Abstract
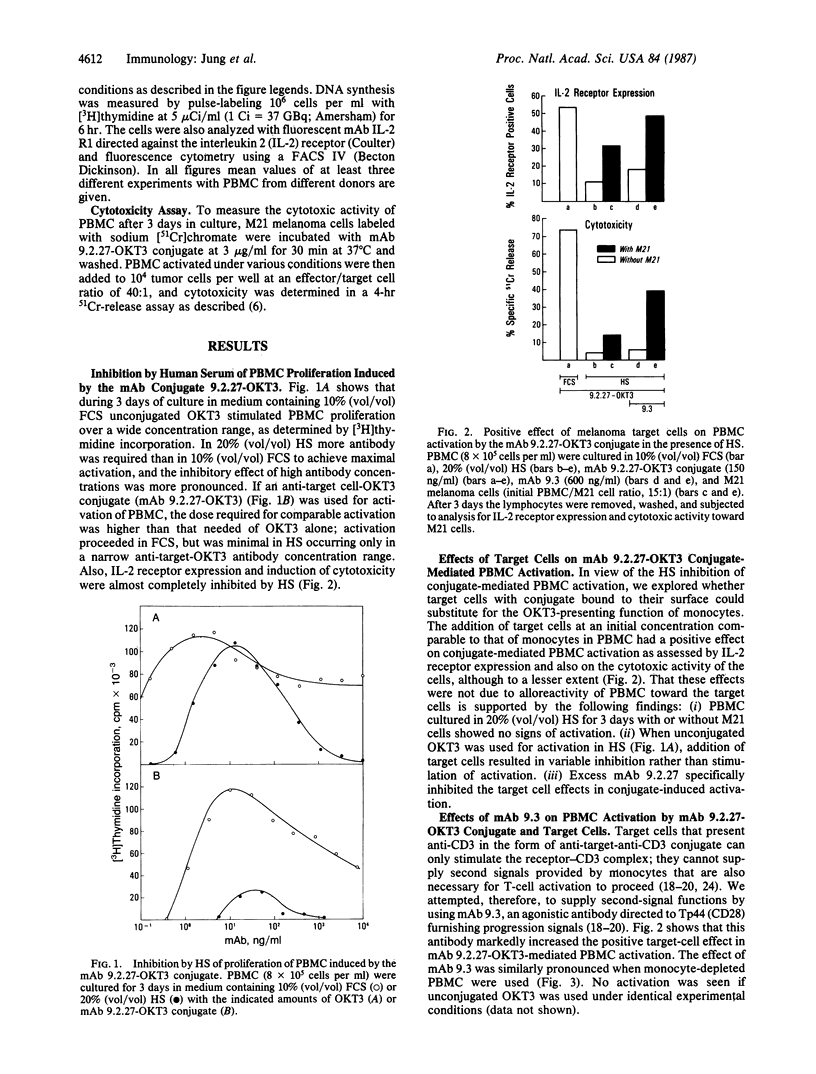
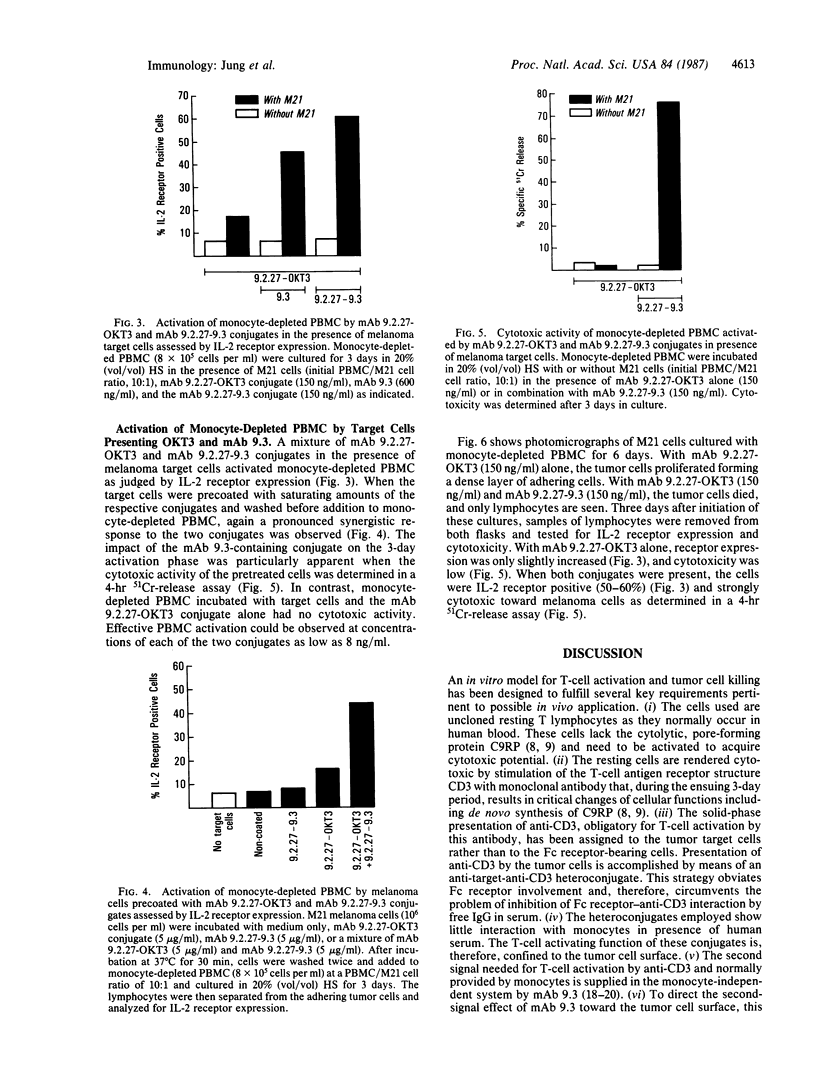
An in vitro model for peripheral human T-cell activation and resultant tumor cell killing is described. Cytotoxic T lymphocytes may be generated from resting lymphocytes by incubation of human peripheral blood mononuclear cells for 3 days with the anti-CD3 monoclonal antibody OKT3. Cytotoxicity in peripheral blood mononuclear cells can also be induced by adding an anti-target-OKT3 antibody conjugate and 10% (vol/vol) fetal calf serum to the culture medium. Conjugate activation of T cells was almost completely blocked, however, when 20% (vol/vol) human serum was added to the medium. Conjugate-mediated peripheral blood mononuclear cells activation was restored to some extent by the addition of melanoma target cells to the culture and was markedly enhanced by a second conjugate containing anti-target cell and anti-CD28 antibody. Monoclonal antibody 9.3 (anti-CD28) provides a progression signal in T-lymphocyte activation when used in combination with anti-CD3. Thus, presentation by the tumor target cells of anti-CD3 and anti-CD28 to resting human lymphocytes causes T-cell activation, which is independent of monocytes, proceeds in the presence of human serum, and results in tumor cell killing.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bumol T. F., Reisfeld R. A. Unique glycoprotein-proteoglycan complex defined by monoclonal antibody on human melanoma cells. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1245–1249. doi: 10.1073/pnas.79.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Gmünder H., Lesslauer W. A 45-kDa human T-cell membrane glycoprotein functions in the regulation of cell proliferative responses. Eur J Biochem. 1984 Jul 2;142(1):153–160. doi: 10.1111/j.1432-1033.1984.tb08263.x. [DOI] [PubMed] [Google Scholar]
- Hara T., Fu S. M., Hansen J. A. Human T cell activation. II. A new activation pathway used by a major T cell population via a disulfide-bonded dimer of a 44 kilodalton polypeptide (9.3 antigen). J Exp Med. 1985 Jun 1;161(6):1513–1524. doi: 10.1084/jem.161.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G., Honsik C. J., Reisfeld R. A., Müller-Eberhard H. J. Activation of human peripheral blood mononuclear cells by anti-T3: killing of tumor target cells coated with anti-target-anti-T3 conjugates. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4479–4483. doi: 10.1073/pnas.83.12.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegren U., Andersson J., Wigzell H. Mechanism of T lymphocyte activation by OKT3 antibodies. A general model for T cell induction. Eur J Immunol. 1984 Apr;14(4):325–328. doi: 10.1002/eji.1830140409. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Grosmaire L. S., Rabinovitch P. S. Crosslinking of surface antigens causes mobilization of intracellular ionized calcium in T lymphocytes. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1384–1388. doi: 10.1073/pnas.84.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Martin P. J., Spooner C. E., Hansen J. A., Meier K. E. Valency of CD3 binding and internalization of the CD3 cell-surface complex control T cell responses to second signals: distinction between effects on protein kinase C, cytoplasmic free calcium, and proliferation. J Immunol. 1986 Jun 1;136(11):3945–3952. [PubMed] [Google Scholar]
- Lesslauer W., Koning F., Ottenhoff T., Giphart M., Goulmy E., van Rood J. J. T90/44 (9.3 antigen). A cell surface molecule with a function in human T cell activation. Eur J Immunol. 1986 Oct;16(10):1289–1296. doi: 10.1002/eji.1830161017. [DOI] [PubMed] [Google Scholar]
- Liu M. A., Kranz D. M., Kurnick J. T., Boyle L. A., Levy R., Eisen H. N. Heteroantibody duplexes target cells for lysis by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8648–8652. doi: 10.1073/pnas.82.24.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney R. J., Abraham G. N. The Fc portion of intact IgG blocks stimulation of human PBMC by anti-T3. J Immunol. 1984 Jul;133(1):154–156. [PubMed] [Google Scholar]
- Manger B., Weiss A., Weyand C., Goronzy J., Stobo J. D. T cell activation: differences in the signals required for IL 2 production by nonactivated and activated T cells. J Immunol. 1985 Dec;135(6):3669–3673. [PubMed] [Google Scholar]
- Martin D. E., Zalman L. S., Jung G., Müller-Eberhard H. J. Induction of synthesis of the cytolytic C9 (ninth component of complement)-related protein in human peripheral mononuclear cells by monoclonal antibody OKT3 or interleukin 2: correlation with cytotoxicity and lymphocyte phenotype. Proc Natl Acad Sci U S A. 1987 May;84(9):2946–2950. doi: 10.1073/pnas.84.9.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Meyer zum Büschenfelde K. H. T cell receptor triggering induces responsiveness to interleukin 1 and interleukin 2 but does not lead to T cell proliferation. J Immunol. 1986 Jun 1;136(11):4106–4112. [PubMed] [Google Scholar]
- Pantaleo G., Olive D., Harris D., Poggi A., Moretta L., Moretta A. Signal transducing mechanisms involved in human T cell activation via surface T44 molecules. Comparison with signals transduced via the T cell receptor complex. Eur J Immunol. 1986 Dec;16(12):1639–1642. doi: 10.1002/eji.1830161228. [DOI] [PubMed] [Google Scholar]
- Perez P., Hoffman R. W., Shaw S., Bluestone J. A., Segal D. M. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature. 1985 Jul 25;316(6026):354–356. doi: 10.1038/316354a0. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Kanagawa O., Bevan M. J. Hybrid antibodies can target sites for attack by T cells. Nature. 1985 Apr 18;314(6012):628–631. doi: 10.1038/314628a0. [DOI] [PubMed] [Google Scholar]
- Thiele D. L., Kurosaka M., Lipsky P. E. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol. 1983 Nov;131(5):2282–2290. [PubMed] [Google Scholar]
- Van Wauwe J., Goossens J. Mitogenic actions of Orthoclone OKT3 on human peripheral blood lymphocytes: effects of monocytes and serum components. Int J Immunopharmacol. 1981;3(3):203–208. doi: 10.1016/0192-0561(81)90014-x. [DOI] [PubMed] [Google Scholar]
- Weiss A., Manger B., Imboden J. Synergy between the T3/antigen receptor complex and Tp44 in the activation of human T cells. J Immunol. 1986 Aug 1;137(3):819–825. [PubMed] [Google Scholar]
- Williams J. M., Deloria D., Hansen J. A., Dinarello C. A., Loertscher R., Shapiro H. M., Strom T. B. The events of primary T cell activation can be staged by use of Sepharose-bound anti-T3 (64.1) monoclonal antibody and purified interleukin 1. J Immunol. 1985 Oct;135(4):2249–2255. [PubMed] [Google Scholar]
- Zalman L. S., Brothers M. A., Chiu F. J., Müller-Eberhard H. J. Mechanism of cytotoxicity of human large granular lymphocytes: relationship of the cytotoxic lymphocyte protein to the ninth component (C9) of human complement. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5262–5266. doi: 10.1073/pnas.83.14.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalman L. S., Martin D. E., Jung G., Müller-Eberhard H. J. The cytolytic protein of human lymphocytes related to the ninth component (C9) of human complement: isolation from anti-CD3-activated peripheral blood mononuclear cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2426–2429. doi: 10.1073/pnas.84.8.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]




