Abstract
Background
Epidemiologic studies of BMI in relation to mortality commonly exclude persons with health conditions and/or a history of smoking in order to prevent bias resulting from illness-related weight loss (“reverse causation”). Analysis of BMI from an earlier time period may minimize reverse causation without requiring exclusion of participants based on disease or smoking history.
Methods
We prospectively examined BMI based on technician measurements of weight and height from 10 years prior to start of follow-up in relation to subsequent mortality in a cohort of 50,186 women who were 40 to 93 years old at baseline in 1987–1989. Deaths were ascertained through the U.S. National Death Index. Proportional hazards regression was used to estimate hazard ratios of mortality, adjusted for age, education, race/ethnicity, income, menopausal hormone use, smoking, and physical activity.
Results
During 10 years of follow-up through 1997, 5,201 women died. Overall, we observed a J-shaped association between BMI and mortality, with increased risk for women who were underweight, overweight, or obese. The hazard ratios and 95% confidence intervals of mortality for BMI categories of <18.5, 18.5–20.9, 21.0–23.4 (reference), 23.5–24.9, 25.0–27.4, 27.5–29.9, 30.0–34.9, and 35.0+ kg/m2 were 1.43 (1.19, 1.72), 1.07 (0.98, 1.17), 1.0 (reference), 1.10 (1.00, 1.20), 1.20 (1.11, 1.31), 1.23 (1.11, 1.37), 1.60 (1.44, 1.77), and 1.92 (1.64, 2.24). There was little evidence that preexisting conditions (heart disease, diabetes, and/or cancer) or smoking history modified the past BMI and mortality relation (p=0.54 and 0.76).
Conclusions
In this large cohort of women, BMI based on technician measurements of weight and height from 10 years prior to baseline showed increased risk for mortality across the range of overweight and obesity, regardless of disease and smoking history. Observed associations between overweight, obesity, and mortality in healthy individuals may also apply to persons with a history of disease or smoking.
Keywords: Body mass, weight, obesity, overweight, mortality, life-expectancy, epidemiology
INTRODUCTION
The association between body weight and mortality remains controversial despite several decades of research. Conflicting results regarding the shape of the relationship have included a positive (1), a J-shaped (2) or U-shaped (3, 4), an inverse (5), and a null (6) association. Rising prevalence of overweight and obesity in the United States (7) and other parts of the world (8) in recent decades underscores the timeliness of understanding the relation between body mass index (BMI; the weight in kilograms divided by the square of height in meters) and mortality.
Much of the uncertainty underlying the nature of the relation between BMI and mortality is the issue of reverse causation. As it relates to the BMI and mortality association, reverse causation refers to chronic illness present at the time of weight assessment causing both previous weight loss and subsequent death (9, 10). To mitigate this type of potential bias, some analyses exclude at baseline all study participants with diagnosed chronic illness and those with recent weight loss (1, 11). However, those restrictions may not eliminate all deaths attributable to undetected chronic illness at study entry, especially in populations which typically experience high levels of morbidity such as smokers (12). Moreover, some have suggested that findings based on restriction of the analysis to apparently healthy individuals may yield results of uncertain applicability to those with chronic conditions and smokers (13). Studies of the BMI and mortality relation which incorporate such populations but which still take steps to account for potential reverse causation would be of particular value.
An approach that may help account for reverse causation is to examine BMI from a prior period, such as 10 years prior to mortality follow-up. A BMI measured from a prior period is more likely than later BMI to capture weight unaffected by serious chronic illness (10). Previous investigations have examined the BMI and mortality relation using body weight prior to follow-up and have generally found that past weight results in a stronger positive association with mortality than more recent weight (14–19), possibly suggesting that past weight mitigates reverse causation.
We examined the relation of BMI to mortality in a large cohort of U.S. women for whom historical data on BMI from 10 years prior to study baseline were available. Our data on BMI from 10 years in the past were based on a series of clinical assessments of weight and height, providing us with a very accurate measure of BMI. Investigation of past BMI may help reduce reverse causation in analyses of mortality while still allowing individuals surviving with chronic conditions, or who currently or formerly smoked to remain in the analysis.
METHODS
Study population
Study participants were selected from the Breast Cancer Detection Demonstration Project (BCDDP), a mammography screening program conducted from 1973 through 1980 as a joint project between the National Cancer Institute and the American Cancer Society. Annual breast cancer screening examinations were attended by 283,222 women at 29 screening sites throughout the United States (20).
In 1979–1981, the BCDDP follow-up study was initiated when 61,430 of 64,182 (96%) questionnaires eliciting information on family history of breast cancer, hormone use, and screening history were returned by selected women from the original breast cancer screening project. The group included 1) a sample of women who did not receive a breast biopsy or a recommendation for biopsy (N=24,196) 2) all women who were recommended a surgical consultation but did not have a biopsy performed (N=9,103); 3) all women with benign breast disease confirmed by biopsy during screening (N=24,403); and 4) all women with incident breast cancer during screening (N=3,729). The sample of women without breast biopsy or recommendation for a biopsy was individually matched to women who were recommended for a consultation for surgery (groups 3 and 4 above) by age at entry into the screening program (5-year age groups), screening center, race, data of enrollment (6-month periods), and duration of participation in the screening. Of the 61,430 women enrolled, we excluded 1,857 women with missing or implausible values for weight or height and 42 women with a BMI below 15 or greater than 60, resulting in a cohort of 59,531 women. Follow-up questionnaires that were designed to update information on participants’ exposures to selected risk factors and to identify history of chronic diseases were mailed to members of the BCDDP follow-up study in 1987, 1993 and 1995.
In the current analysis, we considered the 50,186 women who responded to the 1987 questionnaire that first requested information on smoking, physical activity, and diet. For these participants, BMI was assessed approximately 10 years prior to the start of follow-up. The participants in our analysis were similar to the women of the overall analytic cohort with respect to year of birth, education, race, level of income, and menopausal hormone use. The design and methods of the BCDDP follow-up study have been described previously (21). The BCDDP follow-up study was approved by the Institutional Review Board of the National Cancer Institute. Completion of each questionnaire was implied to indicate informed consent.
Measurement of weight, height, and covariates
As part of the original breast cancer screening project, clinical measures of weight and height were assessed using a beam-balance and stadiometer, respectively (22). For the present study, BMI was calculated from the weight and height measured at the most recent screening exam. For the majority of participants, the most recent weight and height measurements occurred approximately ten years prior to baseline.
Data on race, annual household income, and highest attained level of education were reported by participants upon entry into the screening program. The remaining covariates were assessed on the baseline questionnaire. Smoking history was assessed by asking participants to indicate if they had ever smoked and if so, the age they started smoking, the average number of cigarettes smoked per day, and if and when they had quit smoking. The physical activity assessment asked women to report the time spent per day engaging in moderate and vigorous activities during the past year. We calculated a physical activity index by multiplying the number of hours of moderate and vigorous physical activity by estimated intensity levels of 4 METS and 6 METS per hour, respectively (23). Our physical activity assessment closely resembles the instrument used in the Framingham Heart Study that was validated using indirect calorimetry (24).
End point ascertainment
Vital status of cohort members was ascertained by regular searches of the National Death Index through December 31, 1997, concurrent with administration of each follow-up questionnaire. The accuracy of the National Death Index in determining vital status is estimated to exceed 95% (25). The cause of death was coded as listed on the death certificate using the ICD-9 system.
History of coronary heart disease, diabetes, and cancer were self-reported on the 1987 questionnaire. Self-reported cancers were confirmed using pathology reports. Information about participants’ history of coronary heart disease or diabetes was ascertained by self-report alone.
Data analysis
For the primary analysis, subjects were followed from the date the 1987 questionnaire was returned until the date of death, date of last contact, or December 31, 1997, whichever occurred first. We used Cox proportional hazards regression with age as the underlying time metric to estimate hazard ratios (HRs) and corresponding 95 percent confidence intervals (95% CIs) of mortality. BMI was divided into 8 categories [15.0–18.4, 18.5–20.9, 21.0–23.4 (reference group), 23.5–24.9, 25.0–27.4, 27.5–29.9, 30.0–34.9, and ≥35.0] that encompass the definitions of under weight (<18.5), normal weight (18.5–24.9), overweight (25.0–29.9), and obesity (≥30.0) recommended by the World Health Organization (26). We selected 21.0–23.4 as the reference group because it represents the intermediate category of the normal range. We also report results for WHO categories.
All multivariate models were adjusted for age, income, education, race/ethnicity, reason for selection into the cohort, menopausal hormone use, smoking and physical activity. We also constructed alternative models adjusting for marital status, parity, and several dietary factors such as fruit and vegetable intake, meat intake, alcohol intake, and recommended food score (a score that assessed adherence to current dietary guidelines, i.e. intakes of fruits, vegetables, whole grains, lean meats and low-fat dairy products) (27). However, those variables were not included in the final models because they did not alter the BMI and mortality association. We assigned missing indicator variables for participants lacking data on smoking (N=785), education (N=381), physical activity (N=4,223), and menopausal hormone use (N=689). We also conducted separate analyses that excluded participants with missing values; this resulted in no substantial changes in beta estimates (all changes less than 10%).
In order to depict the shape of the dose-response relation on a continuous scale, we used cubic restricted spline models (28) with knots based on the 5th, 25th, 75th, and 95th percentiles of past BMI, i.e. at BMI values of 19.2, 21.3, 25.8, and 32.0, respectively. The reference BMI was set at the 12.5th percentile of past BMI or a value of 20.2.
We conducted analyses stratified by age at start of follow-up (<65, ≥65 years), age at assessment of BMI (<65, ≥65 years), history of chronic illness (any coronary heart disease, diabetes, or cancer prior to assessment of BMI versus no coronary heart disease, diabetes, or cancer prior to assessment of BMI), and smoking status (never, current, past). We formally tested for potential interactions of the BMI and mortality association using the likelihood ratio test, i.e. comparing the likelihood of models with and without multiplicative interaction terms. Interaction terms were calculated using the cross product of the BMI categories and the factor of interest (e.g. any history of chronic illness). The cohort included a substantial number of women diagnosed with breast cancer during screening, therefore we examined the BMI and mortality relation in this group separately.
We calculated the life expectancy according to BMI category of women 40 years of age by selecting the median life expectancy (the age at which 50% of the population would be expected to have died) from direct adjusted survival curves (29, 30). Confidence intervals were obtained using a bootstrap estimator. We first calculated the median life expectancy of each BMI category in 500 distinct datasets created by sampling with replacement. We then used the reflection method (31) and the distribution of median life-expectancies to derive confidence intervals. All statistical analyses were conducted using PHREG in SAS version 9.0.
RESULTS
During 10 years of follow-up, we ascertained 5,201 deaths. The mean ages at study baseline and death were 62.6 years [standard deviation (SD=8.4)] and 75.2 (SD=9.5) years, respectively. Age at study entry ranged from 40 to 93. The mean BMI during screening was 24.1 kg/m2 (SD=4.0). A majority of women (57.0%) had never smoked and only a minority (12.3%) were current smokers. As compared with women of normal weight (BMI of 18.5–24.9), women who were overweight (BMI of 25.0–29.9) or obese (BMI ≥ 30.0) were less likely to report graduation from high school, Caucasian ethnicity, high annual household income, menopausal hormone use, current smoking, and physical activity (Table 1).
Table 1.
| Body Mass Index | ||||||||
|---|---|---|---|---|---|---|---|---|
| 15.0–18.4 | 18.5–20.9 | 21.0–23.4 | 23.5–24.9 | 25.0–27.4 | 27.5–29.9 | 30.0–34.9 | 35.0+ | |
| No. of participants (%) | 1,054 (2.1) | 9,323 (18.6) | 15,794 (31.5) | 7,336 (14.6) | 8,350 (16.6) | 3,737 (7.5) | 3,370 (6.7) | 1,224 (2.4) |
| Age (years) | 60.9 | 60.9 | 62.3 | 63.2 | 63.8 | 64.0 | 63.2 | 61.2 |
| Education (% high-school or more) | 87.9 | 92.5 | 90.5 | 87.6 | 84.5 | 82.3 | 80.0 | 77.1 |
| Caucasian, Non-Hispanic (%) | 84.0 | 89.1 | 89.7 | 88.6 | 87.2 | 86.3 | 84.4 | 83.2 |
| Annual household income at screening, 1973–1978‡ | ||||||||
| Less than $10,000 | 22.9 | 16.0 | 16.7 | 19.0 | 22.3 | 25.2 | 28.3 | 34.7 |
| $10,000–$14,999 | 22.6 | 21.3 | 23.6 | 25.8 | 27.9 | 27.1 | 29.3 | 28.8 |
| $15,000–$29,999 | 37.7 | 43.7 | 44.6 | 43.1 | 39.9 | 39.3 | 34.7 | 30.6 |
| $30,000 or more | 16.8 | 19.0 | 15.1 | 12.1 | 10.0 | 8.4 | 7.7 | 5.9 |
| Menopausal hormone use (ever/never; %) | 70.1 | 72.9 | 70.3 | 66.9 | 64.3 | 60.8 | 56.9 | 49.8 |
| Smoking | ||||||||
| Former smoker (%)§ | 27.3 | 32.6 | 32.0 | 30.4 | 29.3 | 27.4 | 28.2 | 29.9 |
| < 10 cigarettes/day | 7.7 | 10.3 | 10.0 | 9.2 | 8.8 | 8.1 | 8.3 | 8.2 |
| 10–20 cigarettes/day | 9.2 | 9.9 | 9.5 | 8.8 | 8.2 | 7.9 | 7.9 | 7.0 |
| More than 20 cigarettes/day | 9.4 | 10.7 | 11.0 | 11.2 | 10.7 | 10.1 | 10.8 | 13.0 |
| Current smoker (%) | 19.3 | 14.5 | 12.7 | 11.8 | 11.6 | 10.5 | 9.2 | 8.6 |
| < 10 cigarettes/day | 2.3 | 2.3 | 2.0 | 1.7 | 1.7 | 1.5 | 1.3 | 1.8 |
| 10–20 cigarettes/day | 6.6 | 4.8 | 3.9 | 3.8 | 3.3 | 3.0 | 2.5 | 2.4 |
| More than 20 cigarettes/day | 10.3 | 7.3 | 6.8 | 6.2 | 6.4 | 6.0 | 5.3 | 4.4 |
| Physical activity index** | 28.1 | 29.4 | 29.7 | 29.8 | 29.5 | 28.5 | 27.6 | 24.3 |
| History of chronic disease prior to enrollment (%) | 14.4 | 12.1 | 11.8 | 12.7 | 13.2 | 14.6 | 16.6 | 19.1 |
| Coronary heart and/or diabetes (%) | 5.6 | 3.5 | 3.2 | 4.0 | 4.7 | 6.0 | 8.0 | 10.6 |
| Cancer (%) | 9.2 | 9.1 | 8.9 | 9.0 | 9.0 | 9.2 | 9.5 | 10.1 |
Education, ethnicity, and income were assessed during screening. Menopausal hormone use, smoking status, physical activity, and chronic disease history were reported on the 1987 questionnaire.
Based on the final technician measured height and weight from annual examinations during screening phase (1973–1978). The median difference between the date of the final BMI assessment and baseline was approximately 10 years
Due to missing data, catgeories may not add to 100%
Some former and current smokers did not report their smoking intensity, thus these categories may not add up to the total
The physical activity index was calculated by multiplying average daily hours of moderate and vigorous weekday activity by estimated metabolic equivalent (MET) levels of 4 and 6, respectively (4*hours moderate activity + 6*hours vigorous activity)
We observed a J-shaped association between BMI and mortality, with elevated risk among underweight women and monotonically increasing risks across the entire range of overweight and obesity (Table 2). For WHO categories of <18.5, 18.5–24.9 (reference), 25.0–29.9, 30.0–34.9, and ≥35.0 kg/m2 the HRs and 95% CIs were 1.30 (1.03, 1.65), 1.0 (reference), 1.19 (1.10, 1.28), 1.55 (1.38, 1.75), and 1.92 (1.60, 2.30), respectively.
Table 2.
Hazard ratios of mortality in relation to body mass index* among all women and by age-group in the BCDDP cohort†
| Body Mass Index (kg/m2) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 15.0–18.4 | 18.5–20.9 | 21.0–23.4 | 23.5–24.9 | 25.0–27.4 | 27.5–29.9 | 30.0–34.9 | 35.0+ | |
| All women | ||||||||
| No. of deaths | 127 | 783 | 1,377 | 768 | 1,000 | 471 | 495 | 180 |
| Person-years | 8,825 | 79,274 | 133,943 | 61,813 | 69,812 | 31,153 | 27,674 | 10,077 |
| Age-adjusted HR | 1.57 | 1.09 | 1.00 | 1.11 | 1.22 | 1.25 | 1.64 | 2.10 |
| 95% CI | 1.31, 1.89 | 1.00, 1.19 | ref | 1.02, 1.21 | 1.12, 1.32 | 1.13, 1.39 | 1.48, 1.82 | 1.79, 2.45 |
| Multivariate‡ HR | 1.43 | 1.07 | 1.00 | 1.10 | 1.20 | 1.23 | 1.60 | 1.92 |
| 95% CI | 1.19, 1.72 | 0.98, 1.17 | ref | 1.00, 1.20 | 1.11, 1.31 | 1.11, 1.37 | 1.44, 1.77 | 1.64, 2.24 |
| Age at start of follow-up | ||||||||
| Age <65 yrs | ||||||||
| No. of deaths | 41 | 281 | 454 | 228 | 263 | 127 | 158 | 86 |
| Person-years | 6,316 | 57,821 | 89,745 | 38,937 | 42,010 | 18,431 | 17,410 | 7,356 |
| Age-adjusted HR | 1.44 | 1.02 | 1.00 | 1.11 | 1.19 | 1.30 | 1.76 | 2.36 |
| 95% CI | 1.05, 1.99 | 0.88, 1.19 | ref | 0.95, 1.31 | 1.02, 1.38 | 1.07, 1.58 | 1.47, 2.11 | 1.87, 2.97 |
| Multivariate HR | 1.37 | 1.03 | 1.00 | 1.10 | 1.17 | 1.28 | 1.66 | 2.14 |
| 95% CI | 1.00, 1.89 | 0.88, 1.19 | ref | 0.94, 1.30 | 1.01, 1.37 | 1.05, 1.56 | 1.39, 2.00 | 1.70, 2.71 |
| Age 65+ yrs | ||||||||
| No. of deaths | 86 | 502 | 923 | 540 | 737 | 344 | 337 | 94 |
| Person-years | 2,509 | 21,453 | 44,198 | 22,876 | 27,802 | 12,722 | 10,624 | 2,721 |
| Age-adjusted HR | 1.65 | 1.13 | 1.00 | 1.11 | 1.23 | 1.24 | 1.60 | 1.89 |
| 95% CI | 1.32, 2.06 | 1.01, 1.26 | ref | 1.00, 1.23 | 1.12, 1.36 | 1.09, 1.40 | 1.41, 1.81 | 1.53, 2.34 |
| Multivariate HR | 1.51 | 1.12 | 1.00 | 1.10 | 1.22 | 1.22 | 1.57 | 1.74 |
| 95% CI | 1.21, 1.88 | 1.00, 1.25 | ref | 0.99, 1.22 | 1.11, 1.35 | 1.08, 1.38 | 1.38, 1.78 | 1.40, 2.15 |
| Age at assessment of BMI | ||||||||
| Age <65 yrs | ||||||||
| No. of deaths | 86 | 550 | 915 | 481 | 627 | 318 | 360 | 147 |
| Person-years | 8,198 | 74,373 | 123,482 | 56,156 | 62,568 | 27,823 | 25,313 | 9,587 |
| Age-adjusted HR | 1.62 | 1.10 | 1.00 | 1.08 | 1.23 | 1.39 | 1.78 | 2.22 |
| 95% CI | 1.30, 2.02 | 0.99, 1.22 | ref | 0.97, 1.21 | 1.11, 1.36 | 1.22, 1.58 | 1.57, 2.01 | 1.87, 2.64 |
| Multivariate HR | 1.48 | 1.08 | 1.00 | 1.08 | 1.22 | 1.37 | 1.73 | 2.04 |
| 95% CI | 1.18, 1.85 | 0.97, 1.20 | ref | 0.97, 1.20 | 1.10, 1.35 | 1.21, 1.56 | 1.53, 1.96 | 1.71, 2.44 |
| Age 65+ yrs | ||||||||
| No. of deaths | 41 | 233 | 462 | 287 | 373 | 153 | 135 | 33 |
| Person-years | 627 | 4,901 | 10,461 | 5,656 | 7,244 | 3,330 | 2,361 | 490 |
| Age-adjusted HR | 1.50 | 1.07 | 1.00 | 1.15 | 1.19 | 1.03 | 1.36 | 1.71 |
| 95% CI | 1.09, 2.07 | 0.91, 1.25 | ref | 1.00, 1.34 | 1.04, 1.37 | 0.86, 1.24 | 1.13, 1.65 | 1.20, 2.43 |
| Multivariate HR | 1.44 | 1.08 | 1.00 | 1.13 | 1.17 | 1.01 | 1.34 | 1.52 |
| 95% CI | 1.04, 1.98 | 0.92, 1.26 | ref | 0.97, 1.31 | 1.02, 1.34 | 0.84, 1.21 | 1.10, 1.63 | 1.06, 2.17 |
Body mass index was measured by technician approximately 10 years prior to study baseline.
HR=hazard ratio, CI=confidence interval
Multivariate models are adjusted for for age, menopausal hormone use (ever/never), annual household income (less than $10,000, $10,000–$14,999, $15,000–$29,999, and $30,000+), education level (% graduated from high-school or further education), race/ethnicity (caucasian/non-caucasian), smoking (never smoking; quit smoking ≥ 10 years ago with categories of 1 to 10; 11 to 20; and > 20 cigarettes per day; quit smoking 1 to 9 years ago with categories of 1 to 10; 11 to 20; and > 20 cigarettes per day; current smoking with categories of 1 to 10; 11 to 20; and > 20 cigarettes per day), physical activity (quintiles of physical activity index).
The hazard ratio of mortality for overweight and obesity was similar for women younger than 65 years of age and older than 65 years of age at study baseline (P for interaction=0.39) (Table 2). Furthermore, in strata defined by age at BMI measurement (younger than 65 vs. older than 65), we found that the hazard ratio of overweight was similar (P for interaction=0.22). However, the hazard ratio of obesity was distinctly less pronounced among women whose BMI was assessed after age 65 than among those whose BMI was assessed before age 65 (P for interaction=0.004).
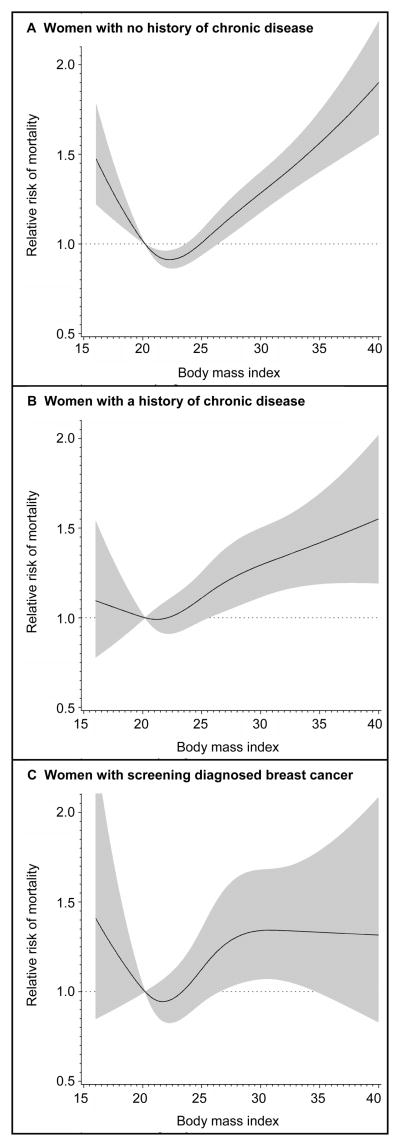
The relation of BMI to mortality among women with and without a history of chronic illness differed little from that of the cohort as a whole (Table 3), indicating no major effect modification (p for interaction=0.54). Examination of the dose response curves showed that women with no history of chronic disease had a J-shaped relationship with mortality, with evident excess risk among the underweight, overweight, and obese (Figure 1a). The dose-response curve among women with a disease history at the time of weight assessment was relatively comparable, though there was little association between very light body weight (i.e. BMI lower than the reference BMI) and increased risk of mortality in this group (Figure 1b). Among women with screen detected breast cancers, women in the overweight BMI categories (25.0–27.4 and 27.5–29.9) experienced a 1.31 fold and 1.57 fold increased risk of mortality relative to women of the reference BMI (see Table 3, also Figure 1c). Cancer mortality was the predominant cause of death in women with screen detected breast cancer (50% of deaths vs. 38% in other women).
Table 3.
Hazard ratios of mortality in relation to body mass index* according to selected subgroups of women in the BCDDP cohort†
| Body Mass Index (kg/m2) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 15.0–18.4 | 18.5–20.9 | 21.0–23.4 | 23.5–24.9 | 25.0–27.4 | 27.5–29.9 | 30.0–34.9 | 35.0+ | |
| Chronic disease history‡ | ||||||||
| Any chronic disease history | ||||||||
| No. of deaths | 26 | 193 | 309 | 210 | 265 | 140 | 148 | 52 |
| Person-years | 1,138 | 8,518 | 14,901 | 7,621 | 9,290 | 4,588 | 4,461 | 1,721 |
| Age-adjusted HR | 1.17 | 1.22 | 1.00 | 1.29 | 1.24 | 1.40 | 1.65 | 1.79 |
| 95% CI | 0.79, 1.75 | 1.01, 1.45 | ref | 1.08, 1.54 | 1.05, 1.46 | 1.14, 1.71 | 1.35, 2.00 | 1.33, 2.40 |
| Multivariate HR | 1.10 | 1.21 | 1.00 | 1.29 | 1.21 | 1.34 | 1.58 | 1.68 |
| 95% CI | 0.74, 1.65 | 1.01, 1.45 | ref | 1.08, 1.54 | 1.02, 1.42 | 1.09, 1.64 | 1.29, 1.92 | 1.25, 2.26 |
| No chronic disease history | ||||||||
| No. of deaths | 101 | 590 | 1,068 | 558 | 735 | 331 | 347 | 128 |
| Person-years | 7,678 | 70,299 | 118,045 | 53,838 | 59,973 | 26,198 | 22,820 | 8,126 |
| Age-adjusted HR | 1.69 | 1.05 | 1.00 | 1.05 | 1.20 | 1.17 | 1.58 | 2.09 |
| 95% CI | 1.38, 2.07 | 0.95, 1.16 | ref | 0.94, 1.16 | 1.09, 1.31 | 1.04, 1.33 | 1.40, 1.78 | 1.74, 2.51 |
| Multivariate HR | 1.53 | 1.04 | 1.00 | 1.04 | 1.19 | 1.17 | 1.57 | 1.93 |
| 95% CI | 1.25, 1.88 | 0.94, 1.15 | ref | 0.94, 1.16 | 1.08, 1.31 | 1.03, 1.32 | 1.38, 1.77 | 1.61, 2.33 |
| Screen-detected breast cancer | ||||||||
| No. of deaths | 9 | 95 | 141 | 96 | 116 | 60 | 52 | 18 |
| Person-years | 449 | 3,753 | 6,742 | 3,357 | 3,541 | 1,668 | 1,579 | 446 |
| Age-adjusted HR | 1.14 | 1.35 | 1.00 | 1.26 | 1.34 | 1.59 | 1.47 | 2.15 |
| 95% CI | 0.58, 2.23 | 1.04, 1.75 | ref | 0.97, 1.64 | 1.05, 1.72 | 1.18, 2.16 | 1.07, 2.03 | 1.31, 1.51 |
| Multivariate HR | 1.04 | 1.30 | 1.00 | 1.24 | 1.33 | 1.57 | 1.39 | 2.05 |
| 95% CI | 0.53, 2.04 | 1.00, 1.69 | ref | 0.95, 1.61 | 1.04, 1.71 | 1.16, 2.13 | 1.00, 1.92 | 1.25, 3.38 |
| Smoking status | ||||||||
| Never smokers | ||||||||
| No. of deaths | 50 | 348 | 690 | 400 | 538 | 293 | 293 | 103 |
| Person-years | 4,676 | 41,194 | 72,859 | 35,552 | 41,193 | 19,316 | 17,379 | 5,985 |
| Age-adjusted HR | 1.32 | 1.04 | 1.00 | 1.10 | 1.21 | 1.34 | 1.68 | 2.25 |
| 95% CI | 0.99, 1.76 | 0.91, 1.19 | Ref | 0.98, 1.25 | 1.08, 1.35 | 1.17, 1.54 | 1.47, 1.93 | 1.83, 2.77 |
| Multivariate§ HR | 1.32 | 1.07 | 1.00 | 1.09 | 1.18 | 1.28 | 1.58 | 1.94 |
| 95% CI | 0.99, 1.75 | 0.94, 1.21 | ref | 0.97, 1.24 | 1.05, 1.32 | 1.11, 1.47 | 1.38, 1.82 | 1.58, 2.40 |
| Former smokers | ||||||||
| No. of deaths | 38 | 252 | 430 | 249 | 289 | 115 | 144 | 50 |
| Person-years | 2,385 | 25,453 | 42,174 | 18,382 | 19,934 | 8,309 | 7,510 | 3,063 |
| Age-adjusted HR | 1.67 | 1.07 | 1.00 | 1.19 | 1.26 | 1.21 | 1.90 | 2.01 |
| 95% CI | 1.20, 2.33 | 0.91, 1.25 | ref | 1.02, 1.39 | 1.09, 1.47 | 0.98, 1.48 | 1.57, 2.29 | 1.50, 2.70 |
| Multivariate HR | 1.50 | 1.05 | 1.00 | 1.13 | 1.19 | 1.13 | 1.70 | 1.65 |
| 95% CI | 1.07, 2.09 | 0.90, 1.23 | ref | 0.97, 1.32 | 1.02, 1.38 | 0.92, 1.38 | 1.40, 2.06 | 1.22, 2.22 |
| Current smokers | ||||||||
| No. of deaths | 38 | 169 | 221 | 106 | 140 | 49 | 47 | 21 |
| Person-years | 1,680 | 11,542 | 16,875 | 6,930 | 7,451 | 2,944 | 2,418 | 872 |
| Age-adjusted HR | 1.68 | 1.17 | 1.00 | 1.12 | 1.29 | 1.17 | 1.46 | 2.36 |
| 95% CI | 1.19, 2.37 | 0.95, 1.42 | ref | 0.89, 1.41 | 1.04, 1.60 | 0.86, 1.59 | 1.07, 2.00 | 1.51, 3.69 |
| Multivariate HR | 1.72 | 1.19 | 1.00 | 1.11 | 1.26 | 1.09 | 1.33 | 2.21 |
| 95% CI | 1.22, 2.43 | 0.97, 1.45 | ref | 0.88, 1.40 | 1.02, 1.57 | 0.80, 1.50 | 0.96, 1.82 | 1.40, 3.47 |
Body mass index was measured by technician approximately 10 years prior to study baseline.
HR=hazard ratio, CI=confidence interval
Women with chronic disease history includes women with diabetes, coronary heart disease, or cancer diagnosed prior to the technician measurement of height and weight
Multivariate models are adjusted for for age, menopausal hormone use (ever/never), annual household income (less than $10,000, $10,000–$14,999, $15,000–$29,999, and $30,000+), education level (% graduated from high-school or further education), race/ethnicity (caucasian/non-caucasian), smoking (never smoking; quit smoking ≥ 10 years ago with categories of 1 to 10; 11 to 20; and > 20 cigarettes per day; quit smoking 1 to 9 years ago with categories of 1 to 10; 11 to 20; and > 20 cigarettes per day; current smoking with categories of 1 to 10; 11 to 20; and > 20 cigarettes per day), physical activity (quintiles of physical activity index).
Figure 1. Multivariate relative risks of mortality as related to BMI.
In each panel, the lines are cubic restricted spline models depicting the dose-response relationship of BMI to mortality on a continuous basis. Relative risks are indicated by the solid lines, and 95 percent confidence intervals by the gray band. The reference BMI is set at 20.2, which is the BMI at the 12.5th percentile of the cohort. The spline modeling was restricted to BMI values between 16 and 40. Panel A depicts the BMI and mortality relation among 43,649 women with no history of chronic disease at the time of weight assessment, including 3,856 deaths. Chronic disease was defined as any prior diagnosis by a physician of heart-disease, diabetes, or cancer. Panel B shows the BMI and mortality relation among 6,506 women with a history of chronic disease at the time of weight assessment, including 1,342 deaths. Panel C demonstrates the BMI and mortality relation among 2,693 women diagnosed with breast cancer during screening, including 587 deaths.
The BMI and mortality relation was also relatively similar between never, former, and current smokers (p for interaction=0.76). Among former and current smoking women, we observed that overweight and obesity were associated with a magnitude of elevated risk of mortality similar to that of other women, but the increased risk did not attain statistical significance in some overweight categories (Table 3). Among never smokers, the relative risk of mortality associated with underweight was somewhat lower than among former or current smokers.
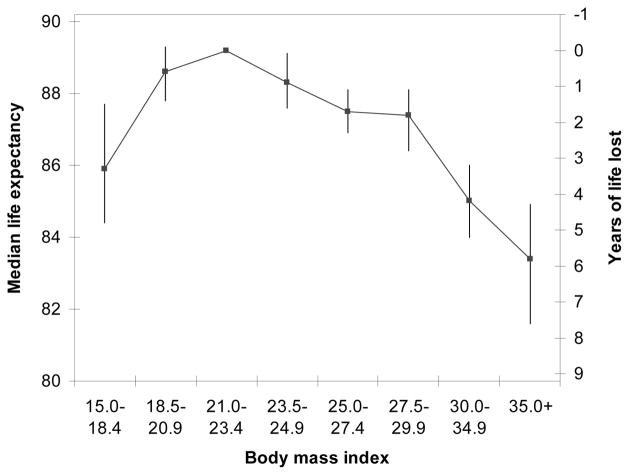
Women who were underweight, overweight, and obese had shorter median life expectancies even after adjustment for education, race/ethnicity, income, menopausal hormone use, smoking and physical activity as compared to women of the reference BMI (21.0–23.4) (Figure 2). For women 40 years of age, estimated life expectancy (with 95% confidence intervals) for the reference BMI of 21.0–23.4 was 89.2 (88.5, 89.7). Relative to women of the reference BMI, we estimated that women in BMI categories of <18.5, 18.5–20.9, 23.5–24.9, 25.0–27.4, 27.5–29.9, 30.0–34.9, and 35.0+ kg/m2 lost 3.3 (1.5, 4.8), 0.6 (−0.1, 1.4), 0.9 (0.1, 1.6), 1.7 (1.0, 2.3), 1.8 (1.1, 2.8), 4.2 (3.2, 5.2), and 5.8 (4.4, 7.7) years of life, respectively.
Figure 2. Life expectancy and years of life lost according to BMI category for women 40 years of age.
For each BMI category, the line above shows the estimated life expectancy. We estimated life expectancy from direct adjusted survival curves using proportional hazards models with age as the underlying time metric. The 95% confidence intervals for each BMI category were derived using a bootstrap estimator.
DISCUSSION
In this large cohort of women aged 40 to 93 years at entry, we observed a J-shaped association between technician measured BMI and mortality, with elevated risk among underweight women and monotonically increasing risks across the entire range of overweight (BMI 25.0–29.9) and obesity (BMI ≥ 30). Both overweight and obesity were associated with increased mortality among younger and older women, among women with and without a history of chronic illness, and among women who never smoked. Among women with screen detected breast cancers, women in the overweight BMI categories (25.0–27.4 and 27.5–29.9) experienced a 31% and 57% increase in mortality. Our study complements previous studies by demonstrating that excess weight, including modest overweight, may be associated with increased risk of mortality among persons with chronic illness and/or a history of smoking as well as healthy individuals.
Both overweight and obese women had shorter life expectancies relative to women of normal weight (reference BMI of 21.0–23.4). For women of 40 years of age, we estimated that overweight, obese (BMI 30.0–34.9), and very obese women (BMI ≥ 35.0) results in a loss of 1.7, 4.2, and 5.8 years of life-expectancy respectively (Figure 2). Our estimates are intermediate to those of the two prior studies which estimated approximately 1–2 (32) and 6 (33) years of life lost due to obesity among women of 40 years of age.
Most previous investigations of BMI in relation to mortality have relied on self-reported height and weight (1, 2, 19). This may have resulted in various degrees of overestimation of the adverse effect of adiposity on mortality because the true magnitude of excess weight is underreported (34). Because BMI in our study was based on measured weight and height, our findings cannot be attributed to measurement error due to inaccurate self-reporting.
We found a simple, positive association between overweight (based on measurements from 10 years in the past) and mortality without excluding participants based on disease, weight, or smoking history. In contrast, most studies reporting a statistically significant positive association between overweight and subsequent mortality risk are based upon analyses which excluded participants with prevalent chronic illness, those with recent weight loss, or smokers. As a consequence, conclusions of these studies were based on subsets as small as 11% (1, 19) and 17% (2) of the total number of available deaths, potentially limiting the generalizability of findings.
The association of overweight to mortality among persons with a history of chronic conditions (2, 19, 35) or a history of smoking (2, 19, 36) is currently a question of intense debate. On the whole, prior publications have not supported a positive association between overweight and mortality among these groups (2, 19, 35, 36). However, due to the high prevalence of morbidity in these populations, illness-induced weight loss may potentially introduce bias to the BMI and mortality relation.
Our finding of a positive association of overweight and obesity with mortality among women with chronic conditions may be due to our use of a BMI that preceded baseline by 10 years. Prior evidence shows that the potential for reverse-causation (i.e. illness-induced weight loss) does not necessarily apply to all persons with chronic illness (10). Rather, evidence suggests that the potential for reverse-causation is greatest in those chronically ill participants who die within the first few years of assessment of BMI (10). Thus, by not including deaths during the 10 years following BMI assessment, our study may selectively eliminate from analysis those chronically ill participants most likely to have lost weight before the BMI measurement, and to have led to bias from reverse causation.
Several previous investigations have examined the BMI and mortality relation using weights assessed prior to follow-up and have generally found that a past weight results in a stronger positive association with mortality than more recent weight (14–19). For example, early actuarial studies noted that increasing time since measurement of weight resulted in a strengthening of mortality risk for individuals of above average weight (14, 15). More recently, the NIH-AARP Diet and Health Study (19) reported that the strength of the association between overweight and mortality increased by about 10% when considering past weight as opposed to recent weight. A similar increase in the strength of the association between BMI and mortality when examining past weight was also observed in other previous studies among women 50 years of age and older (17, 37, 38). In younger women (i.e. aged 24 to 44 years at baseline), the relative risk of mortality associated with overweight increased substantially (by an additional 37%) when considering overweight at age 18 as compared with baseline overweight (18). However, the association of past measured weight and mortality has been examined only in the actuarial studies mentioned above (14, 15).
In a study reviewing the effect of excluding early deaths, Allison and colleagues reported that such exclusions resulted in statistically significant but relatively modest strengthening of the association between BMI and mortality (39). However, the studies reviewed typically excluded deaths only through the first two or three years after baseline, as opposed to the 10 years of deaths excluded in our study. Moreover, exclusion of early deaths may be mostly relevant to persons with chronic disease, but such groups were not specifically examined in the Allison study (39).
The positive association of overweight with mortality among women with a history of chronic conditions may reflect the predominating causes of mortality for individuals with these conditions. For example, among women with breast cancer, our findings are driven by the association of BMI with cancer mortality as opposed to cardiovascular mortality and therefore are not generalizable to all individuals. Chronic conditions in our cohort primarily consisted of coronary heart disease, diabetes, and breast cancer. The BMI and mortality association may be different for individuals with other specific conditions.
The association between overweight and mortality among smokers in our study may be due to reasons other than elimination of reverse-causation when examining past BMI. In previous studies, overweight may have had an artificially weak association with mortality due to residual confounding. In our cohort, current and former smoking women reported relatively light smoking intensity on average, potentially minimizing the impact of residual confounding by smoking in our study.
Our study did not include deaths during the initial 10 years following assessment of weight, thus it may not capture the relation of more recent BMI to mortality from acute conditions. In prior studies, researchers have observed decreased mortality from some acute diseases such as pneumonia (40) and tuberculosis (41) among the overweight and obese. The relation of overweight and obesity to mortality from acute disease is a subject of ongoing investigation and debate.
Body mass index does not discriminate between fat mass and fat free mass. We lacked data on body fat distribution, such as central adiposity, a known predictor of premature mortality (37). Although we adjusted for numerous potential confounding variables, it is possible that unmeasured or unknown factors associated with both weight and mortality partly explain the associations observed. Because participants in our study represent white women of middle and upper income who consented to participate in a screening program, our findings may not apply to all women. Our assessment of physical activity and smoking status occurred 10 years after the clinical assessment of weight. However, this may not be important if the strength of the association of BMI with physical activity and smoking status remained relatively consistent over that 10 year time frame.
In conclusion, we found excess risk of mortality and decreased life-expectancy among the underweight, overweight, and obese regardless of preexisting disease or smoking status. Unlike many previous prospective studies that found a statistically significant association between overweight and mortality only amongst groups of participants with no history of chronic disease or smoking, our findings are based on technician measured weight and height in a cohort which includes these groups.
Acknowledgments
Supported by the Intramural Research Program and TU2CA105666 from the National Cancer Institute, National Institutes of Health. We have no conflict of interest relevant to this article. The views expressed are those of the authors. We would like to thank Leslie Carroll and Matthew Butcher at Information Management Services and Tawanda Roy at the Nutritional Epidemiology Branch for research assistance.
References
- 1.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, et al. Body weight and mortality among women. N Engl J Med. 1995;333:677–85. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 2.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–7. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 4.Gu D, He J, Duan X, Reynolds K, Wu X, Chen J, et al. Body weight and mortality among men and women in China. JAMA. 2006;295:776–83. doi: 10.1001/jama.295.7.776. [DOI] [PubMed] [Google Scholar]
- 5.Dey DK, Rothenberg E, Sundh V, Bosaeus I, Steen B. Body mass index, weight change and mortality in the elderly. A 15 y longitudinal population study of 70 y olds. Eur J Clin Nutr. 2001;55:482–92. doi: 10.1038/sj.ejcn.1601208. [DOI] [PubMed] [Google Scholar]
- 6.Breeze E, Clarke R, Shipley MJ, Marmot MG, Fletcher AE. Cause-specific mortality in old age in relation to body mass index in middle age and in old age: follow-up of the Whitehall cohort of male civil servants. Int J Epidemiol. 2006;35:169–78. doi: 10.1093/ije/dyi212. [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 8.Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol. 2006;35:93–9. doi: 10.1093/ije/dyi272. [DOI] [PubMed] [Google Scholar]
- 9.Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341:427–34. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- 10.Stevens J, Juhaeri, Cai J. Changes in body mass index prior to baseline among participants who are ill or who die during the early years of follow-up. Am J Epidemiol. 2001;153:946–53. doi: 10.1093/aje/153.10.946. [DOI] [PubMed] [Google Scholar]
- 11.Stevens J. Impact of age on associations between weight and mortality. Nutr Rev. 2000;58:129–37. doi: 10.1111/j.1753-4887.2000.tb01847.x. [DOI] [PubMed] [Google Scholar]
- 12.Cigarette smoking-attributable morbidity---United States, 2000. MMWR Morb Mortal Wkly Rep. 2003;52:842–4. [PubMed] [Google Scholar]
- 13.Flegal KM, Graubard BI, Gail MH, Williamson DF. Underweight, Overweight, Obesity, and Excess Deaths--Reply. JAMA. 2005;294:552-b. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 14.Build Study 1979. 1st ed. Chicago: Society of Actuaries and the Association of Life Insurance Medical Directors of America; 1980. [Google Scholar]
- 15.Blair BF, Haines LW. Mortality experience according to build at the higher durations. Transactions of Society of Actuaries. 1966;18:35–45. [Google Scholar]
- 16.Calle EE, Teras LR, Thun MJ. Obesity and mortality. N Engl J Med. 2005;353:2197–9. doi: 10.1056/NEJM200511173532020. [DOI] [PubMed] [Google Scholar]
- 17.Losonczy KG, Harris TB, Cornoni-Huntley J, Simonsick EM, Wallace RB, Cook NR, et al. Does weight loss from middle age to old age explain the inverse weight mortality relation in old age? Am J Epidemiol. 1995;141:312–21. doi: 10.1093/aje/141.4.312. [DOI] [PubMed] [Google Scholar]
- 18.van Dam RM, Willett WC, Manson JE, Hu FB. The relationship between overweight in adolescence and premature death in women. Ann Intern Med. 2006;145:91–7. doi: 10.7326/0003-4819-145-2-200607180-00006. [DOI] [PubMed] [Google Scholar]
- 19.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–78. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 20.Report of the Working Group to Review the National Cancer Institute-American Cancer Society Breast Cancer Detection Demonstration Projects. J Natl Cancer Inst. 1979;62:639–709. [PubMed] [Google Scholar]
- 21.Schairer C, Byrne C, Keyl PM, Brinton LA, Sturgeon SR, Hoover RN. Menopausal estrogen and estrogen-progestin replacement therapy and risk of breast cancer (United States) Cancer Causes Control. 1994;5:491–500. doi: 10.1007/BF01831376. [DOI] [PubMed] [Google Scholar]
- 22.Munoz KA, Ballard-Barbash R, Graubard B, Swanson CA, Schairer C, Kahle LL. Recall of body weight and body size estimation in women enrolled in the breast cancer detection and demonstration project (BCDDP) Int J Obes Relat Metab Disord. 1996;20:854–9. [PubMed] [Google Scholar]
- 23.Colbert LH, Lacey JV, Jr, Schairer C, Albert P, Schatzkin A, Albanes D. Physical activity and risk of endometrial cancer in a prospective cohort study (United States) Cancer Causes Control. 2003;14:559–67. doi: 10.1023/a:1024866827775. [DOI] [PubMed] [Google Scholar]
- 24.Albanes D, Conway JM, Taylor PR, Moe PW, Judd J. Validation and comparison of eight physical activity questionnaires. Epidemiology. 1990;1:65–71. doi: 10.1097/00001648-199001000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–9. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee World Health Organization. Tech Rep Ser. 1995;1995:1–452. [PubMed] [Google Scholar]
- 27.Kant AK, Schatzkin A, Graubard BI, Schairer C. A prospective study of diet quality and mortality in women. JAMA. 2000;283:2109–15. doi: 10.1001/jama.283.16.2109. [DOI] [PubMed] [Google Scholar]
- 28.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 29.Ghali WA, Quan H, Brant R, van Melle G, Norris CM, Faris PD, et al. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286:1494–7. doi: 10.1001/jama.286.12.1494. [DOI] [PubMed] [Google Scholar]
- 30.Korn EL, Graubard BI. Analysis of health surveys. 1. New York: Wiley; 1999. [Google Scholar]
- 31.Efron B, Tibshirani RJ. An introduction to the bootstrap. 1. New York: Chapman & Hall; 1993. [Google Scholar]
- 32.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–93. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 33.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 34.Perry GS, Byers TE, Mokdad AH, Serdula MK, Williamson DF. The validity of self-reports of past body weights by U.S. adults. Epidemiology. 1995;6:61–6. doi: 10.1097/00001648-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–78. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 36.Effect of smoking on the body mass index-mortality relation: empirical evidence from 15 studies. BMI in Diverse Populations Collaborative Group. Am J Epidemiol. 1999;150:1297–308. doi: 10.1093/oxfordjournals.aje.a009961. [DOI] [PubMed] [Google Scholar]
- 37.Folsom AR, Kaye SA, Sellers TA, Hong CP, Cerhan JR, Potter JD, et al. Body fat distribution and 5-year risk of death in older women. JAMA. 1993;269:483–7. [PubMed] [Google Scholar]
- 38.Corrada MM, Kawas CH, Mozaffar F, Paganini-Hill A. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163:938–49. doi: 10.1093/aje/kwj114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allison DB, Faith MS, Heo M, Townsend-Butterworth D, Williamson DF. Meta-analysis of the effect of excluding early deaths on the estimated relationship between body mass index and mortality. Obes Res. 1999;7:342–54. doi: 10.1002/j.1550-8528.1999.tb00417.x. [DOI] [PubMed] [Google Scholar]
- 40.Lange P, Vestbo J, Nyboe J. Risk factors for death and hospitalization from pneumonia. A prospective study of a general population. Eur Respir J. 1995;8:1694–8. doi: 10.1183/09031936.95.08101694. [DOI] [PubMed] [Google Scholar]
- 41.Sacks LV, Pendle S. Factors related to in-hospital deaths in patients with tuberculosis. Arch Intern Med. 1998;158:1916–22. doi: 10.1001/archinte.158.17.1916. [DOI] [PubMed] [Google Scholar]