Abstract
Objective
To investigate associations between MRI brain morphology, cerebrovascular risk (VR), clinical diagnosis and cognition among elders living in urban Shanghai.
Design
Cross-sectional study.
Setting
Memory Disorders Clinic and community normal control (NC) subject recruitment.
Participants
Ninety-six older subjects, 32 with normal cognition, 30 with amnestic MCI (aMCI) and 34 with dementia.
Main outcome measures
Each subject received medical history, neurological/physical exams, neuropsychological evaluations, brain MRI and apolipoprotein E-ε4 (APOE -ε4) genotype test. MRI volumes were assessed using a semi-automatic method.
Results
Brain volume (BV) was significantly smaller in the demented compared with NC (p < 0.001) or aMCI (p = 0.043). Hippocampal volume (HV) was lower, and white matter hyperintensity volume (WMH) was higher, in aMCI (HV: p = 0.028; WMH: p = 0.041) and dementia (HV: p < 0.001; WMH: p = 0.002) compared with NC. APOE -ε4 presence was significantly associated with reduced HV (p = 0.02). Systolic blood pressure was positively associated with VR score (p = 0.037); diastolic blood pressure (p = 0.021) and VR score (p = 0.036) were both positively associated with WMH. WMH (p = 0.029) and VR (p = 0.031) were both higher among the demented than NC.
Conclusion
MRI brain morphology changes were significantly associated clinical diagnosis, in addition, blood pressure was highly associated with VR score and WMH. These results suggest that MRI is a valuable measure of brain injury in a Chinese cohort and can serve to assess the effects of various degenerative and cerebrovascular pathologies.
Keywords: Dementia, Mild Cognitive Impairment, Magnetic Resonance Imaging, white matter hyperintensities, hippocampal volume, cerebrovascular risk, apolipoprotein E genotype, cognition
Introduction
As the worldwide population of older adults increases, age-related diseases such as cerebrovascular (CVD) and Alzheimer’s (AD) diseases present an increasing public health issue. Substantial numbers of longitudinal and cross-sectional studies have been published comparing the etiology, epidemiology and pathology of dementia, MCI and aging across race and ethnic groups [1–13]. Studies of different races are particularly important as recent census data show increasing racial and ethnic diversity in the elderly population of the United States [1, 14]. However, relatively few studies have been performed on persons of Chinese ethnicity.
Because the largest increase in dementia cases is expected to occur primarily in developing countries [15–17], early diagnosis will be needed for effective treatment or prevention. Structural brain imaging is widely used to study the morphological changes of the brain, particularly those associated with AD and CVD processes [8–21]. Neuroimaging also can help in predicting the probability of developing future dementia as well as measure progression of underlying neurodegenerative diseases [22].
In this study, we compared quantitative magnetic resonance imaging (MRI) measures and cerebrovascular risk factors among three cognitive groups in the Shanghai Community Brain Health Initiative–pilot phase (SCOBHI-P). The SCOBHI-P is based on community-dwelling controls and cases seen at a memory disorders clinic who are residents of Shanghai, China. The goals of SCOBHI-P were to investigate the biological and cognitive changes among elders with normal cognition, mild cognitive impairment (MCI) and dementia and evaluate associations between MRI markers and performance on neuropsychological tests..
Design & Methods
Participants
Subject Recruitment
Cases with MCI and dementia were evaluated at Hua Shan Hospital (HH) in Shanghai, China. One hundred and nine cases of dementia and MCI from the Memory Disorders Clinic (MDC) at HH who were initially diagnosed from May 2007 to November 2008 were invited to participate. Of these, 54 were recruited (53.2%), 42 refused, 8 were unreachable and 1 had a stroke. Controls with normal cognition were obtained from a name list provided by the government, which contained information on all residents. We selected a resident group in the Jing’an district of Shanghai (smaller than a neighborhood) consisting of five buildings in the Jingansi Temple Community (JTC). Potential controls were approached at the door to describe the study. Of 71 potential participants from the name list, 10 refused (14%). An additional three names on the name list were unreachable. The recruitment rate in the community was 81.6%. When the 58 residents in the community were clinically evaluated, two met study criteria for dementia (3.5%) and 12 met Petersen criteria for MCI (20.6%). These 14 individuals were added to the case pool. Of the 112 cases and controls, 12 had the MRI evaluation but did not complete the proxy interview and neuropsychological tests and therefore were excluded from the study. The remaining 100 subjects included 32 normal controls, 34 MCI and 34 dementia cases. Of the 34 participants with MCI, 4 (12%) were determined to have non-amnestic MCI (naMCI), while 30 (88%) had amnestic MCI (aMCI). Because the number of naMCI was too small to study as a group, we restricted analysis to 96 subjects, 32 of whom were cognitively normal, 30 were aMCI, and 34 were demented.
Clinical Evaluation
All participants received a multidisciplinary clinical evaluation in the HH MDC. These evaluations included detailed medical history, physical and neurological examinations. They were also evaluated with the Clinical Dementia Rating [23] (CDR) scale. A neuropsychological battery was administered by the study psychometrist that included the modern Chinese Cognitive Abilities Screening Instrument (mc-CASI)[24], WAIS-R Digit Span [25], Bell Cancellation Test [26], WMS Logical Memory Test [27] (immediate and delayed recall), Rey-Osterrieth Complex Figure test (ROCF) [28] (copying and recall), Stroop Test [29], Auditory Verbal Learning Test (AVLT) [30], Category Verbal Fluency Test, WAIS-R Similarities Test [27], Trail-making Test [31], Clock-Drawing Test [32], Boston Naming Test [33] and Chinese version of the Mattis Dementia Rating Scale (Mattis DRS) [34]. All participants were genotyped for Apolipoprotein E [35]. In addition, all subjects received two blood pressure measurements in a seated position. Information about diagnosed stroke, TIA, hypertension, diabetes and coronary artery disease were elicited in the interview. Each subject (case proxy, control proxy and control) was administered a risk factor questionnaire to obtain data on demographics, socioeconomic status (SES), physical activity, mental activity, smoking/alcohol consumption, family history of memory problems and other diseases, social activity, personal medical history, interviewer-inspected prescription and non-prescription medications, hormonal history (women), quality of life and sleep, perceptions of stigma associated with dementia, food preferences at age 50, and activities of daily living (IADL and ADL). We interviewed controls about themselves and control informants about the control in a separate room. None of the cases (dementia or MCI) were interviewed directly; instead data for cases were collected from proxy informants.
Diagnosis (normal cognition, MCI, dementia) was made according to standardized criteria. Each participant was initially diagnosed by four study neurologists (ZH, QZ, QG, DD) in a consensus diagnostic conference. In addition, two international consensus diagnostic teleconferences were held, including U.S. team members (DG, DS, RP, ARB, JM) for 9 MCI subjects and 8 difficult-to-diagnose cases. Dementia was diagnosed using DSM-IV [36] criteria for dementia. AD was diagnosed using NINCDS-ADRDA [37] criteria. Vascular dementia was diagnosed using the NINDS-AIREN [38] criteria. MCI was diagnosed using Petersen MCI criteria [39, 40]. Normal cognitive function was diagnosed if there was no clinically significant cognitive impairment.
MRI Acquisition
Brain images were obtained at HH in Shanghai. We used a series of MRI acquisition protocols developed at the UCD Imaging of Dementia & Aging Laboratory (IDeA Lab), which are suitable for the GE 1.5T MRI system. Imaging parameters were as follows:
Axial spin echo, T2 weighted double echo image with TE1 equal to 20 ms, TE2 equal to 90 ms, TR equal to 2420 ms, a field of view of 24 cm and a slice thickness of 3 mm.
Coronal 3D spoiled gradient recalled echo (IR-prepped SPGR) acquisition, T1 weighted image with TR equal to 9.1 ms, a flip angle of 15 degrees, a field of view of 24 cm and a slice thickness of 1.5 mm.
Axial high resolution Fluid Attenuated Inversion Recovery (FLAIR) image with a TE of 120 ms, a TR of 9000 ms, a TI of 2200 ms, a 24 cm field of view, and a slice thickness of 3 mm.
Qualitative assessment of all available image sequences was used to assist with clinical diagnosis, but the clinical diagnostic team was blind to results of quantitative analyses. The images were sent to the IDeA Laboratory and image quantification was performed by a rater who was blinded to age, sex, educational achievement and diagnostic status.
Image Analysis
Brain and WMH Volumes
Analysis of brain and WMH volumes was based on the FLAIR sequence, which was designed to enhance WMH segmentation [41]. Brain and WMH segmentation was performed in a two-step process according to previously reported methods [42]. In brief, non-brain elements were manually removed from the image by operator guided tracing of the dura matter within the cranial vault including the middle cranial fossa, but excluding the posterior fossa and cerebellum. The resulting measure of the cranial vault was defined as the total cranial volume (TCV) to correct for differences in head size amongst the subjects. Image intensity nonuniformities [43] were then removed from the image and the resulting corrected image was modeled as a mixture of two Gaussian probability functions with the segmentation threshold determined at the minimum probability between these two distributions. Then, a single Gaussian distribution was fitted to the image data using an a priori threshold of 3.5 standard deviations in pixel intensity above the mean to identify WMH. Intra and inter rater reliability for these methods are high and have been published previously [44].
Hippocampal volumes
Boundaries for the hippocampus were manually traced according to previously reported methods [1] which emphasize analysis of the anterior 2/3 of the hippocampus. Intra-rater reliability for right and left hippocampus using this method was excellent, with intraclass correlation coefficients of 0.98 for right side and 0.96 for left side.
MRI Infarctions
Cerebral infarction on MRI was determined according to previously published protocols [44, 45]. MRI infarction was determined from the size, location and imaging characteristics of the lesion based on review of the double echo, FLAIR and 3-dimensional T1 high-resolution image. Lesions 3 mm or larger qualified for consideration as cerebral infarcts. Other necessary imaging characteristics included: 1) CSF density on T1 weighted or FLAIR image and 2) If the infarct was in the basal ganglia area, distinct separation from the circle of Willis vessels. Previously reported kappa values for agreement between the three raters were generally very good, ranging from 0.73 to 0.90 [44].
Cerebrovascular risk factors
Medical histories as well as review of medical records were used to create a summed composite score for cerebrovascular risk. The presence or absence of five cerebrovascular risk factors (i.e, stroke, TIA, hypertension, diabetes and coronary artery disease) was systematically assessed from the informant interview and the subject’s medical record. Blood pressure was measured twice and averaged. Hypertension was defined as measured systolic or diastolic pressures exceeding 140/90 mm Hg or controlled by medication (informed from medical history). The total VR score ranged from 0 to 60%, with a mean of 22.9% (0.189 SD) in all subjects.
Data Analyses
Since MRI measures of brain volume, WMH and hippocampal volume are each known to vary by sex and age [1, 20, 21], all MRI variables were divided by total cranial volume (TCV) [1, 44]. The distribution of normalized WMH was skewed. Therefore WMH volumes were first divided by TCV and then log transformed to better approximate a normal distribution for analysis as previously described [1].
Data were then analyzed in JMP8 (SAS institute, Cary, NC). Analyses of variance (ANOVA) models with clinical diagnosis as the grouping variable were used to detect group differences in demographic variables, MRI measures and cerebrovascular risk factor scores. Chi-square analysis was used to test group differences in sex and MRI infarct prevalence. Analyses of covariance (ANCOVA) were used to further assess associations between cerebrovascular risk factors, presence of MRI infarcts, APOE -ε4 genotype, and cognitive syndromes for each of the 3 MRI measures while controlling for age, education and gender. An ANCOVA approach was also used to evaluate the association of the 3 MRI measures with neuropsychological tests. The Tukey–Kramer method was used for all post-hoc analyses. Logistic regression was used to investigate the association between risk factors and cognitive syndromes or presence of MRI infarcts. P values < 0.05 were considered statistically significant.
Results
Subject Characteristics
Subject characteristics are summarized in Table 1. There were no significant differences across diagnostic groups in age, sex, and education.
Table 1.
Subject characteristics in SCOBHI-P.
| Controls (n = 32) | aMCI (n = 30) | Demented (n = 34) | |
|---|---|---|---|
| Sex (F/M) (number) | 16/16 | 15/15 | 18/16 |
| Age (yr) (mean ± SD) | 73.41 ± 5.51 | 74.8 ± 3.95 | 74.41 ± 5.56 |
| Education Level (mean ± SD) | 2.67 ± 1.38 | 2.93 ± 1.41 | 2.23 ± 1.72 |
| BV*(mean ± SD) | 0.80 ± 0.03a | 0.77 ± 0.04a | 0.75 ± 0.05b |
| HV*(*100) (mean ± SD) | 0.39 ± 0.05a | 0.35 ± 0.05b | 0.33 ± 0.08b |
| WMH**(mean ± SD) | −5.55 ± 0.99a | −5.27 ± 1.24b | −4.63 ± 0.80b |
| VR*** (mean ± SD) | 0.21 ± 0.13a | 0.27 ± 0.18ab | 0.33 ± 0.17b |
| MR Infarct (percent) | 18.8% | 24.1% | 34.4% |
BV: Brain volume; HV: Hippocampal volume. Reported as the percentage of intracranial volume.
WMH: White matter hyperintensity volumes. Reported as the percentage of intracranial volume, then log transformed to normalize variance.
VR: Cerebrovascular risk factor score. Reported as the percentage of five risk factors.
Means with different superscripts indicate significant group differences after Tukey-Kramer adjustments for multiple comparisons (p < 0.05)
Quantitative MRI
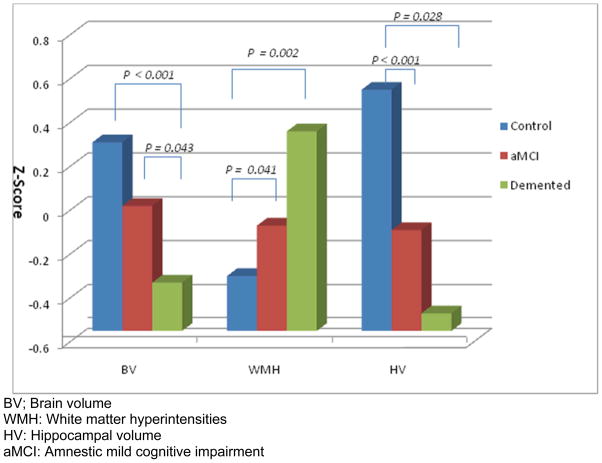
For each MRI measure, ANOVA models were carried out with clinical diagnosis as the outcome variable. Demented subjects had significantly smaller BV than normal controls (p < 0.001) and aMCI subjects (p = 0.04). HV was significantly lower, and WMH was significantly higher, for aMCI (HV: p = 0.03; WMH: p = 0.04) and demented subjects (HV: p < 0.001; WMH: p < 0.01) as compared to normal controls (Figure 1). Chi-square testing indicated that the percent with MRI infarct did not significantly differ across cognitive syndrome groups.
Figure 1.
Brain Volume Measures in controls, aMCI and dementia
BV; Brain volume
WMH: White matter hyperintensities
HV: Hippocampal volume
aMCI: Amnestic mild cognitive impairment
Secondary analyses used ANCOVA models to examine the association between diagnosis, age, APOE-ε4, VR, MRI infarcts, and MRI measures, after controlling for education level and sex (Table 2). Older age was significantly associated with decreased BV (p < 0.001) and HV (p = 0.01) and increased WMH (p = 0.001); APOE-ε4 presence was associated with decreased HV (p = 0.02); history of MRI infarct was significantly associated with increased WMH (p = 0.004). Post-hoc Tukey-Kramer analysis showed that, after adjusting for covariates, demented subjects had significantly smaller BV than normal controls (p < 0.001) and aMCI (p = 0.017), and had significantly higher WMH volumes than normal controls (p = 0.01). HV volume was also significantly lower among NC compared to demented, but did not differ between demented and aMCI.
Table 2.
Associations between risk factors, diagnosis and MRI measures, after adjusting for education and sex (Estimate, SE)a
| BVb | WMHc | HVd | |
|---|---|---|---|
| Demented/NC | −0.038 (0.009) p < 0.001 |
0.72 (0.24) p = 0.01 |
−0.059 (0.016) p < 0.001 |
| Demented/aMCI | −0.026 (0.009) p = 0.017 |
||
| aMCI/NC | |||
| Age | −0.003 (0.001) p < 0.001 |
0.068 (0.020) p = 0.001 |
−0.003 (0.001) p = 0.012 |
| APOE-ε4 (+/−) | −0.037 (0.015) p = 0.019 |
||
| Vascular Risk | |||
| MRI Infarcts (+/−) | 0.66 (0.22) p = 0.004 |
||
Sex and education level were not significant in any of the models
BV: Brain volume
WMH: White Matter Hyperintensities
HV: Hippocampal volume Blanks: not significant.
Vascular Risk Factors
Given the findings of increased WMH in association with cognitive impairment, we further explored the association between VR, cognitive status and MRI measures. VR scores ranged from 0 to 60% with the average VR score being 27% ± 17%; 85.4% of all participants had at least one vascular risk factor; of subjects with a VR greater than zero, 97.6% had hypertension. In addition, 81 (84.4%) members of this sample were hypertensive (average BP: 152.16 ± 21.62/77.57 ± 10.39 mmHg) at baseline, despite the fact that most received treatment. The prevalence of MRI infarcts in this sample was 25.8%. ANOVA results revealed that the mean VR score in demented patients was significantly higher than in controls (p = 0.026; Table 1). After adjusting for age, sex and education, ANCOVA estimates showed that higher systolic pressure was associated with increased VR score (p = 0.037); higher diastolic pressure (p = 0.021) and VR score (p = 0.036) were both associated with increased WMH. Using WMH, blood pressure and VR score together as independent variables in a logistic regression model predicting MRI infarct, we found that increased WMH were significantly associated with the an increased risk of MRI infarct (p = 0.008; Table 3).
Table 3.
Association between vascular risk score, hypertension, white matter hyperintensities and MRI infarcts, after adjusting age, sex, education (Estimate, SE)
| VR score* | WMH** | MRI infarct (+/−) | |
|---|---|---|---|
| Systolic BP*** | 0.002 (0.001) p = 0.037 |
||
| Diastolic BP*** | 0.027 (0.011) p = 0.021 |
||
| VR score* | --- | 1.35 (0.64) p = 0.036 |
|
| WMH** | --- | --- | 0.89 (0.34) p = 0.008 |
VR score: Cerebrovascular factor score
WMH: White matter hyperintensities
BP: Blood pressure
Blank: not significant; Ellipse: unnecessary self comparisons.
Associations with Clinical Syndrome
Logistic regression analyses were used to examine the effects of all risk factors (demographic, MRI measures, vascular risk factors and APOE ε4) in modifying the risk of possessing the cognitive syndromes. Three separate models were fit to allow comparisons between demented and NC, aMCI and NC, and demented and aMCI groups (see Table 4). When comparing demented and normal control groups, we found reduced BV (p = 0.002) and HV (p = 0.024), and increased WMH (p = 0.029) and VR (p = 0.031) to be independently and significantly associated with an increased risk of dementia. With each increase of 1% of BV, the odds of dementia were reduced nearly 40% (OR: 0.61, 95% CI: 0.42–0.80). With each 0.01% increase in HV, the odds for dementia were 16% lower (OR: 0.84, 95% CI: 0.70 – 0.96). Conversely, a 1% increase in WMH volume was associated with a three-fold increase in the odds of dementia (OR: 3.34, 95% CI: 1.33 – 10.28). Similarly, the presence of each VR was associated with a nearly four times increased odds of dementia (OR: 3.78, 95% CI 1.26 – 16.03).
Table 4.
Associations between individual risk factors and diagnostic outcomes from logistic regression models (OR, 95% CI), adjusting for age, sex and education#
| * Demented vs Controls | * aMCI vs Controls | * Demented vs aMCI | |
|---|---|---|---|
| BV1 | 0.61 (0.42 – 0.80) | 0.80 (0.66 – 0.94) | |
| HV2 | 0.84 (0.70 – 0.96) | 0.88 (0.76 – 1.0) | 0.89 (0.78 – 0.99) |
| WMH3 | 3.34 (1.33 – 10.28) | 2.43 (1.09 – 6.11) | |
| VR4 | 3.78 (1.26 – 16.03) | ||
| APOE -ε4 | |||
| MRI infarct |
Blanks: not significant;
We report only the significant associations (p < 0.05)
Reported as odds-ratio for 1 unit increase in continuous predictor variables
BV = Brain volume divided by total cranial volume, then multiplied by 100.
HV = Hippocampal volume divided by total cranial volume and multiplied by 10000
WMH = White matter hyperintensity volume divided by total cranial volume, and then multiplied by 100.
VR = the number of vascular risk factors
Similarly, when comparing aMCI to dementia every 1% increase in BV was associated with 20% lower odds for dementia (OR: 0.80, 95% CI: 0.66 – 0.94) and every 0.01% increase in HV was associated with a 11% decrease in the odds for dementia (OR: 0.89, 95% CI: 0.78–0.99). Conversely each 1% increase in WMH was associated with 2.43 times greater odds of dementia (OR: 2.43, 95% CI: 1.09 – 6.11)..
When comparing aMCI to controls, we found that normalized HV volume increase of 0.01% was associated with a 12% reduction in the odds for aMCI (OR: 0.88, 95% CI: 0.76 – 1.0) (Table 4).
Neuropsychological test scores and MRI measures
Finally, separate ANCOVA models controlling for age, education and gender were used to assess the associations between MRI measures (BV, WMH and HV) and performance on individual neuropsychological tests listed in Table 5. We found that increased BV was significantly associated with higher scores for Bell Cancellation Test (p = 0.003), ROCF copying (p < 0.001), Stroop Test color-word (p = 0.001), WAIS-R Similarities Test (p = 0.001), Mattis DRS (p < 0.001), WAIS Digit Span Test total score (p < 0. 01) and Category Verbal Fluency Test (p < 0.001), and lower scores for Trail-making Test (p = 0.005). Increased WMH was associated with worse performance on ROCF coping (p = 0.04) and delayed recall (p = 0.04) tests, WAIS-R Similarities Test (p < 0.001), Mattis DRS (p = 0.005) and Category Verbal Fluency (p = 0.01), and higher score in Trail-making Test (p < 0.001). Increased HV was significantly associated with higher scores for WMS Logical Memory delayed recall test (p = 0.03), ROCF delayed recall test (p = 0.006) and AVLT short (p = 0.01) and long delayed recall (p = 0.006) tests.
Table 5.
Associations between brain morphology and scores on neuropsychological tests, adjusted for age, sex and education (Estimate, SE)
| Neuropsychological tests | Brain Volume | White matter hyperintensity | Hippocampal volume |
|---|---|---|---|
| WMS Logical memory (delayed recall) | 12.73 (5.74) p = 0.03 |
||
| WMS Logical memory (immediate recall) | |||
| Bell Cancellation | 19.70 (6.33) p = 0.003 |
||
| * ROCF copy | 94.71 (22.96) p < 0.001 |
−1.89 (0.90) p = 0.04 |
|
| * ROCF delay | −1.47 (0.69) p = 0.04 |
31.23 (11.09) p = 0.006 |
|
| Stroop (color-word) | 109.57 (32.74) p = 0.001 |
||
| WAIS-R Similarities | 45.79 (13.56) p = 0.001 |
−1.85 (0.51) p < 0.001 |
|
| ** AVLT (short delayed recall) | 8.64 (3.27) p = 0.01 |
||
| ** AVLT (long delayed recall) | 9.20 (3.28) p = 0.006 |
||
| Trail-making (Test B) | −845.76 (290.56) p = 0.005 |
42.74 (9.25) p < 0.001 |
|
| Mattis Dementia Rating Scale | 225.76 (36.81) p < 0.001 |
−3.90 (1.34) p = 0.005 |
|
| WAIS-R Digit Span Total | 10.98 (4.17) p = 0.01 |
||
| WAIS-R Digit Span Forward | 5.22 (2.0) p = 0.01 |
||
| WAIS-R Digit Span Backward | 5.76 (2.86) p = 0.047 |
||
| *** Category Verbal Fluency | 119.76 (23.94) p < 0.001 |
−2.28 (0.90) p = 0.01 |
|
| Boston Naming | |||
| Clock-Drawing |
Rey-Osterieth Complex Figure
Auditory Verbal Learning Test
Total score of animals, fruits and vegetables tests
Blanks: not significant
Discussion
Imaging-based volumetric measurements are widely used to characterize and assist in the diagnoses of patients with dementia and MCI, particularly of the hippocampus, which is recognized as a brain region where AD pathology is likely to first appear [19, 46–48]. Global brain atrophy and WMH are also recognized as structural brain measures associated with aging and dementia [1, 49–52]. However, most of the studies from which these findings are derived were conducted in Caucasian populations. Our study in a Chinese sample found similar volumetric differences in brain, hippocampus and white matter hyperintensities between diagnostic groups. Furthermore, we found that these MRI measures were also associated with previously described risk factors such as age and vascular risk; and for the hippocampus, APOE-ε4 as well. These findings are similar to those of previously reported MRI studies of predominantly white populations [46, 53]. Since hippocampal atrophy shows the earliest and most consistent morphologic change in AD, our findings support the hypothesis that APOE-ε4 also is also a risk factor for amnestic MCI and dementia in a Chinese population.
Cerebrovascular risk factors such as hypertension were common in this Chinese sample, and systolic blood pressure was found to be significantly and positively associated with VR score. The average VR score (0.27 ± 0.16) was higher than for Caucasians in our previous study (0.22 ± 0.20) [1], and VR was found to be significantly associated with the prevalence of MRI infarcts. Although the prevalence of hypertension in SCOBHI-P (men: 85.1%; women: 83.7%) was similar to that of the Framingham study [44] (men: 87%; women: 82%) and 96.4% of subjects were receiving treatment, their blood pressures were less well controlled (men: 154.8 ± 21.5/80.9 ± 10.2 mmHg; women: 158.5 ± 76.4) than those of Framingham participants (men; 139 ± 18.8/68.2 ± 11.9 mmHg; women: 141 ± 20.8/67.5 ± 10.8 mmHg). We believe that this finding might explain the higher prevalence of MRI infarcts in this Chinese study as compared to the Framingham study [44].
The combinations of MRI volumes and VR score reliably distinguished groups of individuals with normal cognition, aMCI and dementia. Whereas demented subjects differed significantly from the two other groups on measures of BV, WMH and HV, aMCI differed significantly from normal controls only on HV; VR score also differed significantly between demented individuals and controls. MCI has been recognized to be the transitional state between normal cognition and dementia [40], and individuals with aMCI are thought to display early manifestations of AD pathology with 13% per year on average converting to AD [54, 55]. Our analysis of the three diagnostic groups confirmed that hippocampal atrophy is the earliest brain structure change in aMCI, while brain atrophy, WMH burden and vascular risk factors were more strongly associated with the dementia syndrome. Again, these findings are remarkably consistent with previous reports utilizing Caucasian populations.
Our results also showed that brain atrophy was significantly associated with performance on the Bell Cancellation test, ROCF copying test, Stroop copy and word test, WAIS-R Similarities and Digit Span tests, Trail-making Test B, Mattis DRS and Category Verbal Fluency test; WMH volume was significantly associated with ROCF delayed recall and copy tests, WAIS-R similarities, Trail-making test B, Mattis DRS and Category Verbal Fluency test, although the significance of the relationship between WMH and ROCF tests are likely marginal given the number of individual analyses performed (no Bonferroni adjustments were made, since the purpose of this exercise is to look at the pattern of findings, making adjustments for multiple comparisons less relevant.). HV was significantly associated with WMS logical memory delayed recall test, ROCF delayed recall test and AVLT short and long-delayed recall tests. These findings suggest that despite age, educational and cultural differences, structural brain changes are consistently associated with cognitive measures. The presence of a strong association between hippocampal volume and memory performance, especially in delayed recall tests, supports the theory that hippocampus has a relatively specific role in retaining information after a delay [56, 57] and also supports the notion that delayed memory impairments and hippocampal atrophy are cardinal features of AD even in a Chinese population where vascular disease is relatively common. In contrast, hypertension was common in this Chinese cohort and less well treated when compared to a reference Caucasian cohort. Given that blood pressure was positively associated with WMH, and that increasing WMH correlated with more impaired cognitive syndrome, it is possible that poorly controlled blood pressure may have been partially responsible for cognitive impairment in our group. Control of blood pressure and likely other vascular risk factors, therefore, might be expected to decrease the prevalence of dementia in this population.
Our study, however, has several limitations. The participants of this study were recruited not only from the community, but also from the memory disorder clinic and, therefore, may not reflect the general population. In addition, although most of our dementia patients were diagnosed with AD, a minority of subjects had vascular dementia. If the study population were strictly limited to AD dementia we may have found brain differences more characteristic of AD associated with dementia. This limitation, however, is likely to be minimal as we found a significant reduction in hippocampal volume, similar to previous findings in AD cohorts. More likely, these data reflect a much higher prevalence of comorbid cerebrovascular disease among our Chinese study group, even though a high percentage of dementia subjects were diagnosed with AD.
Conclusion
China is a country with a large older population that has received relatively little study. Despite obvious cultural differences from previously reported, predominantly Caucasian studies, we identified similar genetic factors and structural brain differences associated with dementia in this population. The greatest difference between this sample and other Caucasian samples appears to relate to the high frequency of vascular brain injury (e.g. MRI Infarction) and the high frequency of vascular risk factors, especially hypertension. Since cerebrovascular disease is a treatable disorder, further study and possible treatment are warranted.
References
- 1.DeCarli C, et al. Brain Behavior Relationships Among African Americans, Whites, and Hispanics. Alzheimer Disease & Associated Disorders. 2008;22(4):382–391. doi: 10.1097/wad.0b013e318185e7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurland BJ, et al. Rates of dementia in three ethnoracial groups. International Journal of Geriatric Psychiatry. 1999;14(6):481–493. [PubMed] [Google Scholar]
- 3.Mungas D, Reed BR, Tomaszewski FS. Criterion-referenced validity of a neuropsychological test battery: equivalent performance in elderly Hispanics and non-Hispanic Whites. J Int Neuropsychol Soc. 2005;11:620–630. doi: 10.1017/S1355617705050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang MX, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 5.Bachman DL, et al. Comparison of Alzheimer’s disease risk factors in white and African American families. Neurology. 2003;60(8):1372–1374. doi: 10.1212/01.wnl.0000058751.43033.4d. [DOI] [PubMed] [Google Scholar]
- 6.Evans DA, et al. Incidence of Alzheimer disease in a biracial urban community -Relation to apolipoprotein E allele status. Archives of Neurology. 2003;60(2):185–189. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 7.Fillenbaum GG, et al. The prevalence and 3-year incidence of dementia in older Black and White community residents. Journal of Clinical Epidemiology. 1998;51(7):587–595. doi: 10.1016/s0895-4356(98)00024-9. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick AL, et al. Incidence and prevalence of dementia in the cardiovascular health study. Journal of the American Geriatrics Society. 2004;52(2):195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 9.Sandberg G, et al. The prevalence of the neuropathological lesions of Alzheimer’s disease is independent of race and gender. Neurobiology of Aging. 2001;22(2):169–175. doi: 10.1016/s0197-4580(00)00236-0. [DOI] [PubMed] [Google Scholar]
- 10.Sencakova D, et al. Hippocampal atrophy correlates with clinical features of Alzheimer disease in African Americans. Archives of Neurology. 2001;58(10):1593–1597. doi: 10.1001/archneur.58.10.1593. [DOI] [PubMed] [Google Scholar]
- 11.Wilkins CH, et al. The neuropathology of Alzheimer disease in African American and white individuals. Archives of Neurology. 2006;63(1):87–90. doi: 10.1001/archneur.63.1.87. [DOI] [PubMed] [Google Scholar]
- 12.Graves AB, Larson EB, Edland SD, Bowen JD, McCormick WC, McCurry SM, Rice MM, Wenzlow A, Uomoto JM. Prevalence of dementia and its subtypes in the Japanese-American population of King County, WA: The Kame Project. American Journal of Epidemiology. 1996;144:760–771. doi: 10.1093/oxfordjournals.aje.a009000. [DOI] [PubMed] [Google Scholar]
- 13.Borenstein AR, Wu Y, Mortimer JA, Schellenberg GD, McCormick WC, Bowen JD, McCurry S, Larson EB. Developmental and vascular risk factors for Alzheimer’s disease. Neurobiology of Aging. 2005;26(3):325–334. doi: 10.1016/j.neurobiolaging.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Census 2000. Vol. 2000. Washington, DC: US Census Bureau; 2000. [Google Scholar]
- 15.Ferri CP, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam LCW, Tam WC, Victor WCL, et al. Screening of Mild Cognitive Impairment in Chinese Older Adults - A Multistage Validation of the Chinese Abbreviated Mild Cognitive Impairment Test. Neuroepidemiology. 2008;2008(30):6–12. doi: 10.1159/000113300. [DOI] [PubMed] [Google Scholar]
- 17.Wimo A, Jonsson L, Winblad B. An estimate of the worldwide prevalence and direct costs of dementia in 2003. Dementia and Geriatric Cognitive Disorders. 2006;21(3):175–181. doi: 10.1159/000090733. [DOI] [PubMed] [Google Scholar]
- 18.DeCarli C. The role of cerebrovascular disease in dementia. Neurologist. 2003;9(3):123–136. doi: 10.1097/00127893-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Jack C, Petersen RC, O’Brien PC, et al. MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology. 1992;1992(42):183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 20.Jack CR, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49(3):786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy D, DeCarli CD, Daly E, et al. Volumetric magnetic resonance imaging in men with dementia of the Alzheimer type: correlations with disease severity. Biol Psychiatry. 1993;1993(34):612–621. doi: 10.1016/0006-3223(93)90153-5. [DOI] [PubMed] [Google Scholar]
- 22.Petersen RC, et al. Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review) - Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 23.Morris JC. THE CLINICAL DEMENTIA RATING (CDR) - CURRENT VERSION AND SCORING RULES. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 24.Teng EL, Homma HKA, Imai Y, Larson E, Graves A, Sugimoto K, Yamaguchi T, Sasaki H, Chiu D, White LR. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiologic studies of dementia. Int Psychogeriatr. 1994;1994(6):45–56. doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- 25.Wechsler D. WAIS-R Manual: Wechsler Adult Intelligence Scale-Revised Corporation. New York: Psychological; 1981. [Google Scholar]
- 26.Gauthier L, Dehaut F, Joanette Y. THE BELLS TEST - A QUANTITATIVE AND QUALITATIVE TEST FOR VISUAL NEGLECT. International Journal of Clinical Neuropsychology. 1989;11(2):49–54. [Google Scholar]
- 27.Wechsler D. Wechsler Memory Scale - Revised Manual. San Antonio: Psychological Corporation; 1987. [Google Scholar]
- 28.Rey A. L-examen psychologique dans les cas d’encephalopathie traumatique. Arch Psychologie. 1941;1941(28):286–340. [Google Scholar]
- 29.Golden C. Stroop Color and Word Test. Vol. 1978. Chicago: Stoelting Company; [Google Scholar]
- 30.Schmidt M. Rey Auditory Verbal Learning Test. A Handbook. Vol. 1996. Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- 31.Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 32.Rouleau I, et al. QUANTITATIVE AND QUALITATIVE ANALYSES OF CLOCK DRAWINGS IN ALZHEIMERS AND HUNTINGTONS-DISEASE. Brain and Cognition. 1992;18(1):70–87. doi: 10.1016/0278-2626(92)90112-y. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan EFGH, Weintraub S. Boston Naming Test. Vol. 1983. Philadelphia: Lea & Fibiger; 1983. [Google Scholar]
- 34.Chan AS, et al. Clinical validity of the Chinese version of Mattis Dementia Rating Scale in differentiating dementia of Alzheimer’s type in Hong Kong. Journal of the International Neuropsychological Society. 2003;9(1):45–55. doi: 10.1017/s1355617703910058. [DOI] [PubMed] [Google Scholar]
- 35.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with Hhal. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 36.Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: APA; 2000. [Google Scholar]
- 37.McKhann G, et al. CLINICAL-DIAGNOSIS OF ALZHEIMERS-DISEASE - REPORT OF THE NINCDS-ADRDA WORK GROUP UNDER THE AUSPICES OF DEPARTMENT-OF-HEALTH-AND-HUMAN-SERVICES TASK-FORCE ON ALZHEIMERS-DISEASE. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 38.Roman GC, et al. VASCULAR DEMENTIA - DIAGNOSTIC-CRITERIA FOR RESEARCH STUDIES - REPORT OF THE NINDS-AIREN INTERNATIONAL WORKSHOP. Neurology. 1993;43(2):250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 39.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 40.Petersen RC, et al. Mild cognitive impairment - Clinical characterization and outcome. Archives of Neurology. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 41.Jack CR, et al. FLAIR histogram segmentation for measurement of leukoaraiosis volume. Journal of Magnetic Resonance Imaging. 2001;14(6):668–676. doi: 10.1002/jmri.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Decarli C, et al. METHOD FOR QUANTIFICATION OF BRAIN, VENTRICULAR, AND SUBARACHNOID CSF VOLUMES FROM MR IMAGES. Journal of Computer Assisted Tomography. 1992;16(2):274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 43.DeCarli C, et al. Local histogram correction of MRI spatially dependent image pixel intensity nonuniformity. Jmri-Journal of Magnetic Resonance Imaging. 1996;6(3):519–528. doi: 10.1002/jmri.1880060316. [DOI] [PubMed] [Google Scholar]
- 44.DeCarli C, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiology of Aging. 2005;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 45.DeCarli C, et al. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30(3):529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- 46.Lehtovirta M, et al. VOLUMES OF HIPPOCAMPUS, AMYGDALA AND FRONTAL-LOBE IN ALZHEIMER PATIENTS WITH DIFFERENT APOLIPOPROTEIN-E GENOTYPES. NeuroscieNce. 1995;67(1):65–72. doi: 10.1016/0306-4522(95)00014-a. [DOI] [PubMed] [Google Scholar]
- 47.Jack CR, Jr, Xu PRYC, et al. Hippocampal atrophy and apolipoprotein E genotype are ubdeoebdebtkt associated with Alzheimer’s disease. Ann Neurol. 1998;43(3):303–310. doi: 10.1002/ana.410430307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Convit A, et al. Hippocampal atrophy in early Alzheimer’s disease: anatomic specificity and validation. Psychiatr Q. 1993;64:371–387. doi: 10.1007/BF01064929. [DOI] [PubMed] [Google Scholar]
- 49.Petersen RC, et al. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54(3):581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- 50.Killiany RJ, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Annals of Neurology. 2000;47(4):430–439. [PubMed] [Google Scholar]
- 51.Brickman AM, et al. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Archives of Neurology. 2008;65(9):1202–1208. doi: 10.1001/archneur.65.9.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fox NC, Warrington EK, Rossor MN. Serial magnetic resonance imaging of cerebral atrophy in preclinical Alzheimer’s disease. Lancet. 1999;353(9170):2125–2125. doi: 10.1016/S0140-6736(99)00496-1. [DOI] [PubMed] [Google Scholar]
- 53.Cherbuin N, et al. Neuroimaging and APOE genotype: a systematic qualitative review. Dement Geriatr Cogn Disord. 2007;24(5):348–362. doi: 10.1159/000109150. [DOI] [PubMed] [Google Scholar]
- 54.Morris JC, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Archives of Neurology. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 55.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Archives of Neurology. 2005;62(7):1160–1163. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- 56.Kramer JH, Schuff N, Reed BR, et al. Hippocampal volume and retention in Alzheimer’s disease. Journal of the International Neuropsychological Society. 2004;10(4):639–643. doi: 10.1017/S1355617704104050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kohler S, et al. Memory impairments associated with hippocampal versus parahippocampal-gyrus atrophy: an MR volumetry study in Alzheimer’s disease. Neuropsychologia. 1998;36(9):901–914. doi: 10.1016/s0028-3932(98)00017-7. [DOI] [PubMed] [Google Scholar]