the formation of resolvin E or maresin E.
Earlier studies indicated that lysoPC expressed cytotoxic activity through a detergent-like function (Colles and Chisolm, 2000). However, the finding that 1-(15-HEPE)-lysoPC is bioactive at relatively low concentrations, much below the critical micellar concentrations of lysoPCs, excludes this possibility (Haberland and Reynolds, 1975). Recently, lysoPCs, with saturated or monounsaturated acyl group, known to be ligands for CD1d site, were reported to increase cytokine secretion from T cells or monocytes (Fox et al., 2009), in contrast to omega-3 polyunsaturated-lysoPC, which reduced cytokine levels in spleen tissue of mice treated with lipopolysaccharide (Kim et al., unpubl. data). Moreover, this pro-inflammatory effect was expressed at concentrations higher than 10 µM, in contrast to omega-3 polyunsaturated lysoPC, which exhibited anti-inflammatory activity at micromolar concentrations. Although lysoPC can be taken in from extraneous sources such as chow diet, most of the extraneous lysoPC is readily hydrolysed by lipase before absorption into intestines or bound to blood protein such as albumin. Whereas the effect of lysoPC on CD1d site was expressed by lysoPC itself, the anti-inflammatory action of 15-HEPE-lysoPC was expressed by lipase hydrolysis. Moreover, the anti-inflammatory effect of 15-HEPE-lysoPC was studied in the zymosan A-induced acute inflammation model, where lymphocytes do not have a significant impact (Kolaczkowska et al., 2008).
In our previous study (Hung et al., 2010), oral administration of 2-docosahexaehoyl-lysoPC effectively prevented zymosan A-induced peritonitis, and lysoPC has also been shown to be effective at preventing inflammation in other models (Kim et al., unpubl. data). Hence, 15-HEPE-lysoPC might be one of many potent anti-inflammatory lipids in vivo, and our results suggest that it probably induces its anti-inflammatory effects by inhibiting the formation of pro-inflammatory leukotrienes and cytokines, and by enhancing the formation of lipoxin A.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF 2009-0069242).
Glossary
Abbreviations
- 1-(15-HEPE)-lysoPC
1-[(15(S)-hydroxyeicosapentaenoyl]-lysophosphatidylcholine
- 1-(15-HPEPE)-lysoPC
1-[15(S)-hydroperoxypentaenoyl] lysophosphatidylcholine
- 1-(15-HPETE)-lysoPC
1-[15(S)-hydroperoxyeicosatetraenoyl] lysophosphatidylcholine
- 1-(17-HPDHE)-lysoPC
1-[17(S)-hydroperoxydocosahexaenoyl] lysophosphatidylcholine
- 15-HEPE
15-hydroxyeicosapentaenoic acid
- 15-HETE
15-hydroxyeicosatetraenoic acid
- 1-arachidonoyl-lysoPC
1-arachidonoyl-lysophosphatidylcholine
- 1-docosahexaenoyl-lysoPC
1-docosahexaenoyl-lysophosphatidylcholine
- 1-eicosapentaenoyl-lysoPC
1-eicosapentaenoyl-lysophosphatidylcholine
- 1-linoleoyl-lysoPC
1-linoleoyl-lysophosphatidylcholine
- IFN-γ
interferon gamma
- IL-1β
interleukin 1-beta
- IL-6
interleukin-6
- LOX
lipoxygenase
- LTB4
leukotriene B4
- LTC4
leukotriene C4
- MPO
myeloperoxidase
- PGE2
prostaglandin E2
- PLA2
phospholipase A2
- TNF-α
tumor necrosis factor alpha
Conflict of interest
There is no conflict of interest to declare.
Supporting Information
Supporting Information: Teaching Materials; Figs 1–14 as PowerPoint slide.
References
- Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005a;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005b;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg G, Moussignac RL, Gronert K, Devchand PR, Schmidt BA, Guilford WJ, et al. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br J Pharmacol. 2004;143:43–52. doi: 10.1038/sj.bjp.0705912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer's disease. J Lipid Res. 2009;50:400–405. doi: 10.1194/jlr.R800068-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini A, Ottani A, Sandrini M. Dual acting anti-inflammatory drugs: a reappraisal. Pharmacol Res. 2001;44:437–450. doi: 10.1006/phrs.2001.0872. [DOI] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Burkert E, Szellas D, Radmark O, Steinhilber D, Werz O. Cell type-dependent activation of 5-lipoxygenase by arachidonic acid. J Leukoc Biol. 2003;73:191–200. doi: 10.1189/jlb.0702354. [DOI] [PubMed] [Google Scholar]
- Byrum RS, Goulet JL, Snouwaert JN, Griffiths RJ, Koller BH. Determination of the contribution of cysteinyl leukotrienes and leukotriene B4 in acute inflammatory responses using 5-lipoxygenase- and leukotriene A4 hydrolase-deficient mice. J Immunol. 1999;163:6810–6819. [PubMed] [Google Scholar]
- Chakrabarti SK, Cole BK, Wen YS, Keller SR, Nadler JL. 12/15-lipoxygenase products induce inflammation and impair insulin signaling in 3T3-L1 adipocytes. Obesity. 2009;17:1657–1663. doi: 10.1038/oby.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids. 2005;73:163–177. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Colles SM, Chisolm GM. Lysophosphatidylcholine-induced cellular injury in cultured fibroblasts involves oxidative events. J Lipid Res. 2000;41:1188–1198. [PubMed] [Google Scholar]
- Dimitrova P, Ivanovska N. Tyrphostin AG-490 inhibited the acute phase of zymosan-induced inflammation. Int Immunopharmacol. 2008;8:1567–1577. doi: 10.1016/j.intimp.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Doherty NS, Poubelle P, Borgeat P, Beaver TH, Westrich GL, Schrader NL. Intraperitoneal injection of zymosan in mice induces pain, inflammation and the synthesis of peptidoleukotrienes and prostaglandin E2. Prostaglandins. 1985;30:769–789. doi: 10.1016/0090-6980(85)90006-1. [DOI] [PubMed] [Google Scholar]
- Engelmann B, Zieseniss S, Brand K, Page S, Lentschat A, Ulmer AJ, et al. Tissue factor expression of human monocytes is suppressed by lysophosphatidylcholine. Arterioscler Thromb Vasc Biol. 1999;19:47–53. doi: 10.1161/01.atv.19.1.47. [DOI] [PubMed] [Google Scholar]
- Fox LM, Cox DG, Lockridge JL, Wang XH, Chen XX, Scharf L, et al. Recognition of Lyso-Phospholipids by Human Natural Killer T Lymphocytes. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000228. e1000228. DOI: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes L, Hernandez M, Fernandez-Aviles FJ, Crespo MS, Nieto ML. Cooperation between secretory phospholipase A2 and TNF-receptor superfamily signaling: implications for the inflammatory response in atherogenesis. Circ Res. 2002;91:681–688. doi: 10.1161/01.res.0000038341.34243.64. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Periz A, Planaguma A, Gronert K, Miquel R, Lopez-Parra M, Titos E, et al. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20:2537–2539. doi: 10.1096/fj.06-6250fje. [DOI] [PubMed] [Google Scholar]
- Griswold DE, Webb EF, Hillegass LM. Induction of plasma exudation and inflammatory cell infiltration by leukotriene C4 and leukotriene B4 in mouse peritonitis. Inflammation. 1991;15:251–258. doi: 10.1007/BF00917310. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- Haberland ME, Reynolds JA. Interaction of L-alpha-palmitoyl lysophosphatidylcholine with the AI polypeptide of high density lipoprotein. J Biol Chem. 1975;250:6636–6639. [PubMed] [Google Scholar]
- Ho HY, Wong PYK. Transformation of 15-hydroperoxide of eicosapentaenoic acid to lipoxins and trihydroxyeicosatetraenoic acids by 5-lipoxygenase partially purified from potato-tubers. Eicosanoids. 1990;3:99–104. [PubMed] [Google Scholar]
- Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- Huang LS, Kim MR, Sok DE. Linoleoyl lysophosphatidylcholine is an efficient substrate for soybean lipoxygenase-1. Arch Biochem Biophys. 2006;455:119–126. doi: 10.1016/j.abb.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Huang LS, Kim MR, Sok DE. Oxygenation of 1-docosahexaenoyl lysophosphatidylcholine by lipoxygenases; conjugated hydroperoxydiene and dihydroxytriene derivatives. Lipids. 2007;42:981–990. doi: 10.1007/s11745-007-3112-y. [DOI] [PubMed] [Google Scholar]
- Huang LS, Kang JS, Kim MR, Sok DE. Oxygenation of arachidonoyl lysophospholipids by lipoxygenases from soybean, porcine leukocyte, or rabbit reticulocyte. J Agric Food Chem. 2008a;56:1224–1232. doi: 10.1021/jf073016i. [DOI] [PubMed] [Google Scholar]
- Huang LS, Kim MR, Sok DE. Regulation of lipoxygenase activity by polyunsaturated lysophosphatidylcholines or their oxygenation derivatives. J Agric Food Chem. 2008b;56:7808–7814. doi: 10.1021/jf801082x. [DOI] [PubMed] [Google Scholar]
- Huang LS, Kim MR, Sok DE. Enzymatic reduction of polyunsaturated lysophosphatidylcholine hydroperoxides by glutathione peroxidase-1. Eur J Lipid Sci Technol. 2009;111:584–592. [Google Scholar]
- Huang LS, Hung ND, Sok DE, Kim MR. Lysophosphatidylcholine containing docosahexaenoic acid at the sn-1 position is anti-inflammatory. Lipids. 2010;45:225–236. doi: 10.1007/s11745-010-3392-5. [DOI] [PubMed] [Google Scholar]
- Hung ND, Kim MR, Sok DE. Anti-inflammatory action of arachidonoyl lysophosphatidylcholine or 15-hydroperoxy derivative in zymosan A-induced peritonitis. Prostaglandins Other Lipid Mediat. 2009;90:105–111. doi: 10.1016/j.prostaglandins.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Hung ND, Kim MR, Sok DE. Oral administration of 2-docosahexaenoyl lysophosphatidylcholine displayed anti-inflammatory effects on zymosan A-induced peritonitis. Inflammation. 2010 doi: 10.1007/s10753-010-9218-z. DOI: 10.1007/s10753-010-9218-z. [DOI] [PubMed] [Google Scholar]
- Jackson SK, Abate W, Tonks AJ. Lysophospholipid acyltransferases: novel potential regulators of the inflammatory response and target for new drug discovery. Pharmacol Ther. 2008;119:104–114. doi: 10.1016/j.pharmthera.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Jeon SG, Moon HG, Kim YS, Choi JP, Shin TS, Hong SW, et al. 15-lipoxygenase metabolites play an important role in the development of a T-helper type 1 allergic inflammation induced by double-stranded RNA. Clin Exp Allergy. 2009;39:908–917. doi: 10.1111/j.1365-2222.2009.03211.x. [DOI] [PubMed] [Google Scholar]
- Kolaczkowska E, Barteczko M, Plytycz B, Arnold B. Role of lymphocytes in the course of murine zymosan-induced peritonitis. Inflamm Res. 2008;57:272–278. doi: 10.1007/s00011-007-7131-1. [DOI] [PubMed] [Google Scholar]
- Lam BK, Wong PY. Biosynthesis and biological activities of lipoxin A5 and B5 from eicosapentaenoic acid. Adv Exp Med Biol. 1988;229:51–59. doi: 10.1007/978-1-4757-0937-7_5. [DOI] [PubMed] [Google Scholar]
- Lam BK, Hirai A, Yoshida S, Tamura Y, Wong PY. Transformation of 15-hydroperoxyeicosapentaenoic acid to lipoxin A5 and B5, mono- and dihydroxyeicosapentaenoic acids by porcine leukocytes. Biochim Biophys Acta. 1987;917:398–405. doi: 10.1016/0005-2760(87)90118-4. [DOI] [PubMed] [Google Scholar]
- Leite DFP, Echevarria-Lima J, Ferreira SC, Calixto JB, Rumjanek VM. ABCC transporter inhibition reduces zymosan-induced peritonitis. J Leukoc Biol. 2007;82:630–637. doi: 10.1189/jlb.0107042. [DOI] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Maderna P, Godson C. Lipoxins: resolutionary road. Br J Pharmacol. 2009;158:947–959. doi: 10.1111/j.1476-5381.2009.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskrey BH, O'Donnell VB. Analysis of eicosanoids and related lipid mediators using mass spectrometry. Biochem Soc Trans. 2008;36:1055–1059. doi: 10.1042/BST0361055. [DOI] [PubMed] [Google Scholar]
- Matsubara M, Hasegawa K. Benidipine, a dihydropyridine-calcium channel blocker, prevents lysophosphatidylcholine-induced injury and reactive oxygen species production in human aortic endothelial cells. Atherosclerosis. 2005;178:57–66. doi: 10.1016/j.atherosclerosis.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Miller CC, Ziboh VA. Human-epidermis transforms eicosapentaenoic acid to 15-hydroxy 5,8,11,13,17-eicosapentaenoic acid – a potent inhibitor of 5-lipoxygenase. J Am Oil Chem Soc. 1988;65:474–474. [Google Scholar]
- Miller C, Yamaguchi RY, Ziboh VA. Guinea pig epidermis generates putative anti-inflammatory metabolites from fish oil polyunsaturated fatty acids. Lipids. 1989;24:998–1003. doi: 10.1007/BF02544068. [DOI] [PubMed] [Google Scholar]
- Moreno JJ. Differential effects of arachidonic and eicosapentaenoic acid-derived eicosanoids on polymorphonuclear transmigration across endothelial cell cultures. J Pharmacol Exp Ther. 2009a;331:1111–1117. doi: 10.1124/jpet.109.157891. [DOI] [PubMed] [Google Scholar]
- Moreno JJ. New aspects of the role of hydroxyeicosatetraenoic acids in cell growth and cancer development. Biochem Pharmacol. 2009b;77:1–10. doi: 10.1016/j.bcp.2008.07.033. [DOI] [PubMed] [Google Scholar]
- Morgan AH, Dioszeghy V, Maskrey BH, Thomas CP, Clark SR, Mathie SA, et al. Phosphatidylethanolamine-esterified eicosanoids in the mouse: tissue localization and inflammation-dependent formation in Th-2 disease. J Biol Chem. 2009;284:21185–21191. doi: 10.1074/jbc.M109.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralikrishna Adibhatla R, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40:376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council) Guide for the Care and Use of Laboratory Animals of Institute of Laboratory Animal Resources Commission on Life Sciences. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Park CH, Kim MR, Han JM, Jeong TS, Sok DE. Lysophosphatidylcholine exhibits selective cytotoxicity, accompanied by ROS formation, in RAW 264.7 macrophages. Lipids. 2009;44:425–435. doi: 10.1007/s11745-009-3286-6. [DOI] [PubMed] [Google Scholar]
- Perez-Chacon G, Astudillo AM, Balgoma D, Balboa MA, Balsinde J. Control of free arachidonic acid levels by phospholipases A(2) and lysophospholipid acyltransferases. Biochim Biophys Acta Mol Cell Biol Lipids. 2009;1791:1103–1113. doi: 10.1016/j.bbalip.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Pettitt TR, Rowley AF, Barrow SE, Mallet AI, Secombes CJ. Synthesis of lipoxins and other lipoxygenase products by macrophages from the rainbow-trout, oncorhynchus-mykiss. J Biol Chem. 1991;266:8720–8726. [PubMed] [Google Scholar]
- Pouliot M, Clish CB, Petasis NA, Van Dyke TE, Serhan CN. Lipoxin A(4) analogues inhibit leukocyte recruitment to Porphyromonas gingivalis: a role for cyclooxygenase-2 and lipoxins in periodontal disease. Biochemistry. 2000;39:4761–4768. doi: 10.1021/bi992551b. [DOI] [PubMed] [Google Scholar]
- Radmark O, Samuelsson B. Regulation of the activity of 5-lipoxygenase, a key enzyme in leukotriene biosynthesis. Biochem Biophys Res Commun. 2010;396:105–110. doi: 10.1016/j.bbrc.2010.02.173. [DOI] [PubMed] [Google Scholar]
- Rao NL, Dunford PJ, Xue X, Jiang X, Lundeen KA, Coles F, et al. Anti-inflammatory activity of a potent, selective leukotriene A4 hydrolase inhibitor in comparison with the 5-lipoxygenase inhibitor zileuton. J Pharmacol Exp Ther. 2007;321:1154–1160. doi: 10.1124/jpet.106.115436. [DOI] [PubMed] [Google Scholar]
- Rao TS, Currie JL, Shaffer AF, Isakson PC. In-vivo characterization of zymosan-induced mouse peritoneal inflammation. J Pharmacol Exp Ther. 1994;269:917–925. [PubMed] [Google Scholar]
- Rowley AF, Lloydevans P, Barrow SE, Serhan CN. Lipoxin biosynthesis by trout macrophages involves the formation of epoxide intermediates. Biochemistry. 1994;33:856–863. doi: 10.1021/bi00170a002. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Serhan CN. Lipoxins and new lipid mediators in the resolution of inflammation. Curr Opin Pharmacol. 2006;6:414–420. doi: 10.1016/j.coph.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005a;73:141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr Opin Clin Nutr Metab Care. 2005b;8:115–121. doi: 10.1097/00075197-200503000-00003. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Zhang P, Zhang L, Osman H, Mohler ER, 3rd, Macphee C, et al. Role of lipoprotein-associated phospholipase A2 in leukocyte activation and inflammatory responses. Atherosclerosis. 2007;191:54–62. doi: 10.1016/j.atherosclerosis.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Sok DE, Kang JB, Shin HD. 15-Hydroxyeicosatetraenoic acid dehydrogenase-activity in microsomal fraction of mouse-liver homogenate. Biochem Biophys Res Commun. 1988;156:524–529. doi: 10.1016/s0006-291x(88)80873-8. [DOI] [PubMed] [Google Scholar]
- Spite M, Summers L, Porter TF, Srivastava S, Bhatnagar A, Serhan CN. Resolvin D1 controls inflammation initiated by glutathione-lipid conjugates formed during oxidative stress. Br J Pharmacol. 2009;158:1062–1073. doi: 10.1111/j.1476-5381.2009.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl GL, Tsao P, Lefer AM, Ramphal JY, Nicolaou KC. Pharmacologic profile of lipoxins A5 and B5: new biologically active eicosanoids. Eur J Pharmacol. 1989;163:55–60. doi: 10.1016/0014-2999(89)90394-4. [DOI] [PubMed] [Google Scholar]
- Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, et al. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Inoue N, Gao D, Rikitake Y, Kawashima S, Tawa R, et al. Lysophosphatidylcholine enhances superoxide anions production via endothelial NADH/NADPH oxidase. J Atheroscler Thromb. 2000;7:238–246. doi: 10.5551/jat1994.7.238. [DOI] [PubMed] [Google Scholar]
- Ternowitz T, Fogh K, Kragballe K. 15-Hydroxyeicosatetraenoic acid (15-HETE) specifically inhibits LTB4-induced chemotaxis of human neutrophils. Skin Pharmacol. 1988;1:93–99. doi: 10.1159/000210754. [DOI] [PubMed] [Google Scholar]
- Thomas CP, Morgan LT, Maskrey BH, Murphy RC, Kuhn H, Hazen SL, et al. Phospholipid-esterified eicosanoids are generated in agonist-activated human platelets and enhance tissue factor-dependent thrombin generation. J Biol Chem. 2010;285:6891–6903. doi: 10.1074/jbc.M109.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumser AEA, Voysey JE, Wilton DC. The binding of lysophospholipids to rat-liver fatty-acid-binding protein and albumin. Biochem J. 1994;301:801–806. doi: 10.1042/bj3010801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, et al. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Tsunomori M, Fujimoto Y, Muta E, Nishida H, Sakuma S, Fujita T. 15-hydroperoxyeicosapentaenoic acid inhibits arachidonic acid metabolism in rabbit platelets more potently than eicosapentaenoic acid. Biochim Biophys Acta Lipids Lipid Metabol. 1996;1300:171–176. doi: 10.1016/0005-2760(95)00243-x. [DOI] [PubMed] [Google Scholar]
- Vang K, Ziboh VA. 15-lipoxygenase metabolites of gamma-linolenic acid/eicosapentaenoic acid suppress growth and arachidonic acid metabolism in human prostatic adenocarcinoma cells: possible implications of dietary fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2005;72:363–372. doi: 10.1016/j.plefa.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- von der Weid PY, Hollenberg MD, Fiorucci S, Wallace JL. Aspirin-triggered, cyclooxygenase-2-dependent lipoxin synthesis modulates vascular tone. Circulation. 2004;110:1320–1325. doi: 10.1161/01.CIR.0000140985.89766.CB. [DOI] [PubMed] [Google Scholar]
- Wen Y, Gu J, Chakrabarti SK, Aylor K, Marshall J, Takahashi Y, et al. The role of 12/15-lipoxygenase in the expression of interleukin-6 and tumor necrosis factor-alpha in macrophages. Endocrinology. 2007;148:1313–1322. doi: 10.1210/en.2006-0665. [DOI] [PubMed] [Google Scholar]
- Wong PY, Hughes R, Lam B. Lipoxene: a new group of trihydroxy pentaenes of eicosapentaenoic acid derived from porcine leukocytes. Biochem Biophys Res Commun. 1985;126:763–772. doi: 10.1016/0006-291x(85)90250-5. [DOI] [PubMed] [Google Scholar]
- Yuhki KI, Ushikubi F, Naraba H, Ueno A, Kato H, Kojima F, et al. Prostaglandin I-2 plays a key role in zymosan-induced mouse pleurisy. J Pharmacol Exp Ther. 2008;325:601–609. doi: 10.1124/jpet.107.134494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


