Abstract
Objective
The goal of this work was to assess electrophysiologic response changes to acoustic stimuli as an intracochlear electrode impacted cochlear structures in an animal model of hearing preservation cochlear implantation. The ultimate goal is to develop efficient procedures for assessing the status of cochlear physiology for intraoperative use.
Methods
Sixteen gerbils and 18 ears were tested. A rigid electrode was inserted through a basal turn cochleostomy and directed towards the basilar membrane/osseous spiral lamina complex. We recorded acoustically evoked early auditory potentials including cochlear microphonics (CMs) and compound action potentials (CAPs) to a short stimulation sequence consisting of one stimulus frequency and intensity as the electrode was advanced. A microendoscope was used to visualize the electrode insertion progress and to identify the site of electrode impact. After each experiment, the site of intracochlear trauma was confirmed using whole mount preparations.
Results
Electrophysiologic changes correlated well with the degree and location of trauma. We observed four distinct patterns. In addition, the endoscope in conjunction with the short recording sequence allowed for the detection of response changes that were reversible when the electrode was retracted. These cases were associated with less than full-thickness damage on histology.
Conclusions
The short recording sequence to obtain acoustically evoked intracochlear potentials and the microendoscope allowed us to detect various levels of cochlear trauma including minor and reversible damage. Recordings of this type are potentially available using current implant technology. Future improvements in the measurements can be expected to improve the efficiency of the recording paradigm to produce a clinically useful tool.
Keywords: cochlear implant, electrophysiology, micro-endoscope, hearing preservation, residual hearing
INTRODUCTION
Severely hearing impaired individuals can obtain marked improvements in speech understanding using a cochlear implant. Recent research has shown that individuals perform especially well if their residual acoustic hearing remains intact when combined with the cochlear implant in the same ear1-3. In these cases, the combination of electric and acoustic hearing, also termed electric-acoustic stimulation (EAS)1,4,5,6, has been shown to improve speech discrimination scores. These improved scores are especially evident in the setting of background noise - a weakness of conventional cochlear implantation2,7.
Given the potential to improve outcomes, recent research has focused on optimizing the surgical procedure in order to improve the likelihood of hearing preservation8,9. The performance benefit of EAS over electrical stimulation alone warrants the additional time needed to employ the modified surgical technique. However, despite surgeons’ best efforts, hearing is lost or partially compromised in approximately 50% of patients5,9,10. In addition, intracochlear trauma during electrode implantation appears to be an important contributing factor in the success of traditional implantations. Specifically, electrode dislocations into scala vestibuli result in decreased cochlear implant performance compared to patients with electrodes located in scala tympani11,12. This interest in electrode induced trauma lead to temporal bone studies that described the morphological effects of electrode implantation and subsequently lead to improved cochleostomy placement8,13-17. In addition, significant advances have been made with the development of less-traumatic electrodes. The downside, unfortunately, is that these “short electrodes” that are used for EAS have an arbitrarily determined length that may understimulate some patients while compromising residual hearing in others.
Despite these advances, the exact factors contributing to the success or failure of hearing preservation remain enigmatic. Consequently, the ability to monitor the state of cochlear physiology during implantation could potentially signal cochlear trauma as well as identify an optimal site for implantation. Technology to provide such monitoring is present in the current generation of cochlear implants, and is expected to improve in future devices. However, information about cochlear physiology during implantation is limited. Consequently, our group has developed an animal model to identify electrophysiological markers of cochlear damage. Previous data showed that upon electrode impact with cochlear structures small changes in cochlear potentials could be detected across a wide range of frequencies and intensities, rather than restricted to threshold effects18. These results imply that a highly reduced stimulus set should be able to detect damage across a wide array of cochlear length. Such a reduced stimulus could provide an efficient means of assessing impacts and their effects during implantation. Thus, in an effort to develop more clinically relevant methods, the goal for this set of experiments was to use a highly reduced stimulus set and evaluate the ability to detect intracochlear damage. As an aide to determine electrode position relative to cochlear structures, microendoscope was used to directly visualize the electrode within the basal turn of the cochlea19.
METHODS
The gerbil was chosen as the animal model because of its sensitive low frequency hearing and easily accessible cochlea. Eighteen cochleae from 16 gerbils were included. All animals were handled and housed according to the standards described by the National Institutes of Health Committee on Care and Use of Laboratory Animals, using protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the study institution.
Animal handling
Surgeries and recordings were performed under deep urethane anesthesia (25% solution in saline, 1.5 g/kg, i.p.). Once anesthesia was induced, the animal was moved to a double-walled, sound attenuated booth. The core body temperature was monitored with a rectal probe and maintained around 37°C with heating pads. Heart rate was monitored with electrodes placed on the chest and back.
Endoscope
In order to visually assess electrode position within the scala tympani of the basal cochlear turn, a microendoscope was utilized to look through the round window as previously described19. The microendoscope is a flexible, custom built device with a 0.4-mm diameter (including a light channel, Zibracorp, Westport, MA, USA). It houses 3,000 fibers, has a 1-mm depth of focus, a 50° field of view (in saline) and ~50x magnification. It is attached to an adjustable light source (Welch Allyn, Skaneateles Falls, NY, USA) and a television camera (ELMO, Plainview, NY, USA), which subsequently feeds to a computer monitor.
Surgical Procedure
The bony bulla was exposed and opened via a standard postauricular skin incision. The pinna was removed to allow for consistent sound delivery. The round window niche, the stapedial artery, and all other structures of the bulla were identified. The microendoscope, which is adjusted via a micromanipulator, was placed against an intact round window membrane.
Since the round window niche was occupied, a cochleostomy was made in the basal turn of the cochlea adjacent to the stapedial artery using a perforator used for stapes surgery. A recording electrode attached to a hydraulic micromanipulator was advanced into the cochlea through the cochleostomy (Figure 1).
Figure 1.
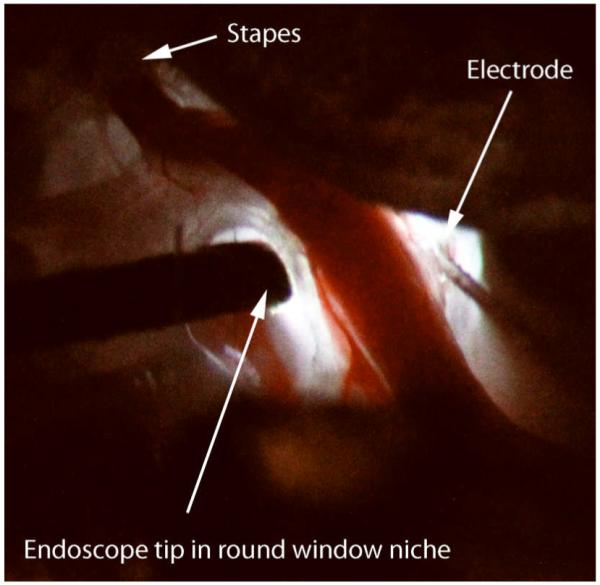
Surgical exposure of the cochlea in the right ear of the gerbil using the endoscope as the only light source. To the left of the spiral modiolar artery, the endoscope can be seen entering the round window niche. To the right and adjacent to the stapedial artery, the cochleostomy can be seen with the electrode entering it. The trajectory of insertion is directed at the BM and/or OSL.
Acoustic Stimulation & Calibration
The stimuli used were 4 kHz tone bursts at 60 or 80 dB SPL. The bursts had a 10 ms plateau and 2 ms rise/fall times and a 30 ms interstimulus interval. Electrical signals were generated and delivered to a well-shielded loudspeaker (Beyer DT-48) using custom software, a National Instruments input/output board (model 6250E), and a Tucker-Davis system III headphone buffer (model HB7). The speaker was 10 cm from the animal’s tympanic membrane. Calibration was performed using a ¼” Bruel and Kjaar microphone placed at the position of the animals eardrum, and levels are presented in dB SPL (re 20 μm Pascal).
Electrode & Recording Configuration
The electrode used was a 50 μm diameter, Teflon-insulated tungsten iridium rigid wire with approximately 50 μm of tip insulation removed. This size was appropriate relative to the gerbil scala tympani in the basal turn, which has a diameter of ~700 μm20. The hydraulic micromanipulator was controlled from outside the sound booth in steps as small as 1 μm. The recording was differential and monopolar, with the electrode connected to the positive input of a preamplifier (Grass Instruments, model P15D), a wire clipped to the neck musculature was the negative input, and the system ground was the animal’s tail. Amplification was 100x and filters were bandpass from 300-50,000 Hz. The output led outside the sound booth where there was additional amplification (10x) and filtering (300-100,000 Hz). The waveform was then digitized (200 kHz sampling rate) and 100 stimulus repetitions were averaged.
Intracochlear Recordings
Baseline recordings were taken as the electrode entered the scala tympani. To isolate the CM and the CAP, digital processing of the averaged waveform was performed with post-filtering. The magnitude of the CM at the stimulus frequency was obtained from a Fast-Fourier analysis of the epoch from 6-11 ms, while the magnitude of the CAP was the root mean square value of the epoch from 1-6 ms after filtering from 500-1500 Hz.
Assessment of Cochlear Status and Electrode Position
Each animal was sacrificed and the cochlea was removed en block. The whole mounts were decalcified, bone was removed for better visualization of cochlear structures, and the block was stained with toluidine blue. Damage was documented using digital photography.
RESULTS
An example of the recordings obtained during an experiment is shown in Figure 2. The experiment consisted of advancing an electrode through the basal turn, observing its progress via the endoscope image, and detecting changes in the CM and/or the CAP as the electrode impacted the BM/OSL complex. When response changes were detected, the electrode could be withdrawn to determine if the effect was reversible, indicative of an impact without lasting damage. In the case shown in Figure 2, using steps as small as 5 μm, controlled and reversible reductions in the CM were observed. A summary of the results from the 18 ears is shown in Table 1. Three patterns of damage during electrode advancement could be distinguished.
Figure 2.
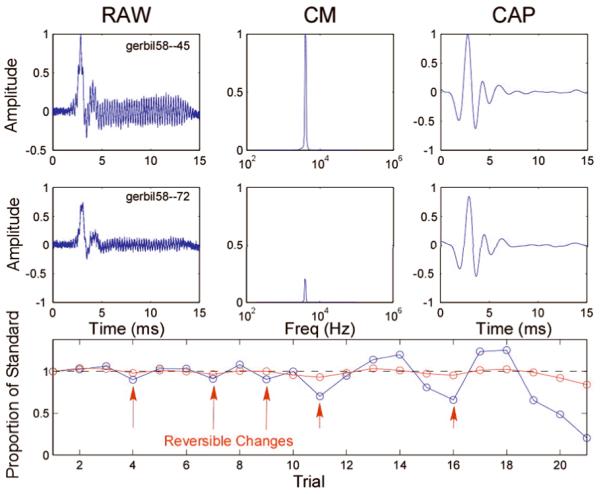
Screenshot of our near real-time recording set-up in gerbil # 58. The top row shows the “standard” or reference recording, obtained at the cochleostomy, or if there had been little change during the penetration, at a depth near where changes first were observed. Shown are the raw averaged response (left), the Fourier spectral magnitude of the CM (middle), and the raw waveform filtered to observe the CAP (right). The middle row represents these same measurements later in the experiment. In this case, it is evident that the magnitudes of the CM and CAP were considerably reduced compared to the standard measurement. The bottom row is a representation of the sequential progress of the experiment, with the abscissa representing the record number and the ordinate the amplitude of the response relative to the standard. The track thus corresponds to time rather than insertion depth, as the electrode was sometimes advanced and sometimes withdrawn to test for reversibility. Examples of reversible changes in the CM (blue) are indicated by arrows. As shown, the data processing software allowed for on-line visualization of the CM and CAP amplitudes and their progression over the course of the experiment. The recording time for each record was ~ 4 seconds, providing almost real-time feedback of the time course of acoustically evoked intracochlear potentials. Once the electrode was close to the OSL or BM (according to the micro endoscopic image), it could be advanced in steps as small as 5 μm to observe reversible changes.
Table 1.
Patterns of electrophysiologic changes with associated intracochlear damage of all 16 animals and 18 ears tested. Two animals underwent bilateral testing (animals #74 and 76). The table categorizes the cases into three types of tracks observed based on physiological patterns (see also Figure 3).The categories are based on changes in the CM, because the CAP was typically stable. The categories were early, irreversible loss of CM, or loss seen unrelated to the advancement of the electrode; late, irreversible loss of CM, or a reduction in the CM that occurred only after electrode advancement and did not recover on electrode retraction; and reversible loss of the CM, where the CM recovered when the electrode was retracted. In some of the RCM cases, after the reversibility was demonstrated the electrode was further advanced until the change became irreversible. Also indicated are the structure impacted and degree of damage
| Classification | Animal | Physiology | Histology | |||||
|---|---|---|---|---|---|---|---|---|
| Pattern | Description | # | Ear | CM | CAP | Structure | Description | |
| #1 | Early irreversible decrease of CM | CAP changes | 47 | right | I | I | BM/OSL | Full thickness |
| 38 | right | I | I | BM/OSL | Full thickness | |||
| 72 | right | I | I | BM/OSL | Full thickness | |||
| 69 | right | I | I | BM | Full thickness | |||
|
|
||||||||
| CAP stable | 74 | left | I | S | BM | Full thickness | ||
|
| ||||||||
| #2 | Irreversible CM decrease, CAP stable | 65 | right | I | S | OSL | Superficial | |
| 76 | left | I | S | OSL/BM | Superficial | |||
| 67 | right | I | S | OSL | Superficial | |||
| 29 | right | I | S | OSL | Superficial | |||
| 36 | right | I | S | BM | Full thickness | |||
|
| ||||||||
| #3 | Reversible CM decrease | 41 | right | R | S | NA | Absent trauma | |
| 64 | right | R | S | OSL | Superficial | |||
| 71 | right | R | S | OSL | Superficial | |||
|
| ||||||||
| Reversible, then irreversible CM decrease |
CAP stable | 58 | right | R/I | S | OSL | Superficial | |
| 76 | right | R/I | S | OSL | Superficial | |||
| 74 | right | R/I | S | BM | Superficial | |||
| 56 | right | R/I | S | OSL | Superficial | |||
|
|
||||||||
| CAP changes | 31 | right | R/I | R/I | BM | Full thickness | ||
Abbreviations: I: irreversible change, R: reversible change, R/I: reversible change, then irreversible change, S: stable.
Early and Irreversible CM Decrease
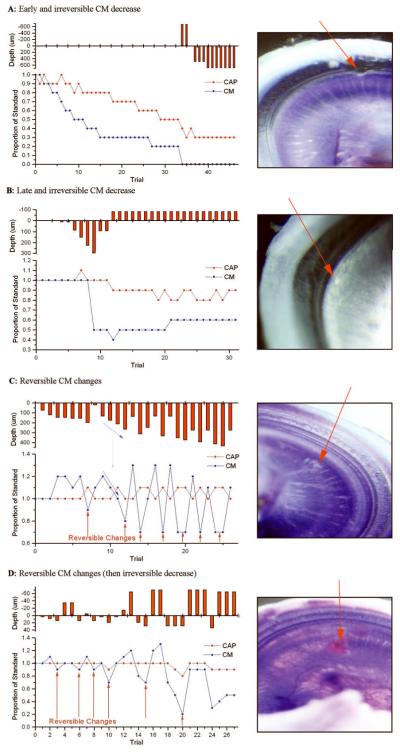
Early, progressive and irreversible reductions in the amplitude of the CM occurred in five ears (Table 1). An example is shown in Figure 3A. The CM and CAP declined while the electrode was stationary, with the CAP declining more slowly. A similar result was seen in four of the five cases. In each case the loss of CM and CAP was essentially complete. In the remaining case only the CM declined, while the CAP was stable. Histological investigation revealed full-thickness trauma to the BM (n=2) or the BM/OSL interface (n=3). Given the early loss of potentials and full thickness breaches on histology, the electrode was probably advanced through the BM/OSL during the initial placement at the cochleostomy.
Figure 3.
Electrophysiologic patterns and corresponding histological damage: Three main types of electrophysiologic changes were observed as shown in Table 1: A: early and irreversible CM decrease: gerbil #72, right ear; full-thickness penetrating trauma to the BM/OSL transition zone with irreversible changes in the CM and CAP. B: Late and irreversible CM decrease: gerbil #65, right ear; irreversible CM changes with stable CAPs and superficial non full-thickness trauma to the OSL. C: Reversible changes: gerbil #64, right ear; reversible changes to the CM with stable CAPs and associated deep scrape on the OSL. D: Reversible changes: gerbil #58, right ear; reversible CM changes before irreversible amplitude reductions were recorded. Associated trauma evidenced a deep scrape in the OSL. Reversible changes and respective site of damage are indicated via red arrows. Blue arrows in C indicate progressive potential loss with increased insertion depth.
Late and Irreversible CM Decrease
Stable CM and CAP amplitudes following the cochleostomy and electrode placement were demonstrated in the remaining 13 ears. Once the stability of the potentials was confirmed, the electrode was advanced stepwise until it approached the BM or OSL, according to the endoscopic image. In five of these cases, an irreversible change in the CM occurred as the electrode was advanced incrementally. In these cases, the electrode was advanced in relatively large steps, and the result was that damage to the cochlear structure was relatively rapid. One such case (Figure 3B) demonstrates the abrupt yet subtotal decrease in the CM amplitude with advancement of the electrode. Upon retraction of the electrode, the CM remained at roughly 50% its starting value. The corresponding whole-mount microdissection showed a superficial scrape in the OSL.
Of the five cases, the microdissections revealed superficial scrapes of the OSL in four and a full thickness breach of the BM in one. It is notable that in most cases the change in the CM was subtotal while the trauma assessed via microdissection was limited to superficial damage, indicating that the degree of potential change corresponds to the degree of cochlear damage.
Reversible CM Changes
The remaining eight ears demonstrated reversible reductions in the magnitude of the CM, i.e., the CM rebounded when the electrode was retracted to shallower insertion depths. In three of the eight cases we stopped the experiment after demonstrating the reversals. The subsequent histology in these cases revealed either no trauma or superficial trauma, only (Table 1). An example is shown in Figure 3C. In this case, several reversals were seen prior to ending the experiment. The amplitude change was proportional to the insertion depth (blue arrows) and when the electrode was only partially withdrawn the potential only partially recovered. The cochlear trauma in this case was a superficial scrape.
In five ears demonstrating reversible CM changes, further electrode advancement ultimately resulted in irreversible decreases. Gerbil #58 exemplifies this pattern of physiological response (Figure 3D). Reversible decreases in the CM were seen with electrode advancements as small as 5 μm, until at 35 μm the decrease in the CM did not rebound with electrode reversal. The correlating histology showed damage to the OSL. This pattern is a prime example of our ability to detect imminent damage to cochlear structures with reversible decreases of intracochlear potentials, as well as irreversible decreases that correspond to trauma to the BM or OSL.
Histological Damage
Additional examples showing types of histological damage are shown in Figure 4. The full thickness breach of Figure 4A was associated with irreversible loss of both CM and CAP, as was true in most EICM cases (Table 1, gerbil #47). However, superficial damage came in different degrees that did not correlate well with the physiology. The BM or OSL could be subject to a relatively deep scrape as in Figure 4B and 4C, but have completely reversible damage (Table 1, gerbil #71 and #64, respectively). Or, damage could appear as relatively superficial (Figure 4D), but produce irreversible physiological damage (Table 1, gerbil #76). It may be significant that in this case the hair cells of the BM appear slightly disrupted.
Figure 4.
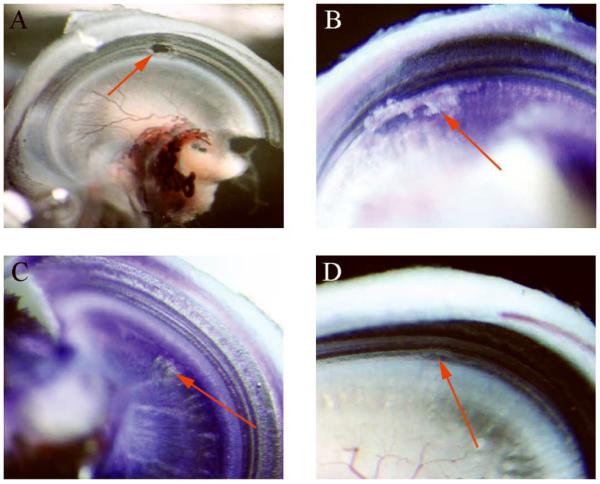
Histological trauma types observed. The arrows depict the damage location. A: gerbil #47, right ear; full thickness trauma indicating an abnormal connection of perilympatic and endolymphatic fluid spaces. Electrophysiology demonstrated early, irreversible CM amplitude decreases with unstable CAPs. B: gerbil #71, right ear; deep and long scrape along the osseous spiral lamina (OSL) with non-full thickness trauma. Electrophysiology from this case showed reversible CM changes with stable CAPs. C: gerbil #64, right ear; small area of deep scrape at the OSL; reversible CM changes with stable CAPs. D: gerbil #76, superficial scrape in the OSL and basilar membrane (BM) transition zone. Irreversible CM amplitude changes with stable CAPs.
Proximity to Source Generators
In 11 of 13 (84.6%) of applicable cases, i.e., all but the early irreversible category, there was a progressive amplitude increase of the CM and CAP as the electrode approached the BM/OSL. An example is shown in Figure 5. Both the CM and CAP increased as the electrode traversed the scala tympani according to the endoscopic image. These increases were most likely due to the increased proximity of the electrode to the source generators of the CM and CAP located in the organ of Corti and OSL, respectively.
Figure 5.
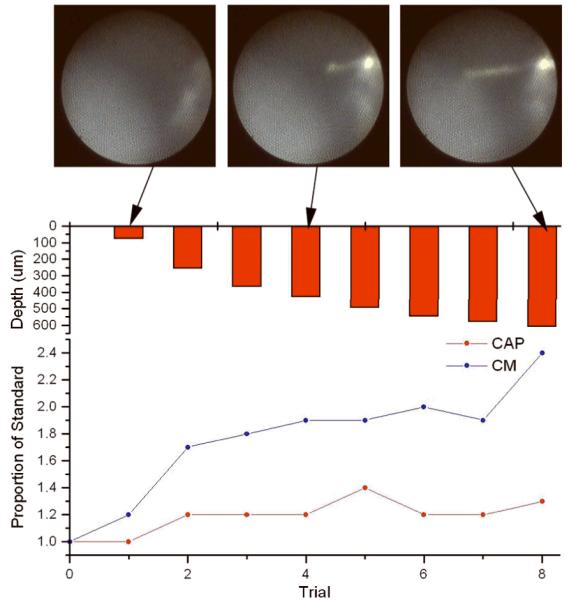
Gerbil #76, left ear. Amplitude increase of both the CM and CAP as the recording electrode approaches the BM and OSL. This pattern has been observed in 11 of the 13 animals with initially stable recordings at the cochleostomy (Patterns #2 and 3, Figure 3B, C, D). The upper row shows the micro endoscopic images of three representative insertion depths (at the cochleostomy, no electrode seen, half way into the scala tympani, and shortly before amplitude decreases were seen). The lower graph depicts the relative amplitude changes based on the recordings taken at the cochleostomy.
DISCUSSION
In this report, we were able to reliably identify impact with cochlear structures through changes in intracochlear potentials. Importantly, these changes were identified with a highly reduced stimulus set consisting of a single frequency and intensity. The recordings were correlated with microendoscopic visualization and with damage to the BM and OSL observed in cochlear whole mount dissections. In some cases, identification of various grades of intracochlear trauma was possible, including those that were reversible when the electrode was withdrawn.
Toward an Intraoperative Recording Paradigm
The recording system has provided a detailed level of control to observe electrode interactions with cochlear structures. In this study a single frequency (4 kHz) and one intensity (60 or 80 dB SPL) was used per experiment. These parameters were chosen because previous reports from our group18 showed that changes in cochlear potentials were widespread over frequency and intensity for a given location of electrode impact. It was therefore reasoned that a frequency/intensity combination where the response is large would provide a sensitive measure to detect response changes, as indeed proved to be the case. In other words, we were able to observe response changes at suprathreshold levels where the signal-to-noise ratio is high, rather than at threshold where the signal is by definition small and difficult to detect. However, we do not know that our particular stimulus is optimal in its ability to detect a change in the shortest time period. Notably, because of the ability to obtain reversible responses, it will be possible to test the changes at a variety of frequencies, levels and number of repetitions to further refine the recording sequence. A primary requirement of an intraoperative recording system is that it be fast, and the results in this study suggest that this requirement is reachable.
Patterns of Damage
Several patterns of damage became apparent during this series of experiments. In some cases early and progressive decreases in the amplitudes of the CM and CAP occurred while the electrode was stationary. Histologically, this pattern of change was associated with full thickness breaches of the basilar membrane alone or in combination with the osseous spiral lamina. Most likely, the electrode was advanced through these structures during its initial placement at the cochleostomy. That is, in some cases, the electrode was advanced too rapidly during initial placement, such that the damage occurred before the experiment began. Presumably, the full thickness breach created an abnormal communication between fluid compartments, thereby allowing a mixing of endolymph and perilymph. During a real cochlear implantation, this magnitude of damage could indicate the need for a full insertion in order to obtain maximum gain from the electric stimulation.
A second pattern of change was an irreversible loss in intracochlear potentials during electrode advancement, with no recovery of the response upon electrode reversal. In these cases it is most likely that the electrode was advanced in steps that were too large to allow for small, reversible changes to be detected. Typically, if the electrode was advanced in steps greater than 20 μm this result was obtained. While such small steps would be impractical during an actual implantation, it must be remembered that the electrode used herein is a rigid wire, which we used because we were seeking to cause damage that could be detected both physiologically and histologically. Clinical electrodes are much softer and more forgiving. In addition, the gerbil cochlea is small, and a greater degree of impact could presumably be tolerated in the larger human cochlea. In all ears, the amplitude decrease was evident in the CM, while the CAP remained stable. It is notable that in the majority of cases, the irreversible change in the CM was subtotal while the damage assessed via microdissection was typically limited to superficial damage of the BM or OSL. Therefore, the degree of change in the intracochlear potentials shows good correlation with the degree of histological damage. During a real cochlear implantation, this magnitude of damage could indicate a glancing blow. Whether such loss is ultimately recoverable over a longer time course can be addressed in future studies.
The last pattern of electrophysiological change was a CM reduction that was reversible with retraction of the electrode. This pattern indicates that physiological recordings can be used to detect potential trauma prior to an irreversible change.
An interesting electrophysiological finding, as described in Figure 5, is that the CM and/or the CAP tended to increase as the electrode approached the BM and/or OSL in 85% of possible cases. Most likely, this is due to the fact that the electrode was approaching the source generators of the potentials in the Organ of Corti and auditory nerve. A similar pattern of increasing response may prove important in the case of an EAS surgery, as an indication that a region of functional hearing is being approached.
CM v. CAP Recordings
In these experiments the CAP remained relatively stable compared to the CM. The only cases where the CAP showed substantial changes was when there was a full breach of the BM and/or OSL. This result was surprising, because in previous experiments the CAP was more affected by electrode contact with cochlear structures than was the CM18. The main differences between the studies were the location of the electrode insertion and the stimulus paradigm. In the previous study, the electrode was inserted through the round window, and thus typically impacted a more basal location than in the current study, which used a basal turn cochleostomy. The difference could produce the different results if it is assumed that the principle source generators of the CM and CAP lie along different areas of the cochlea, as is indeed likely to be the case. For several reasons, the contribution to the CAP in loud sounds (60-80 dB SPL) should be dominated by basal regions of the cochlea, while that of the CM should be dominated by the CF region of the stimulus. One reason is that the traveling wave is faster, and hence more synchronous, in basal parts of the cochlea, so that its contribution to the summed potential recorded by our electrode may be greater. However, this timing effect is likely to be small, and saturation effects are more likely to predominate. That is, the onset response contributing to the CAP is a single action potential so any given CF region saturates quickly with intensity. As sound level increases, the amount of the cochlea responding spreads widely and basally, so that the contribution of the base dominates. With the CM, the response does not saturate until higher intensities. Thus, the cochlear region with CF near that of the stimulus will dominate the response at all intensities. One interpretation of our disparate results is therefore that the penetrations in this study involved a CF region of the gerbil cochlea that was relatively closer to the CF region of the 4 kHz stimulus, although based on frequency mapping in the gerbil cochlea by Muller21 this region lies more apically than the location of contact in either set of experiments. The stimulus paradigm may also have played a role. In the previous study a complete series of frequencies and intensities was obtained at each step, a process requiring ~20 minutes at each position. It may be that the CAP would have been relatively more affected in the current study if the electrode had stayed for a longer time at the site where CM potentials declined.
Improvements Necessary
While these experiments show great promise for advancing this recording paradigm into a clinically useful model, they differ notably from the clinical setting. First, the electrode used in these experiments is a small, rigid metal electrode, not the flexible electrodes used for implantations. This type of electrode was chosen in order to ensure damage and provide feedback about electrophysiological changes associated with such trauma. Therefore, this data set represents the worst-case scenario for trauma. In the future, our group will transition to soft electrodes similar to those used for hearing preservation surgeries. Secondly, in order to determine the electrophysiological changes associated with intracochlear damage in the most sensitive model, these experiments were performed in normal hearing gerbils. Currently, we are developing the gerbil model with residual low frequency hearing and will conduct similar experiments to better simulate the clinical setting. Thirdly, we are not recording using currently available implant technology. While it is possible to record both the CM and the CAP with current systems, this technology has yet to be incorporated into this model. Finally, the electrode is not advanced manually but rather by using a hydraulic micromanipulator. The micromanipulator provides the precise handling necessary for these experiments. While a microadvancement tool is not currently used in the clinical setting, use of robotics in implants may gain popularity in the future22,23.
Acknowledgements
The authors would like to acknowledge Steven Pulver for the excellent technical assistance.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gstoettner WK, van de Heyning P, O’Connor AF, et al. Electric acoustic stimulation of the auditory system: results of a multi-centre investigation. Acta Otolaryngol. 2008;128:968–975. doi: 10.1080/00016480701805471. [DOI] [PubMed] [Google Scholar]
- 2.Gantz BJ, Turner C, Gfeller KE. Acoustic plus electric speech processing: preliminary results of a multicenter clinical trial of the Iowa/Nucleus Hybrid implant. Audiol Neurootol. 2006;11(Suppl 1):63–68. doi: 10.1159/000095616. [DOI] [PubMed] [Google Scholar]
- 3.Fraysse B, Macias AR, Sterkers O, et al. Residual hearing conservation and electroacoustic stimulation with the nucleus 24 contour advance cochlear implant. Otol Neurotol. 2006;27:624–633. doi: 10.1097/01.mao.0000226289.04048.0f. [DOI] [PubMed] [Google Scholar]
- 4.von Ilberg C, Kiefer J, Tillein J, et al. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL J Otorhinolaryngol Relat Spec. 1999;61:334–340. doi: 10.1159/000027695. [DOI] [PubMed] [Google Scholar]
- 5.Gantz BJ, Turner C. Combining acoustic and electrical speech processing: Iowa/Nucleus hybrid implant. Acta Otolaryngol. 2004;124:344–347. doi: 10.1080/00016480410016423. [DOI] [PubMed] [Google Scholar]
- 6.Skarzynski H, Lorens A, Piotrowska A. A new method of partial deafness treatment. Med Sci Monit. 2003;9:CS20–24. [PubMed] [Google Scholar]
- 7.Gstoettner WK, Van De Heyning P, O’Connor AF, et al. Electric acoustic stimulation of the auditory system: results of a multi-centre investigation. Acta Otolaryngol. 2008:1–8. doi: 10.1080/00016480701805471. [DOI] [PubMed] [Google Scholar]
- 8.Adunka O, Kiefer J, Unkelbach MH, Lehnert T, Gstoettner W. Development and evaluation of an improved cochlear implant electrode design for electric acoustic stimulation. Laryngoscope. 2004;114:1237–1241. doi: 10.1097/00005537-200407000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Kiefer J, Gstoettner W, Baumgartner W, et al. Conservation of low-frequency hearing in cochlear implantation. Acta Otolaryngol. 2004;124:272–280. doi: 10.1080/00016480310000755a. [DOI] [PubMed] [Google Scholar]
- 10.James C, Albegger K, Battmer R, et al. Preservation of residual hearing with cochlear implantation: how and why. Acta Otolaryngol. 2005;125:481–491. doi: 10.1080/00016480510026197. [DOI] [PubMed] [Google Scholar]
- 11.Aschendorff A, Kromeier J, Klenzner T, Laszig R. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear. 2007;28:75S–79S. doi: 10.1097/AUD.0b013e318031542e. [DOI] [PubMed] [Google Scholar]
- 12.Skinner MW, Holden TA, Whiting BR, et al. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol Suppl. 2007;197:2–24. [PubMed] [Google Scholar]
- 13.Adunka OF, Pillsbury HC, Kiefer J. Combining perimodiolar electrode placement and atraumatic insertion properties in cochlear implantation -- fact or fantasy? Acta Otolaryngol. 2006;126:475–482. doi: 10.1080/00016480500437393. [DOI] [PubMed] [Google Scholar]
- 14.Adunka O, Gstoettner W, Hambek M, Unkelbach MH, Radeloff A, Kiefer J. Preservation of basal inner ear structures in cochlear implantation. ORL J Otorhinolaryngol Relat Spec. 2004;66:306–312. doi: 10.1159/000081887. [DOI] [PubMed] [Google Scholar]
- 15.Briggs RJ, Tykocinski M, Xu J, et al. Comparison of round window and cochleostomy approaches with a prototype hearing preservation electrode. Audiol Neurootol. 2006;11(Suppl 1):42–48. doi: 10.1159/000095613. [DOI] [PubMed] [Google Scholar]
- 16.Eshraghi AA, Polak M, He J, Telischi FF, Balkany TJ, Van De Water TR. Pattern of hearing loss in a rat model of cochlear implantation trauma. Otol Neurotol. 2005;26:442–447. doi: 10.1097/01.mao.0000169791.53201.e1. discussion 447. [DOI] [PubMed] [Google Scholar]
- 17.Eshraghi AA, Yang NW, Balkany TJ. Comparative study of cochlear damage with three perimodiolar electrode designs. Laryngoscope. 2003;113:415–419. doi: 10.1097/00005537-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Adunka O, Mlot S, Suberman TA, et al. Intracochlear recordings of electrophysiologic parameters indicating cochlear damage. 2010 doi: 10.1097/MAO.0b013e3181f1ffdf. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell AP, Suberman TA, Buchman CA, Fitzpatrick DC, Adunka OF. Flexible cochlear microendoscopy in the gerbil. Laryngoscope. 2010 doi: 10.1002/lary.20979. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorne M, Salt AN, DeMott JE, Henson MM, Henson OW, Jr., Gewalt SL. Cochlear fluid space dimensions for six species derived from reconstructions of three-dimensional magnetic resonance images. Laryngoscope. 1999;109:1661–1668. doi: 10.1097/00005537-199910000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Muller M. The cochlear place-frequency map of the adult and developing mongolian gerbil. Hear Res. 1996;94:148–156. doi: 10.1016/0378-5955(95)00230-8. [DOI] [PubMed] [Google Scholar]
- 22.Labadie RF, Noble JH, Dawant BM, Balachandran R, Majdani O, Fitzpatrick JM. Clinical validation of percutaneous cochlear implant surgery: initial report. Laryngoscope. 2008;118:1031–1039. doi: 10.1097/MLG.0b013e31816b309e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labadie RF, Mitchell J, Balachandran R, Fitzpatrick JM. Customized, rapid-production microstereotactic table for surgical targeting: description of concept and in vitro validation. Int J Comput Assist Radiol Surg. 2009;4:273–280. doi: 10.1007/s11548-009-0292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]