Abstract
Background
There has been limited study of yoga training as a complementary exercise strategy to manage the symptom of dyspnea in patients with chronic obstructive pulmonary disease (COPD).
Purpose
The primary purpose of this pilot study was to evaluate a yoga program for its safety, feasibility, and efficacy for decreasing dyspnea intensity (DI) and dyspnea-related distress (DD) in older adults with COPD.
Methods
Clinically stable patients with COPD (n = 29; age 69.9 ± 9.5; forced expiratory volume in 1 second (FEV1) 47.7 ± 15.6% predicted; female = 21) were randomized to a 12-week yoga program specifically designed for people with COPD or usual-care control (UC). The twice-weekly yoga program included asanas (yoga postures) and visama vritti pranayama (timed breathing). Safety measure outcomes included heart rate, oxygen saturation, dyspnea, and pain. Feasibility was measured by patient-reported enjoyment, difficulty, and adherence to yoga sessions. At baseline and at 12 weeks, DI and DD were measured during incremental cycle ergometry and a 6-minute walk (6MW) test. Secondary efficacy outcomes included physical performance, psychologic well-being, and health-related quality of life (HRQoL).
Results
Yoga training was safe and feasible for patients with COPD. While yoga training had only small effects on DI after the 6MW test (effect size [ES], 0.20; p = 0.60), there were greater reductions in DD in the yoga group compared to UC (ES, 0.67; p = 0.08). Yoga training also improved 6MW distance (+71.7 ± 21.8 feet versus −27.6 ± 36.2 feet; ES = 0.78, p = 0.04) and self-reported functional performance (ES = 0.79, p = 0.04) compared to UC. There were small positive changes in muscle strength and HRQoL.
Conclusions
Elderly patients with COPD participated safely in a 12-week yoga program especially designed for patients with this chronic illness. After the program, the subjects tolerated more activity with less DD and improved their functional performance. These findings need to be confirmed in a larger, more sufficiently powered efficacy study.
Introduction
Dyspnea, or shortness of breath, is a distressing and disabling symptom commonly experienced by people who suffer from chronic pulmonary disease.1,2 A recent review of prevalence studies documented that 10%–70% of patients with terminal cancer and 90%–95% of patients with advanced chronic obstructive pulmonary disease (COPD) experience dyspnea.3 Medical and pharmacologic treatments are of limited efficacy for the relief of dyspnea in people with advanced COPD.1 Therefore, patients must rely on their own self-care strategies to manage their dyspnea on a daily basis.
Home walking and supervised endurance exercise are strategies that have been shown to reduce dyspnea intensity (DI) and dyspnea-related distress (DD).1,4,5 We have shown that a dyspnea self-management program decreased DI and DD in patients with COPD.6,7 Prompted by patients' preferences for participating in different types of exercise, we questioned if yoga training could be suggested as an alternative mode of exercise. While yoga therapy has been shown to have positive effects in healthy patients who are elderly8–10 and people with asthma,11–13 and for various symptoms such as anxiety,14 headache,15 depression16 and back pain,17,18 the evidence to support the efficacy of yoga for relieving DI and DD in patients with COPD is limited. Previous studies did not use standardized measures to assess dyspnea.19–21
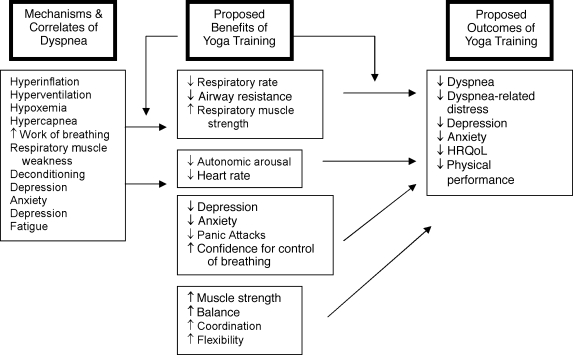
With its focus on achieving slower and deeper breathing, improved breath control, physical fitness, and improved stress management for other patients,22,23 we hypothesized that the pathophysiologic changes in COPD such as hyperinflation, increased work of breathing, and deconditioning could be improved with yoga practice (Fig. 1). Reductions of dyspnea after exercise training without physiologic changes may be attributed to patients' increased feeling of control over their breathing, a response shift in the perception of the symptom, or a decrease in anxiety, which enhances dyspnea.24–26,27 Although patients report using yoga to manage their dyspnea, the safety and feasibility of this therapy have not been studied.
FIG. 1.
Proposed relationships among dyspnea mechanisms, benefits of yoga training, and outcomes of participation in a yoga program. HRQoL, health-related quality of life.
Therefore, the primary purpose of this pilot study was to develop and evaluate a yoga program for its safety, feasibility, and efficacy for decreasing DI and DD in people with COPD. The secondary outcomes of exercise and functional performance, muscle strength, psychologic well-being, and health-related quality of life (HRQOL) were also measured.
Methods
Study design
This randomized pilot study compared the effects of a 12-week yoga training program (Yoga) with a usual-care control intervention (UC) in patients with COPD. Patients in the UC group received an educational pamphlet, “Living with COPD” (Krames Patient Education, San Bruno, CA) and were offered the yoga program at the conclusion of the 12-week period. Primary and secondary outcomes were measured at baseline and at 12 weeks. Safety and feasibility outcomes were measured at the end of each yoga session.
Patients
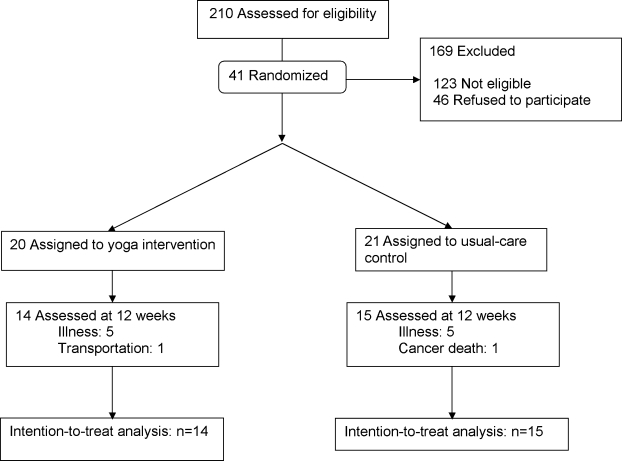
Patients with COPD were recruited between April 2004 and June 2005 from American Lung Association Better Breathers Clubs, by advertisements, and by letters and e-mail messages sent to practicing physicians. We included people who were > 40 years of age and had their activities of daily living (ADL) limited by dyspnea from clinically stable COPD.28 Patients receiving supplemental oxygen were included if their oxygen (O2) saturation could be maintained at ≥ 80% on ≤ 6 L/minute of nasal oxygen during a 6-minute walk test (6MW). Patients who had active symptomatic illness (e.g., ischemic heart disease, neuromuscular disease, and psychiatric illness) and those who had completed a pulmonary rehabilitation, yoga, or exercise training program in the last 6 months were excluded. Over 14 months, 210 individuals were screened by telephone. Forty-one (41) patients were randomly assigned to the Yoga group or to the UC group (Fig. 2). The institutional review board of the University of California, San Francisco (UCSF) approved the study protocol.
FIG. 2.
Study flow.
The yoga training program
The 12-week yoga training program for patients with COPD was developed by an invited panel of expert yoga instructors, who met for a 1-day workshop at the UCSF Osher Center for Integrative Medicine. We chose primarily Iyengar yoga techniques because of the emphasis on the use of props, the availability of Iyengar-trained yoga instructors who had experience with older chronically ill individuals, and documented positive application of Iyengar methods in the treatment of chronic disease.29 Patients were offered a total of 24 1-hour yoga sessions that consisted of yoga asanas (poses) interspersed with visama vritti pranayama (timed breathing) (Table 1).
Table 1.
Sequence of Yoga Poses During the Yoga Sessions
| Poses | Goal |
|---|---|
| Tadasana (mountain pose) | Stretch thoracic spine and use during transitions between poses. |
| Adho Mukha Savasana (half dog pose) | Stretch respiratory accessory muscles and improve flexibility in shoulder joints and thoracic spine. |
| Adho Mukha Virsana (child's pose) | Stretch lower back and increase awareness of breathing in back. |
| Trikonasana (triangle pose) | Stretch intracostal muscles and improve flexibility in the thoracic spine. |
| Bhujaganasana (cobra pose) | Stretch anterior muscles of respiration and improve flexibility in thoracic spine. |
| Bharadvajasana (simple twist) | Stretch and strengthen intracostal muscles and improve the flexibility of the vertebral column. |
| Salamba Setu Bandhasana (supported bridge pose) | Stretch anterior muscles of respiration, improve flexibility in thoracic spine, and calm the nervous system. |
| Dandasana | Release the back of legs and extend spine. |
| Baddha Konasana | Open the chest and improve flexibility of the hips. |
| Savasana with gradual inclusion of sama vritti and pranayama visama vritti (vatiations) | Relax and improve respiratory function. |
Timed breathing pranayama techniques were integrated into the yoga program to maximize the potential for breath improvement in these patients with COPD. The expert panel chose timed breathing because it is gentle, adaptable for daily life practice, and easy to modify based on the needs of the individual. In addition, the focus on prolonged exhalation may counteract the air trapping in the lungs and slow expiratory flow rates that are related to increased dyspnea in patients with COPD. Patients were led in timed breathing, with exhalation twice as long as inhalation and no inspiratory or expiratory pauses. They began with a pattern of 2-0-4-0 and worked up to 6-0-12-0 as tolerated. Patients inhaled through their nostrils, if possible, and then exhaled gently. The yoga instructors focused attention on the breath during all asanas, and all stretching movements were done during exhalation. Although most patients naturally adopted a pursed-lips breathing pattern,30–33 the yoga instructors taught pranayama techniques from a yoga perspective, with no attempt to integrate breathing retraining techniques.34
Patients were given a videotape of one yoga class and were strongly encouraged to practice daily at home. They were asked to document the number of sessions and number of minutes of home practice since the last yoga class during each yoga class session. These subjects were also asked and to describe their home practice during individual exit interviews.
Measurements
Safety and feasibility
Safety of the yoga program was assessed by measuring heart rate, oxygen saturation, pain on a 0–10 point scale, and dyspnea on a modified Borg scale35 before and after each yoga session. Feasibility was assessed by perceived difficulty of the yoga class, and level of enjoyment was rated by patients after each session, using a 0–10 point scale. The percentage of yoga sessions attended was used as a measure of adherence. Patients were asked about their satisfaction and experiences with the program during individual exit interviews, which were conducted by the principal investigator, who was not involved with the study operations.
Primary efficacy outcomes: DI and DD during laboratory exercise
Two laboratory exercises and a questionnaire were implemented to determine efficacy outcomes. In the first exercise, patients performed two 6MW approximately 30 minutes apart in a straight hospital corridor.36 The longer walk was used for analysis. DI and DD were measured before and after each 6MW with a modified Borg scale,35 using the following questions: “How short of breath are you right now?” and “How bothersome or worrisome is your shortness of breath to you right now?”
In the second exercise, patients performed a symptom-limited test on an electronically braked upright bicycle (Ergo-line Ergometrics 800, Bitz, Germany). Heart rate and rhythm were recorded continuously with a 12-lead electrocardiogram. Finger pulse oximetry was monitored continuously (Nonin Onyx 9500, Plymouth, MN). DI and DD were rated every minute using the modified Borg scale.
The questionnaire, a 5-item dyspnea subscale of the paper-and-pencil Chronic Respiratory Disease Questionnaire (CRQ), was used to measure DI with activities of daily living (ADL).37 Patients chose five activities that were most important to them and rated DI with these activities on a 7-point Likert scale.
Secondary efficacy outcomes: Physical performance, sense of well-being, and HRQL
Evaluation of physical performance included administration of a pulmonary function test in which patients performed spirometry 15–20 minutes after 2 puffs of albuterol administered via spacer (Aerochamber; Monaghan, Plattsburg, NY). Spirometry, including the percent of predicted forced expiratory volume in 1 second (FEV1% predicted), forced vital capacity (FVC), and the ratio of FEV1 and FVC (FEV1/FVC), was performed using a KOKO spirometer (Pulmonary Data Services, Louisville, CO) at baseline and at 12 weeks.38 Exercise performance was then determined by total distance walked on the longest 6MW test and watts recorded on the cycle test.36 Finally, muscle strength was evaluated by measurement of the strength of the hamstring and quadriceps muscles with isokinetic muscle testing on the Biodex System 2 (Biodex Medical Systems, Inc., Shirley, NY) with variable resistance at a constant speed. A maximum contraction test (maximum peak torque/body weight), indicating the muscle's maximum strength, and sustained (60 seconds) repeated contraction test (total work in joules), indicating muscle endurance, were performed on the right lower limb at the settings of 90°, 120°, and 180°.
Depression and anxiety were measured as indicators of psychologic well-being. The Center for Epidemiological Studies Depression Scale (CESD),39 was used to measure depressed mood, and the Spielberger State Anxiety Inventory (SSAI) was used to measure state anxiety.40
HRQoL was measured with two instruments. The Medical Outcomes Study 36-Item Short Form Health Survey (SF-36)41–44 has six scales and two composite measures of physical and mental functioning. The Chronic Respiratory Disease Questionnaire (CRQ) is a validated disease-specific health status instrument that includes 20 questions in four domains: dyspnea; fatigue; emotional function; and mastery (feeling of being in control over breathing difficulty).45
Finally, the Functional Performance Inventory, short form (S-FPI) was used to measure functional performance as a component of HRQoL.46–48 The 32-item, validated inventory has six subscales: body care; household maintenance; physical exercise; recreation; spiritual activities; and social activities, which are summed for a total score.
Statistical analysis
Descriptive statistics were used to compare baseline and post-test characteristics of the two groups. To adjust for the “work” associated with ratings of DI and DD, dyspnea slopes and indices were created. The slope expressing the linear relationship between DI, DD, and time during the cycle ergometry test was determined for each patient using data collected at the end of every stage from rest to end of exercise, (i.e., every 60 seconds). The individual slopes were averaged to determine an overall sample mean slope, DI/time or DD/time. The DI and DD indices during the 6MW were calculated by dividing the modified Borg scores at the end of the 6MW by the distance walked in feet during 6MW, and multiplying by 1000. For each dependent variable, a two-way repeated measures analysis of variance was performed with one between-subjects factor, treatment group, and one within-subjects factor, time (before and after 12 weeks). This design allowed for testing of the interaction of treatment group by time. A p-value < 0.05 was considered significant. Because this was a pilot study and all analyses were exploratory, we did not adjust for multiple comparisons. Effect sizes, calculated as 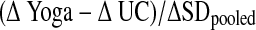 are presented. All analyses were conducted with SPSS version 12.0 (SPSS, Inc., Chicago, IL).
are presented. All analyses were conducted with SPSS version 12.0 (SPSS, Inc., Chicago, IL).
Results
Sample characteristics
Of the 41 patients who were randomized, 12 did not complete the study. Patients who dropped out had worse pulmonary function (FEV1% 37.7 ± 16.0 versus 47.7 ± 15.6; p = 0.07) and higher CESD scores (16.2 ± 5.4 versus 11.1 ± 7.5; p = 0.03) compared to those who completed the study. One patient in the UC group died of pancreatic cancer and (1) patient was unable to attend yoga classes because of transportation difficulties. The remaining 10 subjects withdrew because of illness (5 due to COPD exacerbation and 5 due to comorbid illnesses). The 29 patients in the yoga and UC groups were statistically similar across all parameters at baseline (Table 2).
Table 2.
Baseline Characteristics
| Variable | Yoga (n = 14) | UC (n = 15) |
|---|---|---|
| Gender, female/male (n/n) | 10/4 | 11/4 |
| Age, years | 72.2 ± 6.5 | 67.7 ± 11.5 |
| FEV1, L (% predicted) | 1.3 ± 0.5 (51.2 ± 10.5) | 1.0 ± 0.4 (44.4 ± 19.0) |
| FEV1/FVC % | 46.3 ± 7.8 | 43.3 ± 12.6 |
| Race, Caucasian | 10 (71%) | 13 (87%) |
| Education: | ||
| Partial college or more | 12 (86%) | 13 (87%) |
| High school or less | 2 (14%) | 2 (13%) |
| Marital status | ||
| Never married | 2 (14%) | 7 (47%) |
| Married | 4 (29%) | 3 (20%) |
| Widowed/divorced | 8 (57%) | 5 (33%) |
| Living situation | ||
| Live alone | 4 (29%) | 9 (60%) |
| With spouse or other | 10 (71%) | 6 (40%) |
| Employed | 4 (29%) | 2 (13%) |
| Uses oxygen | 4 (29%) | 1 (7%) |
| Previous rehab attendance | 8 (57%) | 5 (33%) |
| Current smoker | 2 (14%) | 3 (20%) |
UC, usual care control; FEV1, forced expiratory volume in 1 second, FVC, forced vital capacity.
Safety and feasibility
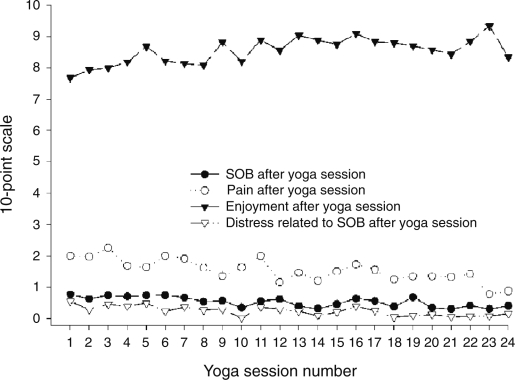
Immediately after each of the 24 yoga sessions, DI ranged from 0.31 ± 0.35 to 0.77 ± 1.04 (Fig. 3), DD ranged from 0.02 ± 0.1 to 0.55 ± 1.1, and pain ratings ranged from 0.78 ± 0.88 to 2.26 ± 2.23 on a 0–10 point scale. Heart rate decreased by 2–6 beats per minute from the beginning to the end of the yoga session and ranged from 79 ± 12 to 88 ± 14. There were no adverse clinical events associated with the yoga training. Enjoyment ratings ranged from 7.68 ± 2.45 to 9.33 ± 1.37, and perceived difficulty of the yoga practice ranged from 0.94 ± 1.0 to 2.97 ± 2.37. Patients attended an average of 20 sessions (range: 17–23) out of 24 sessions. Thirteen (13) of 14 (93%) yoga participants reported that they practiced the yoga program at least some of the time at home, and 4 (29%) practiced at least 5 times per week at home.
FIG. 3.
Yoga session safety and feasibility measures during 24 yoga class sessions. The 10-point scale used was a numerical rating scale.
A majority of patients (77%) reported that the yoga program was beneficial and that their expectations were met or exceeded. The most frequently reported benefits were learning a new strategy for managing dyspnea and increasing the ability to perform ADL. Other reported benefits included improved breathing techniques and bronchial drainage; improved postures, relaxation, and stress reduction; improved feelings of well-being; enjoyable social interactions; pain relief; and increased awareness of breathing.
Measurements
Primary efficacy outcomes: DI and DD during laboratory exercise
In terms of dyspnea intensity, there were no significant differences between the groups at the end of the 6MW, as an index of DI/feet during the 6MW, or during and at the end of the cycle ergometer test (Table 3).
Table 3.
Primary Outcomes: Dyspnea Intensity and Dyspnea-Related Distress
| |
|
Means ± SD |
|||
|---|---|---|---|---|---|
| Time | Yoga (n = 14) | UC (n = 15) | p-Value | Effect size | |
| Six-minute alk (6MW) | |||||
| DI end | Baseline | 3.8 ± 2.3 | 2.9 ± 1.6 | ||
| 3 mo | 3.8 ± 1.5 | 3.3 ± 2.5 | 0.6 | 0.2 | |
| DD end | Baseline | 2.6 ± 2.8 | 1.1 ± 1.1 | ||
| 3 mo | 1.6 ± 1.7 | 1.4 ± 1.5 | 0.08 | 0.67 | |
| DI Index | Baseline | 3.0 ± 2.0 | 2.0 ± 1.2 | ||
| 3 mo | 3.1 ± 2.6 | 2.3 ± 1.7 | 0.77 | 0.11 | |
| DD Index | Baseline | 2.1 ± 2.4 | 0.8 ± 0.7 | ||
| 3 mo | 1.5 ± 2.3 | 0.2 ± 1.0 | 0.07 | 0.71 | |
| Incremental cycle ergometry | |||||
| DI end | Baseline | 4.5 ± 2.5 | 3.6 ± 2.3 | ||
| 3 mo | 4.4 ± 2.4 | 3.8 ± 1.9 | 0.55 | 0.23 | |
| DD end | Baseline | 3.6 ± 3.3 | 2.8 ± 2.9 | ||
| 3 mo | 3.3 ± 3.2 | 2.8 ± 2.5 | 0.46 | 0.28 | |
| DI/time | Baseline | 0.94 ± 0.34 | 0.90 ± 0.49 | ||
| 3 mo | 0.95 ± 0.52 | 1.0 ± 0.53 | 0.2 | 0.56 | |
| DD/time | Baseline | 0.76 ± 0.52 | 0.73 ± 0.70 | ||
| 3 mo | 0.70 ± 0.76 | 0.82 ± 0.76 | 0.44 | 0.30 | |
| CRQ | |||||
| Dyspneaa | Baseline | 14.6 ± 3.0 | 12.9 ± 6.1 | ||
| 3 mo | 16.8 ± 6.3 | 15.2 ± 6.6 | 0.96 | 0.07 | |
Yoga (n = 5); UC (n = 9).
SD, standard deviation; DI; dyspnea intensity; DD; dyspnea-related distress; DI end; DI at the end of the 6MW; DD end; DD at the end of the 6MW; DI and DD index; DI or DD end divided by 6MW distance in feet X 1000; DI/time or DD/time; DI or DD divided by the number of minutes on bicycle ergometer; CRQ; Chronic Respiratory Disease Questionnaire.
Dyspnea-related distress was decreased at the end of the 6MW by 0.9 ± 0.2 in the Yoga group, compared with an increase of 0.3 ± 1.3 in the UC group (p = 0.08). The DD/feet index showed similar differences between groups (p = 0.07). There were no significant differences in DD during or at the end of the cycle ergometer test.
Results from the questionnaire evaluating dyspnea with ADL were affected by measurement errors. Data for 15 patients on the CRQ dyspnea subscale were missing. This occurred primarily as a result of a change from an interview format to a self-administered questionnaire. For the patients with valid data (n = 14), both groups showed improvement, but there were no significant differences between the groups.
Secondary efficacy outcomes: Physical performance, sense of well-being, and HRQL. There were no significant differences in the changes in pulmonary function (FEV1 and FVC) between the two groups. The Yoga patients had significantly greater improvements in distance covered on the 6MW test compared to the UC group (p = 0.04) (Table 4). The Yoga training program resulted in better performance time on the cycle ergometer test, while the UC group performance was unchanged; however, these changes were not significantly different between the groups (p = 0.59). Yoga training only had small-to-medium effects on quadriceps and hamstring strength.
Table 4.
Secondary Physiologic Outcomes
|
Means ± SD | |||||
|---|---|---|---|---|---|
| Time | Yoga (n = 14) | UC (n = 15) | p-Value | Effect size | |
| 6MW distance (feet) | Baseline | 1387.9 ± 408.2 | 1511.0 ± 199.6 | ||
| 3 mo | 1452.9 ± 406.3 | 1483.4 ± 229.0 | 0.04 | 0.78 | |
| Incremental cycle ergometry | |||||
| Duration (watts) | Baseline | 85 ± 29 | 72 ± 19 | ||
| 3 mo | 87 ± 30 | 72 ± 18 | 0.61 | 0.19 | |
| Spirometry | |||||
| FEV1 % predicted | Baseline | 51.2 ± 10.5 | 44.4 ± 19.0 | ||
| 3 mo | 51.2 ± 10.6 | 45.9 ± 20.2 | 0.49 | 0.25 | |
| FEV1/FVC | Baseline | 0.46 ± 0.08 | 0.43 ± 0.13 | ||
| 3 mo | 0.45 ± 0.06 | 0.44 ± 0.12 | 0.43 | 0.3 | |
| Muscle strengtha | |||||
| Hamstring strength | Baseline | 22.9 ± 8.8 | 27.21 ± 6.2 | ||
| (flexion tq/bw 90) | 3 mo | 25.8 ± 9.3 | 28.2 ± 8.5 | 0.29 | 0.41 |
| Quads strength | Baseline | 33.1 ± 9.7 | 36.1 ± 12.1 | ||
| (extension tq/bw 90) | 3 mo | 36.4 ± 9.9 | 38.9 ± 12.2 | 0.8 | 0.1 |
| Hamstring strength | Baseline | 20.3 ± 8.6 | 23.8 ± 5.1 | ||
| (flexion tq/bw 180) | 3 mo | 22.5 ± 7.8 | 24.0 ± 7.1 | 0.19 | 0.52 |
| Quads strength | Baseline | 26.7 ± 10.3 | 26.8 ± 8.5 | ||
| (extension tq/bw 180) | 3 mo | 25.9 ± 6.9 | 27.7 ± 9.3 | 0.42 | 0.31 |
Yoga (n = 13); UC (n = 19).
SD, standard deviation; 6MW; six-minute walk; FEV1% predicted; forced expiratory volume in 1 second, percent predicted; FEV1/FVC; ratio of forced expiratory volume in 1 second to forced vital capacity; tq/bw; torque divided by body weight.
Similarly, there were no significant changes within or differences between the Yoga and UC groups in depressive symptoms, state anxiety, or general or disease-specific HRQoL (Table 5). Improvements in self-reported functional performance were modestly greater for the Yoga group compared to the UC group (p = 0.04).
Table 5.
Secondary Outcomes
| |
|
Means ± SD |
|
|
|
|---|---|---|---|---|---|
| Time | Yoga (n = 14) | UC (n = 15) | p-value | Effect size | |
| CRQ | |||||
| Fatigue | Baseline | 18.0 ± 4.4 | 16.0 ± 3.7 | ||
| 3 months | 16.8 ± 5.1 | 16.3 ± 5.2 | 0.34 | 0.36 | |
| Emotional | Baseline | 35.7 ± 5.0 | 34.1 ± 6.3 | ||
| 3 months | 35.4 ± 5.9 | 35.1 ± 6.2 | 0.28 | 0.41 | |
| Mastery | Baseline | 22.4 ± 3.5 | 19.5 ± 5.0 | ||
| 3 months | 22.3 ± 4.0 | 19.4 ± 5.3 | 0.85 | 0.07 | |
| SF-36 | |||||
| Physical componenta | Baseline | 36.8 ± 10.4 | 38.6 ± 8.4 | ||
| 3 months | 35.4 ± 9.7 | 36.8 ± 8.8 | 0.87 | 0.06 | |
| Mental componenta | Baseline | 54.2 ± 6.1 | 51.5 ± 9.3 | ||
| 3 months | 54.8 ± 8.0 | 52.3 ± 9.6 | 0.93 | 0.03 | |
| FPI total | Baseline | 2.0 ± 0.5 | 2.1 ± 0.5 | ||
| 3 months | 2.2 ± 0.4 | 2.1 ± 0.5 | 0.04 | 0.79 | |
| CESD | Baseline | 9.5 ± 4.5 | 12.6 ± 9.4 | ||
| 3 months | 9.8 ± 7.0 | 11.4 ± 6.0 | 0.48 | 0.27 | |
| SSAI | Baseline | 30.2 ± 8.0 | 33.8 ± 9.0 | ||
| 3 months | 31.0 ± 8.8 | 32.2 ± 9.1 | 0.51 | 0.39 | |
UC (n = 14).
SD, standard deviation; CRQ, Chronic Respiratory Questionnaire; SF-36, Medical Outcomes Study Short-Form 36; FPI, Functional Performance Inventory; CESD, Centers for Epidemiologic Studies of Depression; SSAI, State Anxiety Inventory; UC, usual-care control group.
Discussion
The major findings of this pilot study were that this 12-week yoga program was safe, feasible, and enjoyable for older adults with COPD. In addition, patients who participated in the program improved their exercise performance and self-reported functional performance and decreased their DD more than subjects who received educational pamphlets on COPD. Although the minimal clinically important difference (MCID) has not yet been established for DD measured on the modified Borg scale, the MCID for DI is one point.49 Using this criterion as a proxy, the improvement in DD experienced after participation in the yoga intervention would be considered clinically significant.
DI and pulmonary function did not change; however, the ability of these patients to walk longer without feeling as bothered by dyspnea may indicate an improvement in their perceived ability to control their dyspnea during exercise. This was a pilot feasibility study, not specifically powered to detect changes even in the primary outcome of dyspnea. Therefore, it must be acknowledged that these positive findings may be due to chance, given the small sample, the multiple comparisons, and very modest changes in the secondary outcomes.
Previous studies of yoga in patients with COPD had a number of methodological limitations, which makes comparisons with our study challenging. One study used a single-group design19 whereas the other two used a randomized design.20,21 But because the authors only reported within-group changes in the latter two studies, it is not known if there were statistically significant group differences in pulmonary function and other measured outcomes. We did not find positive changes in maximum work tolerance on a cycle ergometer test, as was reported earlier by Tandon.21 Differences in the sample characteristics, testing protocol, and yoga program may partially account for our negative findings. Unlike our study, none of the published reports included an attention-control group. Without a true attention-control group, one cannot rule out whether the small positive changes in DD were due solely to the participants' being in a supportive group environment or to the yoga training itself.50
The lack of significant differences between the groups in other physiologic and psychologic outcomes is not clear. It is possible that the “dose” of the yoga program may not have been strong enough to bring about clinically significant changes in the measured outcomes. Although we added home practice to increase the intervention dose, patients reported difficulty with performing the yoga independently. Similarly, the yoga style and method may be inadequate to effect changes in physiologic and psychologic outcomes that are important to this clinical population. Other yoga traditions integrate breathing practice with asanas much earlier in the beginner's practice of yoga,51 whereas Iyengar yoga considers pranayama as an advanced technique to be practiced only after asanas are mastered.22, 52
We measured anxiety and depressive symptoms with standardized instruments and did not find improvements with yoga training. A recent systematic review of the effects of yoga on anxiety found that the literature was of poor quality and the effect on anxiety was equivocal.14 Perhaps measurement of more proximal outcomes to yoga practice such as mindfulness53–56 or stress reduction via galvanic skin response will be more promising.57
Limitations of this pilot study include a suboptimal intervention dose and selection of yoga poses/style, the small sample size, the lack of a control for the group effect of the yoga sessions, and the possible lack of sensitive measures of physiologic and psychologic changes that may have occurred in this sample of patients with COPD. Future studies will need to address these methodological weaknesses as well as considering the addition of COPD self-management education and skills training to enhance the effects of the yoga program.4,6,58 Our study has several notable strengths including the use of an expert panel of yoga teachers to design a program specifically for patients with COPD, a randomized controlled design, a study sample that is gender balanced, expert yoga teachers with experience in a Western medical setting, and the measurement of safety variables and symptom distress.
Conclusions
In conclusion, this yoga program was safe and not dangerous, it improved functional performance and decreased DD during exercise while not increasing pain or dyspnea.
Acknowledgments
The yoga program was given at the UCSF Osher Center for Integrative Medicine in San Francisco, CA. The authors would like to acknowledge Bradly Jacobs, M.D., for his knowledgeable thoughts and assistance throughout the development and conduct of the study; the teachers of the yoga classes, Jillian Chelson, P.A., Bonnie Maeda, R.N., and Cara Judea Alhadeff, B.A., for providing individualized and continual support for the participants; and the invited members of the expert panel, especially Judith Lasater, Ph.D., who offered critical and thoughtful recommendations in the initial development of the program. Other panel members included Barbara Benagh, (www.yogastudio.org) Uday Kiran Chaka, B.Tech., Nancy C. Field (patient representative), Suza Francina, R.Y.T., Mamatha Krishnabhat, Matra Majmundar, O.T.R., T.Y.C., C.C.E., and P.K. Vedanthan, M.D.
Disclosure Statement
This study was funded by grant No. R21 AT01168–03 from the National Center for Complementary and Alternative Medicine, National Institutes of Health (NIH), and grant No. 1KL2RR025015–01 from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH. Information on NCRR is available at www.ncrr.nih.gov/. This study was carried out in part in the General Clinical Research Center, Moffitt Hospital, University of California, San Francisco, with funds provided to that institution by the NCRR under grant No. 5 M01 RR-00079 from the U.S. Public Health Service.
References
- 1.American Thoracic Society. Dyspnea: Mechanisms, assessment, and management: A consensus statement. Am J Respir Crit Care Med. 1999;159:321–340. doi: 10.1164/ajrccm.159.1.ats898. [DOI] [PubMed] [Google Scholar]
- 2.Lorenz K. Lynn J. Morton S. Dy S. End-of-Life Care and Outcomes: Summary, Evidence Report/Technology Assessment: Number 110. Rockville, MD: AHRQ Publication Number 05-E004–1, Agency for Healthcare Research and Quality; 2004. [Dec 8;2008 ]. [Google Scholar]
- 3.Solano JP. Gomes B. Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symp Manage. 2006;31:58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Nici L. Donner C. Wouters E, et al. American Thoracic Society/European Respiratory Society Statement on Pulmonary Rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–1413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 5.Troosters T. Casaburi R. Gosselink R. Decramer M. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:19–38. doi: 10.1164/rccm.200408-1109SO. [DOI] [PubMed] [Google Scholar]
- 6.Carrieri-Kohlman V. Nguyen HQ. Donesky-Cuenco D, et al. Impact of brief or extended exercise training on the benefit of a dyspnea self-management program in COPD. J Cardiopulm Rehabil. 2005;25:275–284. doi: 10.1097/00008483-200509000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen HQ. Donesky-Cuenco D. Wolpin S, et al. Randomized controlled trial of an internet-based versus face-to-face dyspnea self-management program for patients with chronic obstructive pulmonary disease: Pilot study. J Med Internet Res. 2008;10:e9. doi: 10.2196/jmir.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bijlani RL. Vempati RP. Yadav RK, et al. A brief but comprehensive lifestyle education program based on yoga reduces risk factors for cardiovascular disease and diabetes mellitus. J Altern Complement Med. 2005;11:267–274. doi: 10.1089/acm.2005.11.267. [DOI] [PubMed] [Google Scholar]
- 9.DiBenedetto M. Innes KE. Taylor AG, et al. Effect of a gentle Iyengar yoga program on gait in the elderly: An exploratory study. Arch Phys Med Rehabil. 2005;86:1830–1837. doi: 10.1016/j.apmr.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Oken BS. Zajdel D. Kishiyama S, et al. Randomized, controlled, six-month trial of yoga in healthy seniors: Effects on cognition and quality of life. Altern Ther Health Med. 2006;12:40–47. [PMC free article] [PubMed] [Google Scholar]
- 11.Manocha R. Marks GB. Kenchington P, et al. Sahaja yoga in the management of moderate to severe asthma: A randomised controlled trial. Thorax. 2002;57:110–115. doi: 10.1136/thorax.57.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabina AB. Williams AL. Wall HK, et al. Yoga intervention for adults with mild-to-moderate asthma: A pilot study. Ann Allergy Asthma Immun. 2005;94:543–548. doi: 10.1016/s1081-1206(10)61131-3. [DOI] [PubMed] [Google Scholar]
- 13.Vedanthan PK. Kesavalu LN. Murthy KC, et al. Clinical study of yoga techniques in university students with asthma: A controlled study. Allergy Asthma Proc. 1998;19:3–9. doi: 10.2500/108854198778557971. [DOI] [PubMed] [Google Scholar]
- 14.Kirkwood G. Rampes H. Tuffrey V, et al. Yoga for anxiety: A systematic review of the research evidence. Br J Sports Med. 2005;39:884–891. doi: 10.1136/bjsm.2005.018069. discussion 891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John PJ. Sharma N. Sharma CM. Kankane A. Effectiveness of yoga therapy in the treatment of migraine without aura: A randomized controlled trial. Headache. 2007;47:654–661. doi: 10.1111/j.1526-4610.2007.00789.x. [DOI] [PubMed] [Google Scholar]
- 16.Pilkington K. Kirkwood G. Rampes H. Richardson J. Yoga for depression: The research evidence. J Affect Disord. 2005;89:13–24. doi: 10.1016/j.jad.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs BP. Mehling W. Avins AL, et al. Feasibility of conducting a clinical trial on Hatha yoga for chronic low back pain: Methodological lessons. Altern Ther Health Med. 2004;10:80–83. [PubMed] [Google Scholar]
- 18.Sherman KJ. Cherkin DC. Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: A randomized, controlled trial. Ann Intern Med. 2005;143:849–856. doi: 10.7326/0003-4819-143-12-200512200-00003. [DOI] [PubMed] [Google Scholar]
- 19.Behera D. Yoga therapy in chronic bronchitis. J Assoc Physicians Ind. 1998;46:207–208. [PubMed] [Google Scholar]
- 20.Kulpati DD. Kamath RK. Chauhan MR. The influence of physical conditioning by yoga asanas and breathing exercises in patients [with] chronic obstructive lung disease. J Assoc Physicians India. 1982;30:865–868. [PubMed] [Google Scholar]
- 21.Tandon MK. Adjunct treatment with yoga in chronic severe airways obstruction. Thorax. 1978;33:514–517. doi: 10.1136/thx.33.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lasater JH. Relax and Renew: Restful Yoga for Stressful Times. Berkeley, CA: Rodmell Press; 1995. [Google Scholar]
- 23.Raman K. A matter of health: Integration of Yoga and Western Medicine for Prevention and Cure. Chennai, India: East-west Books; 1998. [Google Scholar]
- 24.Carrieri Kohlman V. Coping and self-management strategies for dyspnea. In: Mahler D, editor; O'Donnell DE, editor. Dyspnea: Mechanisms, Measurement and Management. Vol. 208. Boca Raton, FL: Taylor & Francis; 2005. pp. 365–396. [Google Scholar]
- 25.Parshall MB. Welsh JD. Brockopp DY, et al. Dyspnea duration, distress, and intensity in emergency department visits for heart failure. Heart Lung. 2001;30:47–56. doi: 10.1067/mhl.2001.112492. [DOI] [PubMed] [Google Scholar]
- 26.Wilson IB. Clinical understanding and clinical implications of response shift. In: Schwartz CE, editor; Sprangers MAG, editor. Adaptation to Changing Health: Response Shift in Quality-of-Life Research. Washington DC: American Psychological Association; 2000. pp. 159–174. [Google Scholar]
- 27.Carrieri-Kohlman V. Gormley JM. Douglas MK, et al. Exercise training decreases dyspnea and the distress and anxiety associated with it: Monitoring alone may be as effective as coaching. Chest. 1996;110:1526–1535. doi: 10.1378/chest.110.6.1526. [DOI] [PubMed] [Google Scholar]
- 28.Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) www.goldcopd.org. [Dec 12;2008 ]. www.goldcopd.org
- 29.Iyengar BKS. Yoga: The Path to Holistic Health. New York: DK Publishing; 2001. [Google Scholar]
- 30.Garrod R. Dallimore K. Cook J, et al. An evaluation of the acute impact of pursed lips breathing on walking distance in nonspontaneous pursed lips breathing chronic obstructive pulmonary disease patients. Chron Respir Dis. 2005;2:67–72. doi: 10.1191/1479972305cd068oa. [DOI] [PubMed] [Google Scholar]
- 31.Nield MA. Soo Hoo GW. Roper JM. Santiago S. Efficacy of pursed-lips breathing: A breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil Prev. 2007;27:237–244. doi: 10.1097/01.HCR.0000281770.82652.cb. [DOI] [PubMed] [Google Scholar]
- 32.Spahija J. de Marchie M. Grassino A. Effects of imposed pursed-lips breathing on respiratory mechanics and dyspnea at rest and during exercise in COPD. Chest. 2005;128:640–650. doi: 10.1378/chest.128.2.640. [DOI] [PubMed] [Google Scholar]
- 33.Tiep BL. Burns M. Kao D, et al. Pursed lips breathing training using ear oximetry. Chest. 1986;90:218–221. doi: 10.1378/chest.90.2.218. [DOI] [PubMed] [Google Scholar]
- 34.Gosselink R. Breathing techniques in patients with chronic obstructive pulmonary disease (COPD) Chron Respir Dis. 2004;1:163–172. doi: 10.1191/1479972304cd020rs. [DOI] [PubMed] [Google Scholar]
- 35.Burdon JG. Juniper EF. Killian KJ, et al. The perception of breathlessness in asthma. Am Rev Respir Dis. 1982;126:825–828. doi: 10.1164/arrd.1982.126.5.825. [DOI] [PubMed] [Google Scholar]
- 36.American Thoracic Society. ATS Statement: Guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 37.Schunemann HJ. Goldstein R. Mador MJ, et al. A randomised trial to evaluate the self-administered standardised chronic respiratory questionnaire. Eur Respir J. 2005;25:31–40. doi: 10.1183/09031936.04.00029704. [DOI] [PubMed] [Google Scholar]
- 38.Miller MR. Hankinson J. Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 39.Eaton WW. Kessler LG. Rates of symptoms of depression in a national sample. Am J Epidemiol. 1981;114:528–538. doi: 10.1093/oxfordjournals.aje.a113218. [DOI] [PubMed] [Google Scholar]
- 40.Spielberger L. STAI Manual. Palo Alto: Psychologists Consultants Press; 1983. [Google Scholar]
- 41.Benzo R. Flume PA. Turner D. Tempest M. Effect of pulmonary rehabilitation on quality of life in patients with COPD: The use of SF-36 summary scores as outcomes measures. J Cardiopulm Rehabil. 2000;20:231–234. doi: 10.1097/00008483-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Jones PW. Bosh TK. Quality of life changes in COPD patients treated with salmeterol. Am J Respir Crit Care Med. 1997;155:1283–1289. doi: 10.1164/ajrccm.155.4.9105068. [DOI] [PubMed] [Google Scholar]
- 43.Mahler DA. Mackowiak JI. Evaluation of the Short-Form 36-item questionnaire to measure health-related quality of life in patients with COPD. Chest. 1995;107:1585–1589. doi: 10.1378/chest.107.6.1585. [DOI] [PubMed] [Google Scholar]
- 44.Mahler DA. Tomlinson D. Olmstead EM, et al. Changes in dyspnea, health status, and lung function in chronic airway disease. Am J Respir Crit Care Med. 1995;151:61–65. doi: 10.1164/ajrccm.151.1.7812573. [DOI] [PubMed] [Google Scholar]
- 45.Guyatt GH. Townsend M. Berman LB. Pugsley SO. Quality of life in patients with chronic airflow limitation. Br J Dis Chest. 1987;81:45–54. doi: 10.1016/0007-0971(87)90107-0. [DOI] [PubMed] [Google Scholar]
- 46.Larson JL. Kapella MC. Wirtz S, et al. Reliability and validity of the Functional Performance Inventory in patients with moderate to severe chronic obstructive pulmonary disease. J Nurs Meas. 1998;6:55–73. [PubMed] [Google Scholar]
- 47.Leidy NK. Psychometric properties of the functional performance inventory in patients with chronic obstructive pulmonary disease. Nurs Res. 1999;48:20–28. doi: 10.1097/00006199-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Leidy NK. Haase JE. Functional status from the patient's perspective: The challenge of preserving personal integrity. Res Nurs Health. 1999;22:67–77. doi: 10.1002/(sici)1098-240x(199902)22:1<67::aid-nur8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 49.Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and Visual Analog Scale. COPD. 2005;2:105–110. doi: 10.1081/copd-200050655. [DOI] [PubMed] [Google Scholar]
- 50.Spiegel D. Butler LD. Giese-Davis J, et al. Effects of supportive-expressive group therapy on survival of patients with metastatic breast cancer: A randomized prospective trial. Cancer. 2007;110:1130–1138. doi: 10.1002/cncr.22890. [DOI] [PubMed] [Google Scholar]
- 51.Kraftsow G. Yoga for Transformation: Ancient Teachings and Holistic Practices for Healing Body, Mind, and Heart. New York: Penguin Compass; 2002. [Google Scholar]
- 52.Francina S. The New Yoga for People Over 50. Deerfield Beach, FL: Health Communications; 1997. [Google Scholar]
- 53.Astin JA. Stress reduction through mindfulness meditation. Effects on psychological symptomatology, sense of control, and spiritual experiences. Psychother Psychosom. 1997;66:97–106. doi: 10.1159/000289116. [DOI] [PubMed] [Google Scholar]
- 54.Baer RA. Smith GT. Allen KB. Assessment of mindfulness by self-report: The Kentucky inventory of mindfulness skills. Assessment. 2004;11:191–206. doi: 10.1177/1073191104268029. [DOI] [PubMed] [Google Scholar]
- 55.Bishop SR. What do we really know about mindfulness-based stress reduction? Psychosom Med. 2002;64:71–83. doi: 10.1097/00006842-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Speca M. Carlson LE. Goodey E. Angen M. A randomized, wait-list controlled clinical trial: The effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosom Med. 2000;62:613–622. doi: 10.1097/00006842-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Bonnet A. Naveteur J. Electrodermal responses to words in chronic low back pain patients: A comparison between pain descriptors, other emotional words, and neutral words. Clin J Pain. 2006;22:686–691. doi: 10.1097/01.ajp.0000210933.66063.ec. [DOI] [PubMed] [Google Scholar]
- 58.American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) Guidelines for Pulmonary Rehabilitation Programs. Champaign, IL: Human Kinetics; 2004. [Google Scholar]