Abstract
BACKGROUND AND PURPOSE
Melatonin is involved in the regulation of colonic motility, and sensation, but little is known about the influence of melatonin on 5-hydroxytryptamine (5-HT) release from colonic mucosa. A tachykinin NK2 receptor-selective agonist, [β-Ala8]-neurokinin A4-10[βAla-NKA-(4-10)] can induce 5-HT release from guinea pig colonic mucosa via NK2 receptors on the mucosal layer. The present study was designed to determine the influence of melatonin on 5-HT release from guinea pig colonic mucosa, evoked by the NK2 receptor agonist, βAla-NKA-(4-10).
EXPERIMENTAL APPROACH
The effect of melatonin was investigated on the outflow of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) from muscle layer-free mucosal preparations of guinea pig colon, using high-performance liquid chromatography with electrochemical detection.
KEY RESULTS
Melatonin caused a sustained decline in the βAla-NKA-(4-10)-evoked 5-HT outflow from the muscle layer-free mucosal preparations, but failed to affect its metabolite 5-HIAA outflow. The specific MT3 receptor agonist, 5-methoxycarbonylamino-N-acetyltryptamine mimicked the inhibitory effect of melatonin on βAla-NKA-(4-10)-evoked 5-HT outflow. A MT3 receptor antagonist prazosin shifted the concentration-response curve of melatonin to the right in a concentration-dependent manner and depressed the maximum effect, but neither a combined MT1/MT2 receptor antagonist luzindole, nor a MT2 receptor antagonist N-pentanoyl-2-benzyltryptamine modified the concentration–response curve to melatonin.
CONCLUSIONS AND IMPLICATIONS
Melatonin inhibits NK2 receptor-triggered 5-HT release from guinea pig colonic mucosa by acting at a MT3 melatonin receptor located directly on the mucosal layer, without affecting 5-HT degradation processes. Possible contributions of MT1/MT2 melatonin receptors to the inhibitory effect of melatonin appear to be negligible. Melatonin may act as a modulator of excess 5-HT release from colonic mucosa.
Keywords: colon, melatonin, MT3 melatonin receptor, NK2 receptors, 5-HT release, serotonin release, tachykinin
Introduction
5-Hydroxytryptamine (serotonin, 5-HT) has long been recognized as an important messenger substance, which regulates colonic motility, secretion or sensation by acting via multiple receptor subtypes (Camilleri, 2002; Gershon and Tack, 2007). Most of the intestinal 5-HT is produced and stored in the mucosal enterochromaffin (EC) cells from which this amine is released into both the intestinal lumen and portal circulation (Racke and Schworer, 1991); therefore, alterations in the release of 5-HT from colonic EC cells affect the colonic function. As alterations of the colonic mucosa 5-HT have been also linked to functional bowel disorder, such as irritable bowel syndrome (IBS) (Miwa et al., 2001; Coates et al., 2004), a comprehensive understanding of the regulatory mechanism of 5-HT release from the colonic mucosa may offer a therapeutic strategy to help treat bowel disorders such as IBS.
Melatonin is a neurohormone derived from 5-HT, which is synthesized in the pineal gland as well as in the gastrointestinal tract (Bubenik, 2008). This indoleamine has been shown to be involved in the regulation of colonic motility and sensation (Lu et al., 2005; 2009; Bubenik, 2008), and pharmacological doses have a beneficial effect on abdominal pain in IBS patients who suffer from disturbed sleep (Song et al., 2005). However, the current knowledge on the role of melatonin in physiological and pathophysiological colonic functions is still poor.
The guinea pig colonic mucosa is not innervated by intrinsic 5-HT-ergic neurones (Wardell et al., 1994); therefore, the mucosal release of 5-HT is not contaminated by neuronal 5-HT. Our previous in vitro studies in the guinea pig colon have demonstrated that a tachykinin NK2 receptor-selective agonist, [β-Ala8]-neurokinin A4-10[βAla-NKA-(4-10)] is capable of inducing tetrodotoxin-resistant and loperamide-insensitive 5-HT release from the colonic mucosa, indicating that βAla-NKA-(4-10) facilitates 5-HT release from the guinea pig colonic EC cells via the activation of tachykinin NK2 receptors located on the mucosal layer (Kojima et al., 2004; 2005;). Consistent with this hypothesis, we have recently shown that this βAla-NKA-(4-10)-evoked 5-HT release is sensitive to the L-type calcium channel blocker nicardipine or the syntaxin inhibitor botulinum toxin type C, indicating that the tachykinin NK2 receptor-triggered 5-HT release is mediated by syntaxin-related exocytosis mechanisms (Kojima et al., 2009). Also, strong NK2 receptor immunoreactivity has been observed on the surfaces of enterocytes at the bases of crypts in the guinea pig proximal colon (Portbury et al., 1996). Overall, these results demonstrate that the guinea pig-isolated colonic mucosa is a useful in vitro preparation for studying non-neuronal regulatory mechanisms involved in the control of 5-HT release from colonic EC cells. In addition, daily melatonin supplementation has been recently shown to decrease the availability of 5-HT at the colonic mucosal surface of older mice: this indicates that melatonin can inhibit 5-HT release (Bertrand et al., 2010). Therefore, we investigated the possibility that melatonin affects the NK2 receptor-triggered 5-HT release from guinea pig colonic mucosa. To accomplish this, we determined whether melatonin affects the release of 5-HT from guinea pig colonic mucosa evoked by the selective NK2 receptor agonist, βAla-NKA-(4-10), using isolated muscle layer-free mucosal preparations.
Methods
Tissue preparation
All procedures were performed in accordance with the Dokkyo University School of Medicine animal care guidelines, which conform to the Guide for the Care and Use of Laboratory animal (NIH publication no. 85-23, revised 1985). Male Dunkin-Hartley guinea pigs (250–500 g body weight) were purchased from the Shizuoka Laboratory Animal Center, Inc. (Shizuoka). Guinea pigs were anaesthetized with enflurane and bled via the femoral artery. A segment of the proximal colon, 3–8 cm distal from the caecum was removed, and the luminal contents were washed out with a modified Tyrode's solution (136.8 mM NaCl, 2.7 mM KCl, 1.8 mM CaCl2, 1.05 mM MgCl2, 0.42 mM NaH2PO4, 11.9 mM NaHCO3, 5.56 mM glucose and 0.06 mM ethylenediaminetetraacetic acid Na2). Muscle layer (longitudinal/circular muscle layer)-free colonic mucosal preparations (1.5 cm in length) from the proximal colon were prepared as described in a previous study (Kojima et al., 2004). The tissue preparations were suspended in a longitudinal direction under a 4.9 mN load in 2-mL tissue baths filled with modified Tyrode's solution at 37°C and were aerated with 95% O2/5% CO2. The tissue preparations were allowed to equilibrate for 90 min with fresh replacement of the bathing medium every 10 min. Following the equilibration period, the experiments were conducted by collecting the bathing medium every 10 min. The medium obtained during the first 90–100 min was discarded. βAla-NKA-(4-10) and melatonin was added to the incubation medium from 120 to 140 min or from 120 to 160 min respectively. At the end of the collection period, the tissue preparations were blotted and weighed.
Measurement of 5-HT, 5-hydroxyindoleacetic acid (5-HIAA) and noradrenaline (NA)
The collected medium was lyophilized, dissolved in 0.4 M perchloric acid (200 µL) and passed through a 0.45-µm filter (Dismic-13CP; Advantec, Tokyo, Japan). 5-HT, 5-HIAA and NA levels in the filtrate were measured by a high-performance liquid chromatography (HPLC) with electrochemical detection (ECD-300; Eicom, Tokyo, Japan) and a pen recticoder (SS250F; Sekonic, Tokyo, Japan), as described previously (Kojima et al., 2004). Known concentrations of 5-HT, 5-HIAA and NA (Sigma, St Louis, MO, USA) were used as standards. The separation of 5-HT, 5-HIAA and NA was achieved by a reverse-phase column [length of 100 mm, inner diameter of 4.6 mm, C-18 (3 µm); Shiseido, Tokyo, Japan], using a mobile phase consisting of 0.1 M monochloroacetic acid, 1 mM ethylenediaminetetraacetic acid, 55 mg·L−1 sodium octylsulphate and 5–10% acetonitrile (pH 3.2) at a flow rate of 0.5 mL·min−1. Aliquots (20 µL) of the filtrate were injected directly into the HPLC column. The limit of detection was between 142 and 283 fmol for 5-HT, between 131 and 261 fmol for 5-HIAA, and between 75 and 148 fmol for NA per injection. The levels of 5-HT, 5-HIAA and NA in the incubation medium are expressed in units or pmol·g−1·10 min−1. The results are expressed as a percentage of the mean outflow observed during the first two collection samples (100–120 min of incubation) of the individual experiments.
Drugs and solutions
The following drugs were used: βAla-NKA-(4-10), melatonin, noradrenaline bitartrate salt hydrate, prazosin hydrochloride (Sigma); N-pentanoyl-2-benzyltryptamine (DH-97), luzindole, 5-methoxycarbonylamino-N-acetyltryptamine (5-MCA-NAT, Tocris, Bristol, UK). All drugs were dissolved in 10–70% dimethylsulphoxide with the following exceptions: β-Ala-NKA-(4-10) (100 µM), noradrenaline bitartrate salt hydrate (100 µM) and prazosin hydrochloride (10 µM) were dissolved in distilled water. All subsequent dilutions of the drugs were made with distilled water. The vehicles had no effects on βAla-NKA-(4-10)-evoked 5-HT/5-HIAA outflow or basal 5-HT/5-HIAA outflow.
Presentation of results and statistical analysis
Data are expressed as the means ± SEM from n experiments. In all cases, n= the number of colonic mucosal preparations from different animals. The significance of differences was evaluated by one-way analysis of variance (anova) followed by unpaired Student's t-test, by the computer programme Prism, where necessary multiple comparisons were conducted using one-way anova with Newman–Keuls post hoc testing. A value of P < 0.05 was considered statistically significant. In some experiments, the inhibitory effect of melatonin or 5-MCA-NAT on βAla-NKA-(4-10)-evoked 5-HT outflow was expressed as the per cent change from the control response. The negative logarithm of the molar concentration of melatonin or 5-MCA-NAT causing 50% of the maximal inhibitory effect (pIC50) was calculated from the concentration–response curves for the inhibitory effect of the agonists, according to Van Rossum's method (Van Rossum, 1963).
Results
Effects of βAla-NKA-(4-10)
The mean spontaneous outflow of 5-HT and 5-HIAA from the muscle layer-free mucosal preparations incubated in modified Tyrode's solution in the absence of test compounds (determined between 100 and 120 min of incubation) amounted to 112.8 ± 21.2 and 214.2 ± 18.6 pmol·g−1·10 min−1 respectively (n= 10). In control experiments, the spontaneous outflow of 5-HT/5-HIAA from the muscle layer-free mucosal preparations did not change significantly during the period of observation up to 160 min (Figure 1A,B). As in previous studies (Kojima et al., 2004; 2005; 2009;), addition of the NK2 receptor-selective agonist βAla-NKA-(4-10) to the incubation medium (1 µM, the maximally effective concentration, from 120 to 140 min of incubation) caused a sustained increase in the outflow of 5-HT: 5-HT outflow was enhanced to 195.9 ± 31.5 pmol·g−1·10 min−1 (n= 10, 188.5 ± 15.1%, compared with the initial outflow, P < 0.01) and then remained significantly elevated compared with the initial outflow after washout of the NK2 agonist (P < 0.05; Figure 1A), but caused a marginal increase in the outflow of 5-HIAA; 5-HIAA outflow was enhanced to 250.6 ± 23.7 pmol·g−1·10 min−1 (n= 10, 116.3 ± 2.9%, compared with the initial outflow, P < 0.05; Figure 1B).
Figure 1.
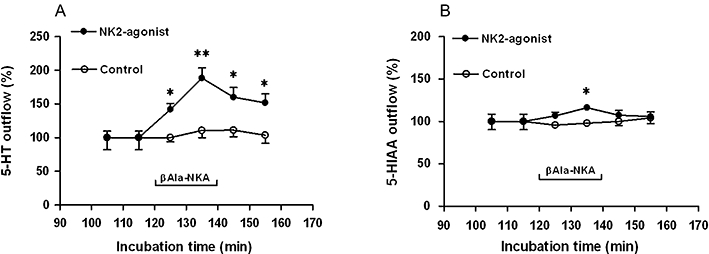
The outflow of 5-HT (A) and 5-HIAA (B) from muscle layer-free mucosal preparations of guinea pig colon in the absence (control) or presence of 1 µM βAla-NKA-(4-10) (NK2-agonist). βAla-NKA-(4-10) was present from 120 to 140 min of incubation, as indicated by the horizontal bar. Ordinates: outflow of 5-HT and 5-HIAA, expressed as % of the mean outflow of first two collections (100–120 min of incubation). Each point represents the means ± SEM from 10 experiments. Abscissae: time after onset of collection of the incubation medium. One-way analysis of variance followed by Newman–Keuls post hoc test, *P < 0.05, **P < 0.01, significantly different from the initial outflow. 5-HT, 5-hydroxytryptamine; 5-HIAA, 5-hydroxyindoleacetic acid; βAla-NKA-(4-10), [β-Ala8]-neurokinin A4-10.
Effects of melatonin or 5-MCA-NAT
In the next series of experiments, we attempted to characterize the effect of melatonin or 5-MCA-NAT on the βAla-NKA-(4-10)-evoked 5-HT/5-HIAA outflow. Addition of melatonin to the incubation medium (1 µM, the maximally effective concentration, from 120 to 160 min of incubation) did not significantly affect the basal outflow of 5-HT and its metabolite 5-HIAA, but caused a sustained decline in the βAla-NKA-(4-10)-evoked 5-HT outflow (67.4 ± 5.3% inhibition, n= 7, P < 0.01; Figure 2A,B). The inhibitory effect of melatonin (10–1000 nM) on the βAla-NKA-(4-10)-evoked 5-HT outflow was concentration-dependent with a pIC50 of 7.78 (Figure 3), but melatonin (10–1000 nM) failed to affect the βAla-NKA-(4-10)-evoked 5-HIAA outflow (Figures 2B and 3). Pre-incubation with melatonin (1 µM, from 100 to 160 min of incubation) also inhibited the βAla-NKA-(4-10)-evoked 5-HT outflow (71.3 ± 7.7% inhibition, n= 4, P < 0.05).
Figure 2.
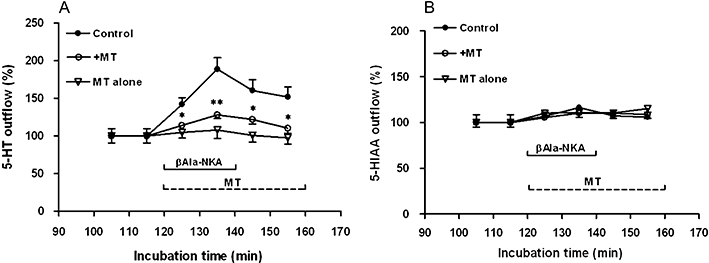
Effect of 1 µM melatonin (MT) on the outflow of 5-HT (A) and 5-HIAA (B) from the muscle layer-free mucosal preparations in the absence (MT alone) or presence (+MT) of 1 µM βAla-NKA-(4-10). βAla-NKA-(4-10) was present from 120 to 140 min of incubation, as indicated by the horizontal bar. Melatonin was present from 120 to 160 min of incubation, as indicated by the horizontal dotted line. Ordinates: outflow of 5-HT (A) and 5-HIAA (B), expressed as per cent of the mean outflow of first two collections (100–120 min). Each point represents the means ± SEM from 6 to 10 experiments. *P < 0.05, **P < 0.01, Significance of difference from the paired control. 5-HT, 5-hydroxytryptamine; 5-HIAA, 5-hydroxyindoleacetic acid; βAla-NKA-(4-10), [β-Ala8]-neurokinin A4-10.
Figure 3.
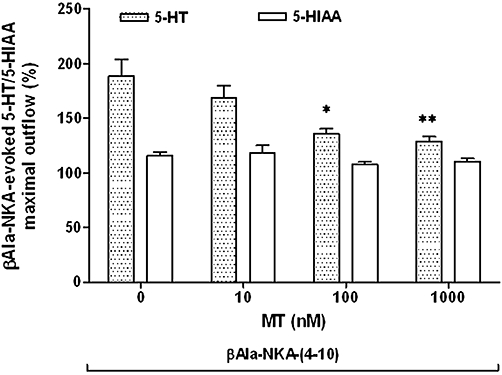
Effect of melatonin (10–1000 nM) on the maximal outflow of 5-HT and 5-HIAA from the muscle layer-free mucosal preparations evoked by 1 µM βAla-NKA-(4-10). Height of columns: βAla-NKA-(4-10)-evoked maximal 5-HT and 5-HIAA outflow, expressed as per cent of the mean outflow of first two collections (100–120 min). Results are the means ± SEM from 6 to 10 experiments. One-way analysis of variance followed by Newman–Keuls post hoc test, *P < 0.05, **P < 0.01, significantly different from the control. 5-HT, 5-hydroxytryptamine; 5-HIAA, 5-hydroxyindoleacetic acid; βAla-NKA-(4-10), [β-Ala8]-neurokinin A4-10.
Addition of a specific MT3 melatonin receptor agonist, 5-MCA-NAT to the incubation medium (100 nM, the maximally effective concentration, from 120 to 160 min) did not significantly affect the basal outflow of 5-HT (Figure 4) and its metabolite 5-HIAA (data not shown), but caused a sustained decline in the βAla-NKA-(4-10)-evoked 5-HT outflow (59.2 ± 5.3% inhibition, n= 7, P < 0.05; Figure 4). The inhibitory effect of 5-MCA-NAT (1–100 nM) was concentration-dependent with a pIC50 of 8.67 (Figure 5). 5-MCA-NAT (1–100 nM) also failed to affect the βAla-NKA-(4-10)-evoked 5-HIAA outflow (Figure 5).
Figure 4.
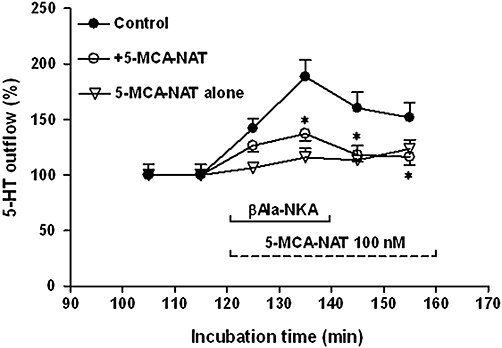
Effect of 100 nM 5-MCA-NAT on the outflow 5-HT from the muscle layer-free mucosa preparations in the absence (5-MCA-NAT alone) or presence (+5-MCA-NAT) of 1 µM βAla-NKA-(4-10). βAla-NKA-(4-10) was present from 120 to 140 min of incubation, as indicated by horizontal bar. 5-MCA-NAT was present from 120 to 160 min of incubation, as indicated by the horizontal dotted line. Ordinates: outflow of 5-HT, expressed as per cent of the mean outflow of first two collections (100–120 min). Each point represents the means ± SEM from 6 to 10 experiments. *P < 0.05, Significance of difference from the paired control. 5-HT, 5-hydroxytryptamine; 5-MCA-NAT, 5-methoxycarbonylamino-N-acetyltryptamine; βAla-NKA-(4-10), [β-Ala8]-neurokinin A4-10.
Figure 5.
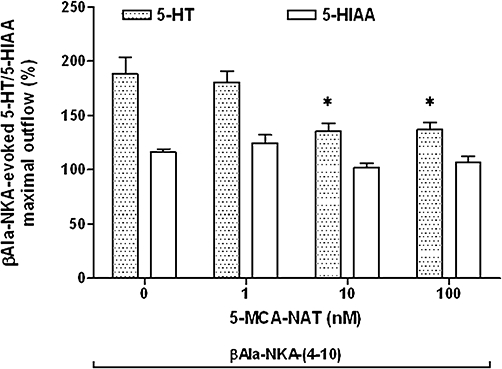
Effect of 5-MCA-NAT (1–100 nM) on the maximal outflow of 5-HT and 5-HIAA from the muscle layer-free mucosal preparations evoked by 1 µM βAla-NKA-(4-10). Height of columns: βAla-NKA-(4-10)-evoked 5-HT and 5-HIAA maximal outflow, expressed as per cent of the mean outflow of first two collections (100–120 min). Results are the means ± SEM from 6 to 10 experiments. One-way analysis of variance followed by Newman–Keuls post hoc test, *P < 0.05, significantly different from the control. 5-HT, 5-hydroxytryptamine; 5-HIAA, 5-hydroxyindoleacetic acid; 5-MCA-NAT, 5-methoxycarbonylamino-N-acetyltryptamine; βAla-NKA-(4-10), [β-Ala8]-neurokinin A4-10.
Effects of antagonists
Several melatonin receptor antagonists were tested against the inhibitory effect of melatonin. None of the antagonists investigated had a significant influence on basal 5-HT/5-HIAA outflow. As shown in Figure 6, a MT3 melatonin receptor antagonist prazosin (0.1 and 1 µM, from the onset of incubation) shifted the concentration–response curve of melatonin to the right in a concentration-dependent manner and depressed the maximum effect at 1 µM. As prazosin is a α1/α2B adrenoceptor antagonist as well as a MT3 receptor antagonist, we also investigated the effect of NA on the βAla-NKA-(4-10)-evoked 5-HT outflow; NA (1 µM, from 120 to 160 min of incubation) had no effect on the βAla-NKA-(4-10)-evoked 5-HT outflow (n= 5, 186.1 ± 18.1%, compared with the initial outflow). Also, no spontaneous outflow of NA from the muscle layer-free mucosal preparations was detected (n= 4).
Figure 6.
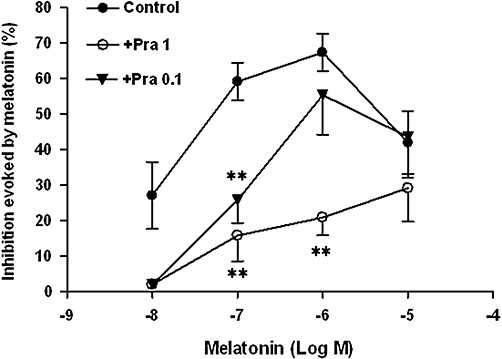
Effect of increasing concentrations of melatonin in the absence (control) or presence of prazosin (+Pra, 0.1 and 1 µM) on the βAla-NKA-(4-10)-evoked maximal 5-HT outflow from the muscle layer-free mucosal preparations. Ordinates: inhibition evoked by melatonin, expressed as the per cent change from the control response. Each point represents the means ± SEM of 5 to 10 experiments. **P < 0.01, significance of difference from the paired control. 5-HT, 5-hydroxytryptamine; βAla-NKA-(4-10), [β-Ala8]-neurokinin A4-10.
Neither the combined MT1/MT2 melatonin receptor antagonist, luzindole (0.3 µM, from the onset of incubation), nor the MT2 melatonin receptor-selective antagonist DH-97 (1 µM, from the onset of incubation) affected the concentration–response curve to melatonin (Figure 7).
Figure 7.
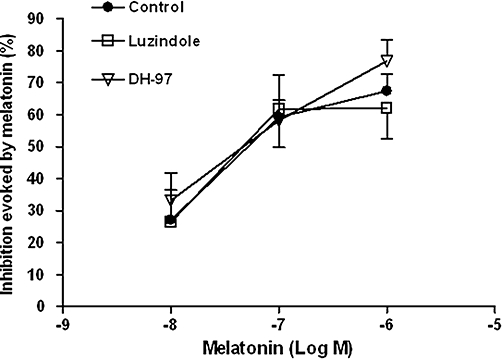
Effect of increasing concentrations of melatonin in the absence (control) or presence of DH-97 (1 µM) or luzindole (0.3 µM) on the βAla-NKA-(4-10)-evoked maximal 5-HT outflow from the muscle layer-free mucosal preparations. Ordinates: inhibition evoked by melatonin, expressed as the per cent change from the control response. Each point represents the mean ± SEM of 5 to 10 experiments. 5-HT, 5-hydroxytryptamine; βAla-NKA-(4-10), [β-Ala8]-neurokinin A4-10; DH-97, N-pentanoyl-2-benzyltryptamine.
Discussion and conclusion
Our previous in vitro studies have repeatedly indicated that the tachykinin NK2 receptor-selective agonist βAla-NKA-(4-10) is capable of inducing 5-HT release from EC cells of the guinea pig colonic mucosa via NK2 receptors on the mucosal layer (Kojima et al., 2004; 2005; 2009;). This agrees with previous findings showing the presence of tachykinin NK2 receptor immunoreactivity on the surfaces of enterocytes at the bases of crypts in the guinea pig proximal colon (Portbury et al., 1996).
The present study deals with the question of whether melatonin affects the NK2 receptor-triggered 5-HT release from guinea pig colonic mucosa.
In our initial experiments, we showed that melatonin inhibits the βAla-NKA-(4-10)-evoked 5-HT outflow with a pIC50 of 7.78, but failed to affect the outflow of 5-HT's metabolite 5-HIAA. This indicates that melatonin inhibits the βAla-NKA-(4-10)-evoked 5-HT outflow via direct interaction with the mucosal layer, without affecting the 5-HT degradation processes. Consistent with this observation, a recent study has shown that daily melatonin supplementation decreases the availability of 5-HT at the colonic mucosal surface of older mice (Bertrand et al., 2010). Direct interaction with the mucosal layer has also been reported in rat distal colon, where melatonin inhibits prostaglandin E2-induced ion secretion (Mrnka et al., 2008). The IC50 value (16.5 nM) obtained from the present study might seem to be higher than physiological levels, as the plasma concentrations of melatonin vary from the picomolar to low nanomolar range. However, the colonic mucosa seems to be capable of releasing melatonin to the lumen and its concentration at the mucosal surface in old mice reaches 6.6 µM (Bertrand et al., 2010), which is higher than the IC50 value reported here. Hence, it is possible that melatonin acts as a physiological modulator of excess 5-HT release from colonic mucosa.
Melatonin receptors are distributed throughout the gastrointestinal tract: effects of melatonin are mediated by specific high-affinity membrane MT1, MT2 and MT3 melatonin receptors (Dubocovich et al., 2003). MT1 and MT2 melatonin receptors are negatively coupled to adenylate cyclase, while the MT3 receptor has been identified as the enzyme quinone reductase 2 (QR2) in some mammalian species (Jockers et al., 2008). Herein, we have also examined the effect of the specific MT3 melatonin receptor agonist 5-MCA-NAT (Pintor et al., 2003), as previous pharmacological data suggested the presence of the MT3 melatonin receptors in guinea pig proximal colon (Santagostino-Barbone et al., 2000). As the second main result of the present study, the specific MT3 receptor agonist 5-MCA-NAT was found to mimic the inhibitory effect of melatonin on the βAla-NKA-(4-10)-evoked 5-HT outflow with an IC50 of 2.1 nM, which is close to the Ki value (6.6 nM) reported for the MT3 binding site in hamster intestinal membranes (Paul et al., 1999). This indicates that melatonin inhibits the βAla-NKA-(4-10)-evoked 5-HT outflow via MT3 melatonin receptors on the mucosal layer. Moreover, we have made use of a selective MT3 melatonin receptor antagonist, prazosin, to define a role for MT3 receptors, because prazosin, familiarly known as an α1/α2B-adrenoceptor antagonist, has been used as a MT3 melatonin receptor antagonist in the guinea pig proximal colon (Lucchelli et al., 1997; Santagostino-Barbone et al., 2000). Hence, the third finding of the present study was that prazosin shifted the concentration–response curve of melatonin to the right in a concentration-dependent manner and depressed the maximum effect. This indicates that prazosin acts non-competitively. Prazosin was also found to be a non-competitive MT3 receptor antagonist in the guinea pig proximal colon (Lucchelli et al., 1997; Santagostino-Barbone et al., 2000). It was further observed that NA failed to affect the βAla-NKA-(4-10)-evoked 5-HT outflow, indicating that α1/α2B-adrenoceptor-mediated effects do not play a role in the inhibitory action of melatonin. Thus, the melatonin receptor that mediates the inhibitory effect of melatonin appears to be a mucosal MT3 melatonin receptor. Also, the possibility that the MT3 melatonin receptor-mediated inhibition of basal 5-HT release is evoked via endogenously released melatonin is unlikely, because prazosin alone had no effect on the basal 5-HT release. Although the MT3 receptor has been identified as the enzyme QR2 in some mammalian species, the relationship between the pharmacological actions of melatonin and the MT3 receptor/QR2 protein is not yet well understood. Thus, the relationship between the inhibitory action of melatonin and the enzyme QR2 remains to be established.
We also made use of the selective MT2 receptor antagonist DH-97 (Ting et al., 1999) to define a role for MT2 melatonin receptors in our experiments, as a recent immunohistochemical study has shown MT2 melatonin receptor immunoreactivity in rat colonic mucosa (Stebelova et al., 2010). However, the concentration–response curve to melatonin was not affected by the presence of the selective MT2 receptor antagonist DH-97; therefore, the role of MT2 melatonin receptors in the inhibitory effect of melatonin is questionable. Furthermore, the lack of effect of a combined MT1/MT2 receptor antagonist, luzindole (pA2= 7.7 in rabbit retina, Dubocovich, 1988) suggests that mechanisms unrelated to MT1/MT2 receptors contribute predominantly to the inhibitory action of melatonin. This further confirms our findings with 5-MCA-NAT as 5-MCA-NAT has been shown to have only micromolar affinity for MT1/MT2 receptors, whereas it has nanomolar affinity for MT3 receptors (Jockers et al., 2008).
Given that colonic mucosal 5-HT may play an important role in the regulation of gut function in IBS patients (Miwa et al., 2001; Coates et al., 2004), it is important to elucidate the role of melatonin in the regulatory mechanism of 5-HT release from the colonic mucosa in order to understand the pathophysiology of IBS. Our observation that melatonin has an inhibitory effect on NK2 receptor-triggered 5-HT release from colonic mucosa may be important from a clinical perspective, as oral melatonin has the potential to be used in the treatment of IBS (Lu et al., 2005; Song et al., 2005).
In conclusion, we demonstrated that melatonin inhibits tachykinin NK2 receptor-triggered 5-HT release from guinea pig colonic mucosa by acting at a MT3 melatonin receptor located directly on the mucosal layer without affecting 5-HT degradation processes. Possible contributions of MT1/MT2 melatonin receptors to the inhibitory effect of melatonin appear to be negligible. Melatonin may act as a modulator of excess 5-HT release from colonic mucosa.
Acknowledgments
This study was supported by a Research Grant of Seki Minato Foundation, Tochigi, Japan.
Glossary
Abbreviations
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-MCA-NAT
5-methoxycarbonylamino-N-acetyltryptamine
- βAla-NKA-(4-10)
[β-Ala8]-neurokinin A4-10
- DH-97
N-pentanoyl-2-benzyltryptamine
- IBS
irritable bowel syndrome
- NA
noradrenaline
Conflicts of interest
None.
Supporting Information
Supporting Information: Teaching Materials; Figs 1–7 as PowerPoint slide.
References
- Bertrand PP, Bertrand RL, Camello PJ, Pozo MJ. Simultaneous measurement of serotonin and melatonin from the intestine of old mice: the effects of daily melatonin supplementation. J Pineal Res. 2010;49:23–34. doi: 10.1111/j.1600-079X.2010.00760.x. [DOI] [PubMed] [Google Scholar]
- Bubenik GA. Thirty four years since the discovery of gastrointestinal melatonin. J Physiol Pharmacol. 2008;59:33–51. [PubMed] [Google Scholar]
- Camilleri M. Serotonergic modulation of visceral sensation: lower gut. Gut. 2002;51(Suppl. 1):i81–i86. doi: 10.1136/gut.51.suppl_1.i81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. Luzindole (N-0774): a novel melatonin receptor antagonist. J Pharmacol Exp Ther. 1988;246:902–910. [PubMed] [Google Scholar]
- Dubocovich ML, Rivera-Bermudez MA, Gerdin MJ, Masana MI. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci. 2003;8:d1093–d1108. doi: 10.2741/1089. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Jockers R, Maurice P, Boutin JA, Delagrange P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what's new? Br J Pharmacol. 2008;154:1182–1195. doi: 10.1038/bjp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Ueda S, Ikeda M, Kamikawa Y. Calcitonin gene-related peptide facilitates serotonin release from guinea-pig colonic mucosa via myenteric neurons and tachykinin NK2/NK3 receptors. Br J Pharmacol. 2004;141:385–390. doi: 10.1038/sj.bjp.0705624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Ikeda M, Kamikawa Y. Loperamide inhibits tachykinin NK3-receptor-triggered serotonin release without affecting NK2-receptor-triggered serotonin release from guinea-pig colonic mucosa. J Pharamcol Sci. 2005;98:175–180. doi: 10.1254/jphs.fpj05011x. [DOI] [PubMed] [Google Scholar]
- Kojima S, Ikeda M, Kamikawa Y. Further investigation into the mechanism of tachykinin NK2 receptor-triggered serotonin release from guinea-pig proximal colon. J Pharmacol Sci. 2009;110:122–126. doi: 10.1254/jphs.09032sc. [DOI] [PubMed] [Google Scholar]
- Lu WZ, Gwee KA, Moochhalla S, Ho KY. Melatonin improves bowel symptoms in female patients with irritable bowel syndrome: a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2005;22:927–934. doi: 10.1111/j.1365-2036.2005.02673.x. [DOI] [PubMed] [Google Scholar]
- Lu WZ, Song GH, Gwee KA, Ho KY. The effects of melatonin on colonic transit time in normal control and IBS patients. Dig Dis Sci. 2009;54:1087–1093. doi: 10.1007/s10620-008-0463-z. [DOI] [PubMed] [Google Scholar]
- Lucchelli A, Santagostino-Barbone MG, Tonini M. Investigation into the contractile response of melatonin in the guinea-pig isolated proximal colon: the role of 5-HT4 and melatonin receptors. Br J Pharmacol. 1997;121:1775–1781. doi: 10.1038/sj.bjp.0701287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa J, Echizen H, Matsueda K, Umeda N. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion. 2001;63:188–194. doi: 10.1159/000051888. [DOI] [PubMed] [Google Scholar]
- Mrnka L, Hock M, Rybova M, Pacha J. Melatonin inhibits prostaglandin E2- and sodium nitroprusside-induced ion secretion in rat distal colon. Eur J Pharmacol. 2008;581:164–170. doi: 10.1016/j.ejphar.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Paul P, Lahaye C, Delagrange P, Nicolas JP, Canet E, Boutin JA. Characterization of 2-[125I]iodomelatonin binding sites in syrian hamster peripheral organs. J Pharmacol Exp Ther. 1999;290:334–340. [PubMed] [Google Scholar]
- Pintor J, Pelaez T, Hoyle CHV, Peral A. Ocular hypotensive effects of melatonin receptor agonists in the rabbit: further evidence for an MT3 receptor. Br J Pharmacol. 2003;138:831–836. doi: 10.1038/sj.bjp.0705118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portbury AL, Furness JB, Southwell BR, Wong H, Walsh JH, Bunnett NW. Distribution of neurokinin-2 receptors in the guinea-pig gastrointestinal tract. Cell Tissue Res. 1996;286:281–292. doi: 10.1007/s004410050698. [DOI] [PubMed] [Google Scholar]
- Racke K, Schworer H. Regulation of serotonin release from the intestinal mucosa. Pharmacol Res. 1991;23:13–25. doi: 10.1016/s1043-6618(05)80101-x. [DOI] [PubMed] [Google Scholar]
- Santagostino-Barbone MG, Masoero E, Spelta V, Lucchelli A. 2-Phenylmelatonin: a partial agonist at enteric melatonin receptors. Pharmacol Toxicol. 2000;87:156–160. doi: 10.1034/j.1600-0773.2000.d01-66.x. [DOI] [PubMed] [Google Scholar]
- Song GH, Leng PH, Gwee KA, Moochhala SM, Ho KY. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances – a randomized double blind placebo controlled study. Gut. 2005;54:1402–1407. doi: 10.1136/gut.2004.062034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebelova K, Anttila K, Manttari S, Saarela S, Zeman M. Immunohistochemical definition of MT(2) receptors and melatonin in the gastrointestinal tissues of rat. Acta Histochem. 2010;112:26–33. doi: 10.1016/j.acthis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Ting KN, Blaylock NA, Sugden D, Delagrange P, Scalbert E, Wilson VG. Molecular and phaemacological evidence for MT1 melatonin receptor subtype in the tail artery of juvenile wistar rats. Br J Pharmacol. 1999;127:987–995. doi: 10.1038/sj.bjp.0702612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rossum JM. Cumulative dose-reponse curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn. 1963;143:299–330. [PubMed] [Google Scholar]
- Wardell CF, Bornstein JC, Furness JB. Projection of 5-hydroxytryptamine-immunoreactive neurons in guinea-pig distal colon. Cell Tissue Res. 1994;278:379–387. doi: 10.1007/BF00414180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


