Summary
Stromal-epithelial interactions may control the growth and initiation of cancers. Here we not only test the hypothesis that bone marrow derived cells may effect development of cancers arising from other tissue cells by forming tumor stroma, but also that sarcomas may arise by transformation of stem cells from the bone marrow and epithelial cancers may arise by transdifferentiation of bone marrow stem cells to epithelial cancers. Lethally irradiated female FVB/N mice were restored with bone marrow (BM) transplants from a male transgenic mouse carrying the polyoma middle T-oncoprotein under the control of the mouse mammary tumor virus promoter (MMTV-PyMT) and followed for development of lesions. Eight of 8 lethally irradiated female FVB/N recipient mice, restored with BM transplants from a male MMTV-PyMT transgenic mouse, developed Ychromosome negative (Y−) cancers of various organs surrounded by Y+ stroma. One of the female FVB/N recipient mice also developed fibrosarcoma and one a diploid breast adenocarcinoma (BCA) containing Ychromosomes. In contrast, only 1 of 12 control female mice restored with normal male bone marrow developed a tumor (lymphoma) during the same time period.. These results indicate not only that the transgenic bone marrow derived stromal cells may indirectly contribute to development of tumors in recipient mice, but also that sarcomas may arise by transformation of bone marrow stem cells and that breast cancers arise by transdifferentiation of bone marrow stem cells, presumably by mesenchymal-epithelial transition.
Keywords: Cancer, Stem Cells, Bone Marrow Transplantation, Breast Cancer
Introduction
Circulating bone marrow derived stem cells (BMDSCs) have been reported to give rise to epithelial cells [1–3] and can also give rise to cancers in various epithelial organs. For example, following the finding in female recipients of male BM grafts that the epithelium of the damaged human GI tract is repopulated by male cells [4], it was reported that C57BL/6 female mice transplanted with ROSA26 male bone marrow cells and infected with Helicobacter pyloris develop gastric carcinomas arising from the ROSA 26 donor male cells [5]. In addition, in a human T-lymphocyte virus transgenic rat model, hematopoietic progenitor cells give rise to epithelial thymomas [6]. A skin carcinoma in a human female receiving a kidney graft from a male donor contained Y+ cells in tumor nests [7], and various other cancers in human females receiving male donor bone marrow transplants have been found to contain about 1:10,000 cancer cells with the Ychromosome, suggesting a process called developmental mimicry [8]. We originally sought to test the hypothesis that BMDSCs could give rise to liver cancer. However, we found that only about 1/20,000 liver nuclei of female recipients could be demonstrated to contain a Ychromosome, even after liver injury {9,10}, and that the model system that we attempted to use for this approach was not suitable. Thus, not only was BMDSC transformation to epithelial cells extremely rare in the liver, but also the hepatocarcinogenic regimens to be used were highly toxic to the DDPIV- rats that we proposed to use as recipients.
Therefore, we changed directions to test whether BMDSCs from a male donor carrying a transgene with a strong oncogene under the control of a promoter activated in the mammary gland, would give rise to breast cancer when they were transplanted into irradiated wild-type female recipients. If the BMDSCs circulate to the breast and transdifferentiate into breast epithelial tissue, then the MMTV promoter would be activated, the PyMT oncoprotein expressed, and BCA would develop. We reasoned that this model would be much more likely to show the rare event of transdifferentiation required for BMDSCs to become epithelial cancers than the BM to liver cancer model.
Bone marrow transplantation to irradiated recipients includes at least two stem cell populations, hematopoietic and mesenchymal. The hematopoietic stem cells restore the blood cells of the irradiated recipient; the mesenchymal stem cells replace the tissue stroma of the recipient, usually over a longer period of time than that required for blood cell replacement. After transplantation of MMTV-PyMT male bone marrow into female recipients we found that much of the mesenchymal stroma and endothelial cells were replaced by donor cells within a few months. Then, 1 recipient mouse developed a Y+ fibrosarcoma, and 1 recipient female mouse developed a Y+ breast cancer. In addition, seven of the 8 recipient mice developed cancers of diverse origin that did not contain the Ychromosome, but were surrounded by Y+ stroma and some developed cancers with little or no stroma. This implies at least five possible mechanisms leading to cancers in the recipient mice: 1) directly from recipient tissue without involvement of transplanted cells 2) from recipient cells influenced by transplanted mesenchymal cells; 3) by fusion of BMDSCs with host cells; 4) by transformation of mesenchymal stem cells; and 5) by transdifferentiation of BMDSCs to breast cells.
Materials and methods
Mice
Transgenic FVB.Cg-Tg(ACTB-EGFP)B5Nagy/J (FVB.EGFP) mice, stock # 003516, and wildtype FVB/NJ (FVB) mice, stock # 001800, were purchased from Jackson Laboratories (Bar Harbor, Maine). Male transgenic FVB/N-Tg(MMTV-PyMT)634Mul (FVB.PyMT) mice, strain # 01XE3, were obtained from the NCI Mouse Repository (Frederick, MD). Genotyping of FVB.PyMT mice for presence of the polyoma virus middle T antigen oncogene was performed by PCR on DNA extracted from tail tips, following standard instructions and with the use of primer sequences obtained from NCI. Primers (5’-GGAAGCAAGTACTTCACAAGGG-3’, 5’-GGAAAGTCACTAGGAGCAGGG-3’) were obtained from Integrated DNA Technologies (San Diego, CA). Mice were housed in the Wadsworth Center’s animal care facility on a 12 hr light/dark cycle, with controlled temperature and humidity, and free access to food and water. All animal experiments were approved by the Wadsworth Center’s Animal Care and Use Committee (IACUC).
Bone Marrow Transplantation
BM cells were isolated from femurs and tibias of 10–12 week-old male FVB.PyMT donors sacrificed by CO2 exposure/cervical dislocation. Erythrocytes were lysed with ammonium chloride, and cell viability was checked by trypan blue exclusion; exclusion was routinely > 95%. Cells were suspended in Hank’s balanced salt solution and injected in a volume of 100 µl via a 27-gauge needle.
A total of eight recipient female FVB mice, 8 weeks old, were irradiated (900–1,050 rads; 137Cs source; Isomedix, Parsippany, NJ) and transplanted intravenously within 2 hrs with 1–2 × 106 unfractionated male-donor BM cells. Engraftment was considered complete at 1 month post-irradiation survival.
Immunohistochemistry
At autopsy, various tissues were collected and either fixed in 10% buffered formalin or snap frozen in Tissue-Tek OCT compound (Sakura Finetek USA, Torrance, CA) by dropping into 2-methylbutane (EMD Chemicals Inc., Gibbstown, NJ) on dry ice; tissues were then stored at −80°C. The following antibodies were used: anti-cytokeratin (CK)14 (catalogue # MCA890; AbD Serotec, Raleigh, NC), anti-CK5/6 (catalogue # MAB1620) and anti-CK18 (catalogue # CBL177); both from Chemicon Corporation, Temecula, CA., anti-panCK (catalogue # VP-C419; Vector Laboratories, Burlingame, CA), anti-p63 (catalogue # MS-1081; Thermo-Fisher Scientific, Fremont, CA), anti-alpha-smooth muscle actin (catalogue # A2547; Sigma Corporation, St. Louis, MO), anti-CD49f (catalogue # 313605, Biolegend Co., San Diego, CA), and anti-Polyoma virus, Middle T-antigen (catalogue # NB100-2749; Novus Biologicals, Littleton, CO); secondary antibody biotinylated goat anti-rat; Jackson ImmunoResearch, West Grove, PA.; catalogue number 112-065-167).
Paraffin-embedded tissues were sectioned at 5 µm, attached to charged slides and rehydrated through xylene and a graded ethanol series to water. Slides were subjected to antigen retrieval by boiling in a pH 6 citrate buffer for 5 min. Sections were blocked in 2% normal donkey serum and then incubated overnight at 4°C with the primary antibody. Following biotinylated secondary antibody incubation (60 min at room temperature) and extravidin-peroxidase (Sigma, catalogue # E2886), slides were developed in diaminobenzidine and mounted in Permount (Fisher Scientific, Pittsburgh, PA).
In Situ Hybridization
Formalin-fixed, paraffin-embedded sections were deparaffinized through xylene washes and rehydrated through a graded ethanol series. A FITC-labeled probe for the mouse Ychromosome (catalogue # 1189-YMF-01; Cambio Ltd., Cambridge, UK) was hybridized to pepsin-treated sections for 10 minutes at 60°C, followed by overnight incubation at 37°C, according to the manufacturer’s directions. Amplification of the probe was performed by primary (goat anti-fluorescein; Rockland, Gilbertsville, PA., catalogue # 600-101-096) and secondary (fluorescein-conjugated donkey anti-goat; Jackson Immunoresearch, West Grove, PA., catalogue number 705-095-147) antibody enhancement. Nuclei were stained with 4’,6-diamidine-2-phenylindole dihydrochloride (catalogue # 236276; Roche Applied Sciences, Indianapolis, IN.). Images were captured on an Olympus BX 51 microscope equipped with fluorescence detection and Optronics PictureFrame Version 1.2 software.
Tumor Harvest and Dissociation
Breast tumors from MMTV-PyMT FVB/N (002374, Jackson Laboratory, Bar Harbor, ME, http://www.jax.org) transgenic mice were harvested when the tumors were approximately 1–2 cm3 (2–2.5 g), minced with a razor blade, and suspended into Dulbecco’s Modified Eagle Medium (DMEM) with 10% calf serum, 10ng/ml mouse epidermal growth factor (EGF) and 5ug/ml bovine insulin for culture.
Cell Staining and Flow Cytometry
Cells were stained at a concentration of 1 X 106 cells per 100 ul of HBSS with 2% heat-inactivated calf serum (HICS). Antibodies at appropriate dilutions (CD24-fluorescein isothiocyanate (FITC), Biolegend Inc; CD49f-Alexa Fluor® 647, Biolegend Inc; CD45-Pacific Blue, Biolegend Inc; CD44-Pacific Blue, Biolegend Inc; CD29-phycoerythrin (PE), Biolegend Inc; Sca-1-phycoerythrin (PE), Biolegend Inc; CD45-phycoerythrin (PE), Biolegend Inc; CD31-phycoerythrin (PE), Biolegend Inc; Ter119-allophycocyanin (APC), Biolegend Inc) were added. Staining duration was for 30 minutes on ice, with light agitation of the staining vessels every 5 minutes. Cells were then washed with staining medium and resuspended in staining medium. The stained specimens were then analyzed using FACSVantage (BD Biosciences, San Diego, http://www.bdbiosciences.com) or FACSAria with either Diva or CellQuest software (BD Biosciences). Cells with appropriate CD24 status were then collected. A small sample of the double-sorted cells was reanalyzed for purity. The cell counter of the flow cytometers was used to determine cell numbers. Cells were collected into HBSS with 2% HICS. Epithelial cells were CD24(+)CD49f(+)CD44lowCD29(+)Sca-1lowCD45(−)CD31(−)Ter119(−); mesenchymal cells were CD24(−)CD49f(−)CD44highCD29(+)Sca-1highCD45(−)CD31(−)Ter119(−).
Culture of Tumor Derived Fibroblasts (Mesenchymal cells)
Over 70 cell lines from MMTV-PyMT and EGFP MMTV-PyMT tumors have been derived, cloned and transplanted in FVB/N mice following proven culture techniques 20. Cells cultured from primary tumors were found to express three phenotypes by flow cytometry: CD 24−44lo; CD24+CD44; and a mixture. The cell lines were characterized as epithelial cell-like (ECL) or mesenchymal cell-like (MCL) by inverted phase microscopy. ECL cells (doubling time 24–48hr) grow more rapidly than MCL cells (doubling time 48–72hr). The ECLs are all CD24+CD44loCD29+CD49f+Sca-1Lo, and the MCLs are CD24−CD44hiCD29+CD49f-Sca-1Hi. The lines of mixed cell origin have been sorted; the CD44lo cells are ECL and the CD44hi cells are MCL (data not shown) indicating a mixture of cell types in the original culture. Mesenchymal cell line 393 was analyzed by PCR and RT-PCR for PyMT and mRNA.
Reverse Transcription Polymerase Chain Reaction (RT-PCR) and Polymerase Chain Reaction (PCR)
Total RNA and DNA were purified from cells using RNeasy Mini Kit and DNeasy Blood & Tissue Kit according to the manufacturer’s instructions (Qiagen). USB two-step RT-PCR kit was used to perform the analysis according to the manufacturer’s instructions (USB). Primers for mouse genes: PyMT transgene, sense primer 5’-GGAAGCAAGTACTTCACAAGGG-3’, antisense primer 5’-AAAGTCACTAGGAGCAGGG-3’; and b-actin, sense primer 5’-CCAGAGCAAGAGAGGTATC-3’, antisense primer 5’-GACCAGAGGCATACAGGGAC-3’.
Laser Capture of tissues
Formalin fixed paraffin embedded tissues were sectioned into 6 um segments and mounted onto Membrane slides (Zeiss). The tissues were rapidly hydrated through two changes of xylene, 100%, 95%, 75% ethanols, and finally dH2O. For contrast, the slides were stained in toluene blue for three minutes before washing in water and dehydrating through two changes of 95% and 100% ethanol and xylene. Xylene exposure was kept to a minimum to prevent the membranes from dissolving. Slides were cut in close temporal proximity to laser capture. Tissues were captured using a PALM MicroBeam workstation (Zeiss). The microdissected tissue fragments were collected into 200 uL AdhesiveCaps, positioned over the slides using the RoboCap arm attachment on the workstation. Samples were placed on ice until purification.
DNA and RNA purification
DNA was extracted from the microdissected tissues using the QIAamp DNA Micro Kit (Qiagen). Samples were incubated at 56° C for four hours to ensure that the proteinase K reaction sufficiently digested the tissues. Carrier RNA was added to the samples to increase binding efficiency to the MinElute columns during the wash steps. Samples were eluted using Buffer ATE from the QIAamp DNA FFPE Tissue Kit (Qiagen), which is a low-EDTA elution buffer optimized for sensitive downstream applications. RNA was isolated from separate samples of microdissected tissues using the RNeasy FFPE Kit (Qiagen). Carrier RNA was also added to these samples, which did not effect downstream applications.
Alternatively, RNA was isolated from whole tissue samples frozen in OCT. Prior to extraction, frozen sections were made from stock samples of experimental tissues to identify key areas of tissue for excision. Quickly, the sample was thawed from OCT and placed on dry ice. Tissue samples were ground to a fine powder by mortar and pestle under liquid nitrogen and lysed immediately using the mirVana total RNA isolation kit (Ambion). This preparation allowed for phenol-chloroform extraction of the RNA, avoiding any complications with fatty tissue hindering column purification. The RNA samples were also DNase treated with Turbo DNA-free (Ambion) prior to any reverse transcriptase reaction to remove any possible DNA contamination.
cDNA prep and qPCR
Reverse transcription of RNA samples was performed using the High Capacity RNA-to-cDNA Master Mix (Applied Biosystems), performing both RT+ (with enzyme) and RT- (without enzyme) reactions. Power SYBR Green PCR Master Mix (Applied Biosystems) was used to amplify the cDNA and DNA isolated from the laser microdissected samples, which contains AmpliTaq Gold DNA Polymerase to give greater specificity with low template concentrations. Primers for qPCR of the Polyomavirus Middle T antigen gene were designed using the Primer-BLAST website, blasting against the representative region in the Murine polyomavirus (a 1.33kb region from base 175 to 1502): Forward PyMT primer: CTG CTG GAA GAA GAC GAA AT; Reverse PyMT Primer: GTT GCA TTG AAT GAG CTC TG; PCR product size 343 base pairs. The PyMT primers were found to be specific to the template, with no other targets found after blasting against the normal mouse genome. The qPCR samples were run on the 7900HT Fast Real-Time PCR System (Applied Biosystems) using the 96-well block, with a dissociation curve at the completion of 40 cycles.
qPCR Data Analysis
Results were analyzed both by the SDS2.2 software and gel electrophoresis of the qPCR products. dCt values were generated normalizing to B-Actin Ct values. The experimental samples were compared for the PyMT primer set, using the positive and negative controls as threshold values. Any values falling between the control range were considered weakly positive. qPCR products for the SRY primer set were run on 1.2% agarose gels to confirm positive results. Weak bands were considered weakly positive. We also used peak analysis to predict results pending confirmation of gel electrophoresis.
Results
Development of tumors
Untreated Female MMTV-PyMT mice begin to develop palpable masses by 4–5 weeks of age and large multifocal breast cancers by 10–12 weeks of age. Male mice develop palpable masses about 3 months of age and large cancers by about 5 months of age. The histologic appearance includes a rage of ductal and glandular structures similar to human breast cancers (Supplemental Figure 1). As expected breast cancer cells of primary tumors arising in male mice stain for Ychromosome, tumors from the female mice are negative (Supplemental Figure 2). Tumors in other organs are not seen at the time that the transgenic mice die from breast cancer.
A summary of the results of an experiment in which a total of 8 female FVB/N mice were irradiated with 900 to 1050 rads at 2 months of age and rescued by transplantation of 1–2 X 106 BM cells from transgenic MMTV-PyMT FVB/N male donors is presented in Table 1. The BM-transplanted mice ranged in age from 14 to 22 months at the time of euthanasia as a result of the size of the tumor masses or weight loss (in accord with the approved IACUC animal welfare procedures). Examples of the tumors found are illustrated in Figure 1, A–H. These include a squamous cell carcinoma of the skin (found in the breast), lung adenomas, a lung adenocarcinoma, adrenal medullary tumors, a Harderian gland adenocarcinoma, a perirenal fibrosarcoma, a diffuse mixed cell cleaved lymphoma [11], and a salivary gland adenocarcinoma, as well as one breast adenocarcinoma (BCA), one cystic adenomatous mammary hyperplasia and two mammary intraductal neoplasias (MIN).
Table 1.
Lesions from female FVB/N mice transplanted with male MMTV-PyMT bone marrow cells.
| Mouse No. |
AGE AT DEATH* (Mos.) |
Gross Description | Glomerulo- Sclerosis |
Histologic Findings | Y+ |
|---|---|---|---|---|---|
| 1 | 14 | Breast mass (1.5cm) Splenomegaly |
+ | Squamous Cell Carcinoma Extramedullary Hematopoiesis Mammary Intraductal neoplasia |
No Yes No |
| 2 | 16 | Breast mass (2mm) Lung mass (3mm) Small spleen Firm lungs |
+++ | Atypical cystic mammary hyperplasia Lung Adenoma Splenic Atrophy. Chronic Pneumonia |
No No ND ND |
| 3 | 19 | Perirenal mass (3mm) | +++ | Perirenal Fibrosarcoma Chronic Pneumonia |
Yes |
| 4 | 19 | Breast masses, 0.7–1.2 cm Adrenal mass (4mm) Splenomegaly |
++ | Breast Adenocarcinoma Adrenal Medullary Tumor Cleaved Mixed Cell Lymphoma |
Yes No No |
| 5 | 19 | Adrenal mass (5mm) | +++ | Adrenal Medullary Tumor | No |
| 6 | 21 | Normal Periorbital mass (7mm) Lung (4mm) |
+++ | Breast Dilated ducts Periorbital Adenocarcinoma (Harderian Gland) Lung Adenocarcinoma |
No No No |
| 7 | 21 | Lung mass (3mm) | +++ | Lung Adenoma | No |
| 8 | 23 | Bilateral neck mass (7mm) | 0 | Salivary gland adenocarcinoma | No |
ND = not done.
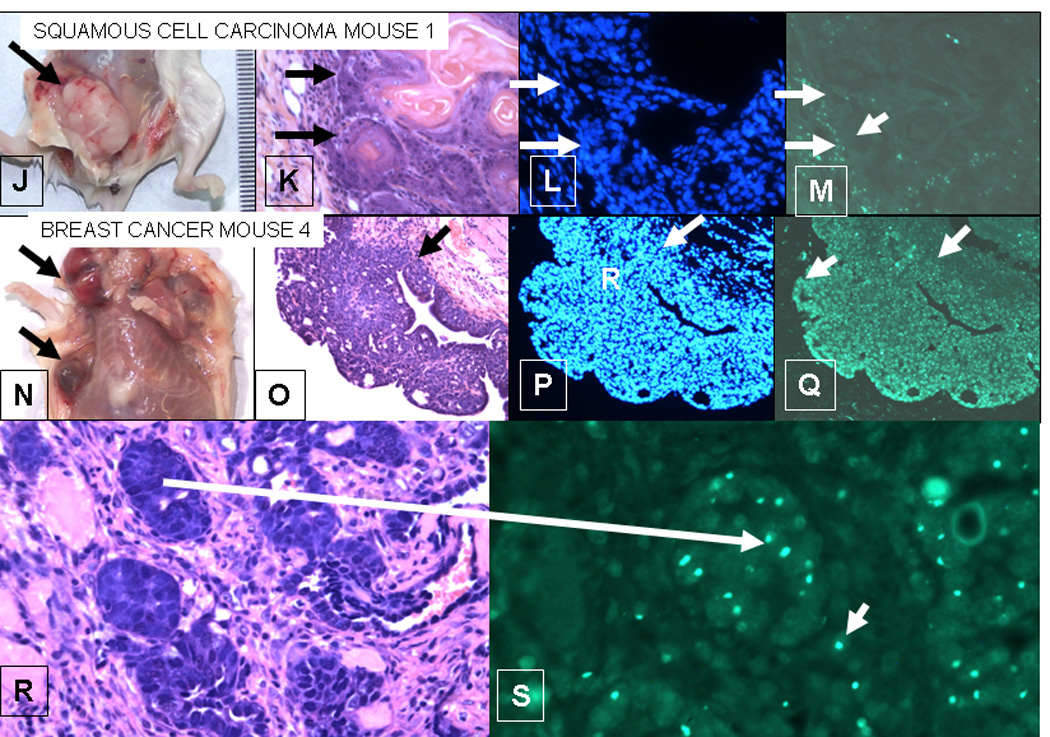
Figure 1.
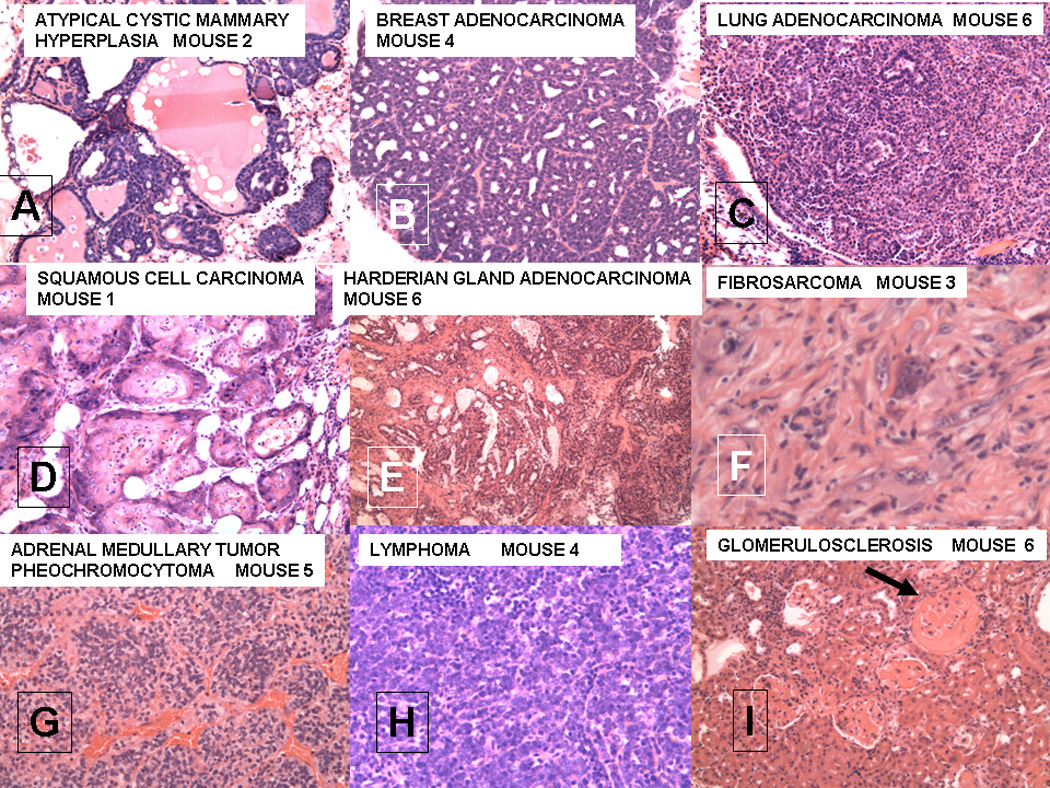
A–I. Various lesions seen in female FVB/N mice after lethal irradiation and bone marrow transplantation from male MMTV-PyMT donors;
J–M. Squamous cell carcinoma from mouse 1;
N–S. Breast cancer from mouse 4.
For A–I, each section is stained with hematoxalin and eosin. All magnifications are 20X except for F which is 40X. The microscopic patterns of the breast adenocarcinoma were varied with medullary, papillary and glandular forms.
J–M Squamous cell carcinoma from mouse 1. The squamous cell carcinoma in the breast of mouse 1 does not contain Y-Chromosomes, whereas the breast adenocarcinoma in mouse 4 (E–F) does. J. Gross lesion from mouse 1. K. H&E, L. DAPI (nuclei), M. in situ for Y-chromosome (40X). The arrows mark the border between the squamous cell carcinoma and the stroma. D shows strong Y-chromosome staining in the stroma of the tumor, but the tumor cells are negative.
N–S. Breast Cancer from mouse 4. N. Gross lesions in breast of mouse 4. The upper arrow point to a cystic lesion; the lower to a solid lesion. O. H&E showing a papillary structure. P. DAPI showing nuclei in the tumor. Q. In situ for Y-chromosomes (20X) showing up to 80% of nuclei in tumor contain Y-chromosome in this section. The arrows in O-Q delineate the border of the stroma and the tumor. In contrast to M, Q shows that the tumor labels strongly for Y-chromosomes, whereas the stroma is weakly labeled. R. H&E and S in situ for Y-chromosome of breast adenocarcinoma (40X). The larger arrow shows a focus of cancer in the H&E section that is labeled for Y-chromosome in a serial section. Note the bright labeling of Y-chromosomes in the tumor. The smaller arrows in J point to Y-chromosome containing cells that could be either single tumor cells or blood derived mononuclear cells in the stroma.
The number of tumors in the experimental mice as compared to age matched untreated controls and to a group of control WT female mice transplanted with WT bone marrow is given in Table 2. Statistical analysis using the two-tailed Mann-Whitney non-parametric test shows that the incidence of tumors in the experimental group is greater that in either control group. The incidence of tumors in the WT to WT BM transplant group is marginally different from that of the untreated controls. In historical controls 61% of 116 female FVB/N mice euthanized at 24 months had cancers, but there were no Harderian gland tumors, fibrosarcomas, salivary gland tumors or breast lesions [12]. In human studies, the cancer risk is 8.3 times higher in bone marrow transplant patients than in untransplanted normal controls [13], but the tissue origins of the human tumors (melanoma, oral cavity, liver, brain, and thyroid) are very different from the types of tumors seen in our study. The incidence of tumors in the WT control group is essentially the same as in the WT to WT group.
Table 2.
Incidence of cancers in control and experimental groups.
| GROUP | INCIDENCE | % | TIME TO DEATH | |
|---|---|---|---|---|
| MEAN | RANGE | |||
| 1. PyMT to WT | 8/8 | 100 | 19 | 14–23 |
| 2. WT to WT | 1/16 | 6 | 13.5 | 6–21 |
| 3. UNTREATED | 7/21 | 33 | 26 | 18–30 |
STATISTICAL ANALYSIS.
1 VS. 2 (p<0.001)
1 VS. 3 (p<0.001)
2 VS. 3 (p<0.065)
Glomeruloscerosis
The finding of severe glomerulosclerosis (Figure 1, I; for grading see Supplemental Figure 3) in the bone marrow recipients is unexpected. None of the sacrificed untreated control mice had glomerulosclerosis and glomerulosclerosis was not reported in the study that we used for comparison [12]. The possible role of irradiation and either normal BM transplantation or transgenic BM transplantation in causing glomerulosclerosis is not known. However, most of the WT to WT BM transplant mice died with glomerulosclerosis and chronic pneumonia. Five of the PyMT to WT experimental group were euthanized due to wasting apparently due to glomerulosclerosis and pneumonia; the tumors in 3 of these mice (#s 2, 5, and 7) were incidental. One of the reasons for the larger number of cancers in the WT compared to the WT to WT BM transplant group is that the mice in the WT group survived much longer than the mice in the WT to WT group, so that the WT to WT BM transplant group may not have lived long enough to get cancer.
Ychromosome positive tumors
Of all of the tumors in the experimental group only the fibrosarcoma in recipient mouse 2 and the breast cancer in recipient mouse 4 were positive for the presence of Ychromosomes by FISH analysis. Over 80% of the cells in these tumors were Y+ (Figure 1,N–S). The fibrosarcoma in mouse 2 contained fibroblastic and liposarcomatous zones, and both zones were Y+ The margin of the tumor contained a layer of lymphocytes. These were clearly separated from the fibroblastic part of the tumor and uniformly contained Y-chromosomes (Supplemental figure 4)..
None of the other 9 tumors had Ychromosomes in the tumor cell nuclei (For examples see Figure 1, J–M), and are most likely recipient derived. In all tissues of the recipients, there were Y+ cells that could be identified by morphology as mononuclear cells, endothelial cells and stromal cells, with by far the greatest number being stromal fibroblasts. For example, after transplantation of male bone marrow cells, the female recipient liver contained many Ychromosome positive endothelial, stellate, and Kuppfer cells, but less than 1/20,000 positive hepatocytes (Figure 2, A,B). It is of interest that some of the recipient derived tumors was surrounded by Y+ stromal cells (Figure 2, C,D), suggesting that the transgenic stromal cells may influence the malignant transformation of the recipient tissues. To determine if there might be ectopic expression of the PyMT oncoprotein, the onco-antigen in tissues was stained by immunoperoxidase (Figure 2, E–H), by analysis of fibroblasts cultured from a primary tumors in a PyMT mouse (Supplemental Figure 5) and by q PCR for mRNA (see below). The antigen was readily seen in the primary breast cancer from the donor mice and in the breast cancer in mouse 4, but was not seen in the other tumors. In addition, oncoprotein was not detected in the tumor stroma.
Figure 2.
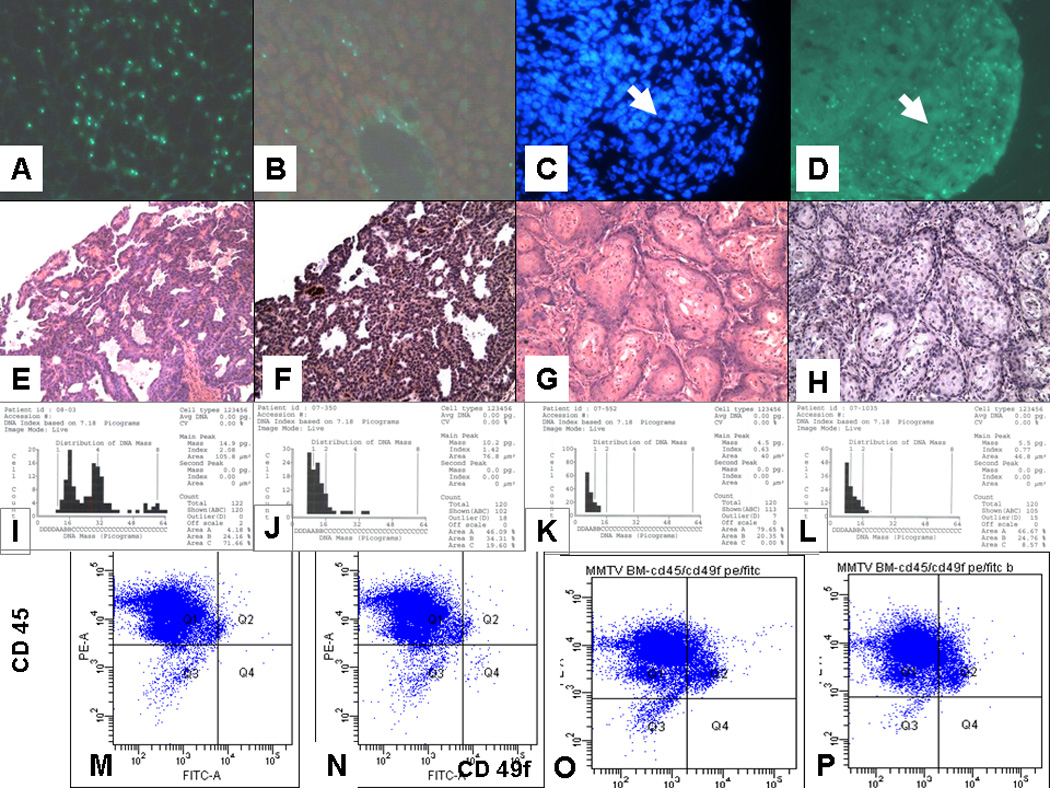
A–D. Y-chromsome labeling of control and experimental lesions. A. Male liver. Note high labeling of hepatocytes (70%). This is the expected frequency as some Y-chromosomes are not in the plane of the tissue section. B. Liver from female recipient of male bone marrow. Hepatocytes are not labeled, but many endothelial cells and Kupffer cells are. C,D Lung adenocarcinoma. C. DAPI; D. FISH. The tumor cells do label for Ychromosomes, whereas the stromal cells are heavily labeled. The arrows show the demarcation between the tumor and the stroma.
E–H. Paired H&E and immunoperioxidase staining for PyMT antigen. E,F. Breast cancer from female FVB/N mouse 4 receiving bone marrow from male MMTV-PyMT donor; G,H. Squamous cell carcinoma from mouse 1. The brown color in F is positive staining for PyMT antigen. There is no brown staining in H. Thus, expression of the PyMT antigen was only seen in the breast cancer cells and not in cancer cells or stroma of the other cancers in the recipient mice.
I–L. DNA Ploidy of I. Normal mouse liver; J. Squamous cell carcinoma from mouse 1; K. Breast cancer from male MMTV-PyMT mouse; L. Breast cancer from mouse 4. The normal liver is known to contain polyploid cells and is included as a positive control. The upper limit of the index for diploid cells is 1.2. By this criterion both the breast cancer from the male mouse (Index 0.63) and from mouse 4 (Index 0,77) are clearly diploid, whereas the control liver is polyploid (Index 2.08 with 3 peaks). The squamous cell carcinoma is also most likely diploid even though the index of 1.42 is greater than 1.2, because there is only one peak. The reason for the high index in the squamous cell carcinoma is most likely due to the fact that the cells are more dense so that some of the cells selected for scanning are overlapping. DNA ploidy analysis is performed with the CAS (Cell Analysis Systems) 200R qDNA analysis application. The instrument is calibrated before each session using a vendor (Cell Analysis Systems) supplied rat hepatocyte calibration slide with known DNA content. A minimum of 120 tumor cells from each specimen slide are identified and analyzed. The established cutoff of a DNA index of 1.2 or greater indicates an aneuploid specimen, under 1.2 indicates a diploid specimen. The DNA ploidy analysis was performed by Merrill Ross of the Department of Pathology, Albany Medical College.
M–P. Detection of breast cancer cells in bone marrow by flow cytometry. M. Normal bone marrow. N. BM cells were spiked by adding 100 breast cancer cells from a transplantable tumor derived from a male MMTV-PyMT mouse to 500,000 normal bone marrow cells. CD45 is a marker for bone marrow cells. CD49f is a marker for breast stem cells. BM derived cells (CD45+CD49f-) are present in Q 1 and breast stem cells (CD45-CD49f+) are detected in Q 4. By this analysis it is possible to detect 1 cancer cell in about 100,000 BM cells.
O. is flow pattern for BM from a normal 5 month old male mouse; P is flow pattern for BM from a 5 month old male MMTV-PyMT mouse. No BCA cells were detected in the BM of the MMTV-PyMT mouse. Since the BM donor was a 12 week old male MMTV-PyMT mouse (BM from 12 week old male MMTV-PyMT mice also had no detectible BCA cells in the BM), it is unlikely that the BCA seen in mouse 6 derived from metastatic cancer cells in the BM. In addition, no BCA cells were seen in the marrow of male mice using CD49f staining of BM cytospins.
Presence of the transgene and transgene product
The transgene was readily identified in tumor stromal cells in cultured in vitro, but mRNA expression was not detected in stromal cells isolated from primary tumors (Supplemental Figure 5). Molecular analysis on laser captured tissue to determine the presence of PyMT DNA and mRNA was not definitive. For example, as expected the breast cancer in mouse 4 was strongly positive for PyMT DNA and moderately positive for mRNA. The Y+ firbosarcoma was moderately positive for DNA, but weakly postitive to negative for mRNA. The squamous cell carcinoma (mouse 1) and the adrenal tumor (mouse 5) were also weakly positive for PyMT DNA, and for mRNA. Detection of PyMT DNA and mRNA at low levels could be due to tissue sampling as it is essentially impossible to extract tumor tissue without stroma. Samples believed to contain only stroma were weakly to moderately positive for DNA, and weakly positive for mRNA. Thus, low levels of production of PyMT by stroma cannot be ruled out, but the accuracy of this method for detecting low levels of mRNA is questionable.
Ploidy Analysis
To test for the possibility of fusion of bone marrow derived cells with breast cells in the breast cancer from mouse 4, DNA ploidy analysis was done on all tumors. The result for selected tumors is shown in Figure 2, I–L). This analysis definitively demonstrated that the breast cancer from mouse 4, as well as the other cancers, was diploid, and no evidence of fusion of donor and recipient cells (14–17) was found.
Tests for tumor cells in the donor bone marrow
Next we tested the possibility that the bone marrow from the donor male mice contained early breast cancer cells that metastasized from occult primary tumors at the time when the BM was taken for transplantation. Using cytokeratin (CK) staining for BCA cells it was shown that CK+ cells in the BM of human patients with BCA, also had a stem cell phenotype [18]. The BM of female MMTV-PyMT mice contains CK+ presumptive BCA metastatic cells as early as 4 weeks of age [19]. The BM used in the present experiment was taken from the male MMTV-PyMT donor at 12 weeks of age when no gross or microscopic breast lesions were found in the donor. To test directly for occult metastatic cells, we examined the BM of 12 week and 5 month old male MMTV-PyMT mice for the presence of CD49f, a well known marker for mouse breast stem cells [20] and for BCAs [21], by direct examination of the BM and by flow cytometry. We were unable to detect any CD49f positive cells in the male BM (Figure 2, M–P), even when the 5 month old mouse had large breast masses. Thus, it is very unlikely that the bone marrow used for transplantation, which was taken at from MMTV-PyMT males at 12 weeks of age when no tumors were palpable, contained tumor cells. From these data we conclude that the breast cancer in female recipient #4 was directly derived from transplanted male bone marrow cells.
Lymphoma
An additional finding of interest was a diffuse cleaved lymphoma [11] present in the spleen of the same mouse that had the BCA. This lymphoma, as well as an adrenal medullar tumor in mouse 4, was negative for Ychromosome. The lymphoma cells had medium sized cleaved nuclei. In the older literature this type of lymphoma in the mouse was called reticulum cell sarcoma. The Y− tumor cells are surrounded by and infiltrated with Y+ lymphocytes in the spleen (Supplemental Figure 6). The adjacent follicles in the spleen contain many Y+ cells and there are positive cells admixed with the lymphoma cells due to inclusion of non-lymphoma lymphocytes within the tumor. The lymphoma cells stained with anti-CD3 and not with anti-CD45R or B220, indicating that it is a T-cell lymphoma. The finding that the lymphoma did not arise from the transplanted male BM suggests that either some female BM stem cells remained after the irradiation, or else that the lymphoma arose from progenitor cells later during lymphocyte development and not directly derived from the bone marrow stem cells.
Discussion
The development of a fibrosarcoma and a breast cancer derived from donor bone marrow after irradiation and transplantation, as well as other cancers not derived from the bone marrow donor suggests both an indirect microenvironmental effect of transgenic mesenchymal stem cells, as well as a direct transformation and a mesenchymal-epithelial transition of pluripotent bone marrow derived stem cells.
The microenvironment is critical for tumor initiation (22,23), as well as for growth (22), invasion (24) and metastasis (25) of cancer. Recently the role of the tissue niche for normal development and tissue renewal has been critically evaluated and a role for the initiation and growth of cancer restated (26,27). For breast cancer (BCA), Bissell and co-workers have termed the relationship between the tissue stromal cells and the mammary epithelial cells (MEC) as “dynamic reciprocity” (28). The development of BCA is associated with a dramatic loss or aberration of basement membrane and myoepithelial cells and the gain of peritumoral myofibroblasts (29–31). The present results suggest that the presence of MMTV-PyMT bone marrow derived stroma induces various cancers in wild type recipient mice. At this time, it is not clear how this is accomplished. So far, we have been unable to demonstrate the presence of the polyoma-middle-T-antigen either in the tumors or in the stroma cells by immunolabeling and cultured transgenic tumor fibroblast cultures do not contain mRNA for PyMT. However, the most likely explanation is that there is a low level of ectopic expression by the stromal cells that induces malignant changes in the associated tissues. This possibility is supported by a very low level of mRNA for PyMT in the Y-tumors and in some of the stroma. Thus, one possible explanation for the effect of the stroma is a low level of PyMT production that somehow induces tumors in recipient tissues. However, it is also possible that qPCR analysis is giving false positives when pushed to detect very low levels. Also, if the stromal cells are producing mRNA for PyMT, we would expect to see Y+ sarcomas as in mouse 3, instead of Y− tumors of other tissues.
The Y+ fibrosarcoma suggests that in this mouse the transplanted BM mesenchymal cell directly transformed to produce this cancer. This suggests that there may have been expression of the PyMT gene in this tumor. However, qPCR for mRNA was equivocal. A low level was detected, much lower than that found in the Y+ BCA.
The presence of a Y+ BCA in the female recipient of a BM transplant from a male mouse proves the principle that cells in the BM marrow can give rise to breast cancer. There are at least four possible explanations for this: 1) mesenchymal-epithelial transition of BM mesenchymal stem cells to breast cells, with activation of the MMTV promoter and expression of the PYMT transgene [5–6]; 2) fusion of cells from the BM with breast cells, and activation of the transgene [14–17); 3) developmental mimicry [8]; and 4) the presence of occult metastatic BCA cells in the BM donor that recirculate and home to the breast of the recipient [18,19. At this point, mesenchymal-epithelial transition is the most likely explanation. If there had been an early fusion event followed by reduction division, the cells of the BCA could be diploid, but this sequence of events would require that the diploid cells of the tumor proliferate at a higher rate and crowd out the polyploid cells. Reduction division has been proposed as a possibility in the liver cells of experimental tyrosinenemic mice with severe liver injury restored by normal BMDCs [32]. However, the liver is known to contain polyploid cells that undergo reduction division in proliferative responses to liver injury [33], a response not seen in most other tissues. In models where fusion events have been described, most of the fused cells persist in the tissues without reduction division [32]. The number of Y+ positive cells in the tumor is much too high to explained by developmental mimicry, in which only about 1:10,000 cells in a tumor is reported to be derived from a BMDC [8]. However, it is possible that a single BMDC was influenced by normal mammary epithelial cells (MEC), such that the BMDC acquired the properties of the MEC and activated the MMTV promoter. Thus fusion, developmental mimicry, and occult tumor cells in the BM each appear highly unlikely.
From these considerations, we conclude that the BCA found in mouse 4 arose from a mesenchymal stem cell in the BM that underwent mesenchymal-epithelial transition to activate the MMTV promoter, expressed the PyMT antigen and underwent malignant transformation. This is not without precedent. After treatment of BM cells with 3’-methycholanthere the transformed BM cells formed many tumor types including epithelial tumors in vitro and on subcutaneous transplantation in vivo [34]. Using DNA chimerism, Golfinopouloss et al. [35] reported a donor-derived breast cancer in a woman who received a BM transplant from her sister 14 years earlier. However in a study of 37 patients who development various cancers after BM transplantation Au et al. [36] found no donor derived tumors.
Epithelial-mesenchymal transition (EMT) is defined as the loss of epithelial characteristics and the acquisition of a mesenchymal phenotype. EMT is an essential component of metazoan embryogenesis [37]. In breast cancer EMT is associated with increased aggressiveness, invasiveness and metastasis [38]. However, it is debated as to whether EMT is an example of transdifferentiation of epithelial cells to mesenchymal cells [39], or an expression of the pluripotency of breast cancer stem cells [40]. In any case, EMT represents a progression of a BCA to a more malignant phenotype, leading to expression of mesenchymal-like products and behavior. One of the observations that confound proponents of EMT is the fact that metastatic breast cancers have histologic and metabolic features similar to the primary epithelial tumor. This is explained by a process whereby EMT-mediated invasion metastases is followed by a reverse mesenchymal-epithelial transition (MET) to allow colonization of secondary sites [40]. The ability of the cancer cells during this process to express both epithelial and mesenchymal phenotypes was referred to as a “metastable phenotype” [41]. Our results support the hypothesis that mesenchymal cells may transdifferentiate into breast cancer cells or contribute to development of BCA by cell-cell interactions or fusion. Fusion of human BCA cells with mouse stromal cells after injection of the human BCA cells into the mammary glands of nude mice has been reported [42]. However, since the BCA in mouse #4 is diploid, there is no evidence for fusion as the mechanism in this study.
The polyoma middle T-antigen transgenic mouse (FVB.PyMT) model used in the present experiments is a widely used model of human breast cancer, enabling the analysis of genes and pathways involved in tumor progression from premalignant disease to metastases [43–47]. Mammary gland tumors occur spontaneously in all homozygous carriers, with palpable tumors appearing in females as early as 4–5 weeks of age and in males at a median age of 83 days [43]. The appearance of BCA correlates with transgene expression in the breast epithelium. Lower amounts of the middle T antigen found in salivary glands, ovaries and epididymis are not accompanied by any growth abnormalities [43], but hyperplasia and tumors in the lung and salivary gland can be induced in MMTV-RARβ4 transgenic mice treated with dexamethasone [48]. We did find one salivary gland adenocarcinoma, but it did not contain Ychromosomes or express PyMT antigen (mouse 8).
Thus we conclude that the transgenic BMD mesenchymal cells may indirectly contribute to development of tumors derived from the recipient mice by creating a transforming microenvironment, and directly contribute by transformation of BMD mesenchymal cells to sarcoma and by mesenchymal-epithelial transition to form breast cancers. However, transdifferentiation of BMD hematopoietic stem cells cannot be ruled out at this time.
Brief Statements.
Bone marrow derived cells may indirectly contribute to development of cancers of other organs by supplying stroma that supports development of cancer.
Bone marrow mesenchymal stem cells may transform to form sarcomas
Bone marrow stem cells may transdifferentiate to become breast cancer.
Supplementary Material
Histology of primary breast cancers in 5.5 month old male (used as donors at 12 weeks of age when tumors are not yet palpable) mouse: A. Arrows point to tumor masses in right axilla and groin; B–D. H&E, primary lesions; E. lung metastasis. The variation of medullary, ductal and glandular histologic patterns of tumors is similar to the patterns seen in human breast cancer.
FISH for Y-chromosomes in a male (top) and female (bottom) in primary breast cancers in MMTV-PyMT mice. A,D. H&E; B,E. DAPI; E,F. FISH for Ychromosome. Approximately 80% of the cells of the male tumor are Y+; whereas none of the cells of the female tumor are Y+.
Grading of degree of glomerulerosclerosis. 0 Normal note prominent red cells in the glomeruli. + About 50% of glomeruli show moderate thickening of basement membrane. ++ 100% of glomeruli show mild to moderate thickening of basement membrane. +++ 100% of glomeruli have marked thickening of the basement membrane.
Fibrosarcoma. A. H & E; B,C. FISH for Y-chromosome. A. shows fibroblastic (top) and lipomatous (bottom) zones of a peri-renal fibrosarcoma from mouse #3; B. is FISH of the edge of the tumor which is heavily infiltrated with lymphocytes; C. is FISH from the densely fibroblastic zone of the tumor. The lymphocyte infiltrate in B contains over 90% Y+ nuclei. Over 80% of the nuclei in the fibroblastic zone of the tumor label for Y, but the labeling is less intense as the density of the nuclei is less than in the lymphocytic infiltrate.
Gel electrophoresis of RT-PCR products from mesenchymal stroma cells cultured from a primary breast cancer in MMTV-PyMT male mouse. Lanes 1 and 2 show that cultures of breast cancer epithelial cells contain both PyMT DNA and mRNA. Lane 3 shows that fibroblastic cultures contain PyMT DNA, but not mRNA.
A H & E; B FISH for Y in lymphoma in spleen of mouse 4. The lymphoma is a cleaved mixed cell lymphoma. The arrow in A points to a mitotic figures. The FISH in B shows Y− tumor cells infiltrated and surrounded by Y+ donor lymphocytes.
Acknowledgements
We thank Merrill Ross of Albany Medical College for the ploidy analysis and Anna Glinsky of Ordway Research Institute for advice on flow cytometry.
Grant Support: The authors thank the NIH for continued support which was used to start this work (1-R01-CA112481 and 1-R01-AG023510), and to NYSTEM C023063, which allowed us to complete it. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NIH or NYSTEM.
Abbreviations used
- BCA
Breast cancer
- BM
Bone marrow
- BMDCs
Bone marrow derived cells
- CD45
Marker for hematopoietic cells
- CD49f
Marker for breast stem cells and breast cancer cells
- cDNA
Complementary DNA
- DNA
Deoxyribonucleic acid
- FISH
Fluorescence in-situ hybridization
- FVB/N
Inbred mouse strain
- GI
Gastrointestinal
- HICS
Heat-inactivated calf serum
- HSC
Hematopoietic stem cells
- IACUC
Institutional animal care and use committee
- MSC
Mesenchymal stem cells
- MMTV
Mouse mammary tumor virus (promoter)
- mRNA
Messenger RNA
- PCR
Polymerase chain reaction
- PyMT
Polyoma virus middle T oncoprotein
- qPCR
Quantitative polymerase chain reaction
- RT-PCR
Reverse transcriptase polymerase chain reaction
References
- 1.Sell S, editor. Adult Stem Cell Plasticity. Stem Cell Reviews. 2005;1:1–77. doi: 10.1385/SCR:1:1:001. [DOI] [PubMed] [Google Scholar]
- 2.Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg LM, Eisenberg CA. Stem cell plasticity, cell fusion, and transdifferentiation. Birth Defects Res Part C Embryo Today. 2003;69:209–218. doi: 10.1002/bdrc.10017. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, Ikeda Y, Hibi T, Inazawa J, Watanabe M. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nature Med. 2002;8:1011–1017. doi: 10.1038/nm755. [DOI] [PubMed] [Google Scholar]
- 5.Houghton JM, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 6.Tsuchikawa T, Ikeda H, Kikuchi K, Tsuji T, Baba T, Ishizu A, Tanaka Y, Kato H, Toshiki T. Hematopoietic progenitor cells as possible origins of epithelial thymoma in a human T lymphocyte virus type I pX gene transgenic rat model. Lab Investig. 2004;84:245–252. doi: 10.1038/labinvest.3700028. [DOI] [PubMed] [Google Scholar]
- 7.Aractingi S, Kanitakis J, Euvard S, Danff CL, Peguillet I, Khosrotehrani K, Lantz O, Carosella ED. Skin carcinoma arising from donor cells in a kidney transplant recipient. Cancer Res. 2005;65:1755–1760. doi: 10.1158/0008-5472.CAN-04-2783. [DOI] [PubMed] [Google Scholar]
- 8.Cogle CR, Theise ND, Fu DT, Ucar D, Lee S, Guthrie SM, Lonergan J, Rybka W, Krause DS, Scott EW. Bone marrow contributes to epithelial cancers in mice and humans as developmental mimicry. Stem Cells. 2007;25:1881–1887. doi: 10.1634/stemcells.2007-0163. [DOI] [PubMed] [Google Scholar]
- 9.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford J, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. 2000. [DOI] [PubMed] [Google Scholar]
- 10.Swenson ES, Guest I, Ilic Z, Mazzeo M, Lizardi P, Hardiman C, Sell S, Krause DS. Hepatocyte nuclear factor-1 as a marker of epithelial phenotype reveals marrow–derived hepatocytes, but not duct cells, after liver injury in mice. Stem Cells. 2008;26:1768–1777. doi: 10.1634/stemcells.2008-0148. PMID: 18467658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukes RJ, Collins RD. Immunologic characterization of human malignant lymphomas. Cancer. 1974;34:1488–1503. doi: 10.1002/1097-0142(197410)34:8+<1488::aid-cncr2820340822>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 12.Mahler JF, Stokes W, Mann PC, Takaoka M, Maronpot RR. Spontaneous lesions in aging FVB/N mice. Toxicologic Pathology. 1996;24:710–716. doi: 10.1177/019262339602400606. [DOI] [PubMed] [Google Scholar]
- 13.Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB, Horowitz MM, Witherspoon RP, Hoover RN, Sobocinski KA, Fraumeni JF, Jr, Boice JD., Jr Solid cancers after bone marrow transplantation. N Eng J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 14.Kerbel RS, Lagarde AE, Dennis JW, Donaghue TP. Spontaneous fusion in vivo between normal host and tumor cells: possible contribution to tumor progression and metastasis studies with a lectin-resistant mutant tumor. Mol Cell Biol. 1983;3:523–538. doi: 10.1128/mcb.3.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larizza L, Schirrmacher V. Somatic cell fusion as a source of genetic rearrangement leading to metastatic variants. Cancer Metastasis Rev. 1984;3:193–222. doi: 10.1007/BF00048385. [DOI] [PubMed] [Google Scholar]
- 16.Pawelek JM. Tumor cell hybridization and metastasis revisited. Melanoma Res. 2000;10:507–514. doi: 10.1097/00008390-200012000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Pawelek JM. Tumour-cell fusion as a source of myeloid traits in cancer. Lancet Oncol. 2005;6:988–993. doi: 10.1016/S1470-2045(05)70466-6. [DOI] [PubMed] [Google Scholar]
- 18.Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, Datar RH, Cote RJ. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 19.Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmuller G, Klein CA. Systemic spread is an early step in breast cancer. Cancer Cells. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Develop Biol. 2007;303:29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 22.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 23.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 24.DeWever O, Marell M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–447. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 25.Fidler IJ. The organ microenvironment and cancer metastasis. Differentiation. 2002;70:498–505. doi: 10.1046/j.1432-0436.2002.700904.x. [DOI] [PubMed] [Google Scholar]
- 26.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 28.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Seminars in Cancer Biology. 2008;18:311–321. doi: 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronnov-Jessen L, Bissell MH. Breast cancer by proxy: can the microenvironment be both the cause and consequence? Trends Mol Med. 2009;15:5–13. doi: 10.1016/j.molmed.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 33.Scott RJ, Chakroborty S, Sell S, Hunt JM, Dunsford HA. Change in the ploidy state of liver cells during chemical hepatocarcinogenesis and its relationship to the increased expression of alphafetoprotein. Cancer Research. 1989;49:6085–6090. [PubMed] [Google Scholar]
- 34.Liu C, Chen Z, Shen X, Zhang T, Lu Y. Multiple tumor types may originate from bone marrow-derived cells. Neoplasia. 2006;8:716–724. doi: 10.1593/neo.06253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golfinopoulos V, Pentheroudakis G, Kamakari S, Metaxa-Mariatou V, Pavlidis N. Donor-derived breast cancer in a bone marrow transplantation recipient. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-9922-7. DOI.1007/s10549-008-9922-7. [DOI] [PubMed] [Google Scholar]
- 36.Au WY, Chan EC, Pang A, Lie AK, Liang R, Yuen AP, Shek TW, Kwong YL. Nonhematological malignancies after allogeneic hematopoietic stem cell transplantation: incidence and molecular monitoring. Bone Marrow Transplant. 2004;34:981–985. doi: 10.1038/sj.bmt.1704674. [DOI] [PubMed] [Google Scholar]
- 37.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling , development and disease. J. Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarrio D, Bodriguez-Pinilla M, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- 39.Thompson EW, Newgreen DF. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005;65:5991–5995. doi: 10.1158/0008-5472.CAN-05-0616. [DOI] [PubMed] [Google Scholar]
- 40.Tarin D. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005;65:5996–6000. doi: 10.1158/0008-5472.CAN-05-0699. [DOI] [PubMed] [Google Scholar]
- 41.Côme C, Arnoux V, Bibeau F, Savagner P. Roles of the transcription factors snail and slug during mammary morphogenesis and breast carcinoma progression. J Mammary Gland Biol Neoplasia. 2004;9:183–193. doi: 10.1023/B:JOMG.0000037161.91969.de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobsen BM, Harrell JC, Jedlicka P, Borges VF, Varella-Garcia M, Horwitz KB. Spontaneous fusion with, and transformation of mouse stroma by, malignant human breast cancer epithelium. Cancer Res. 2006;66:8274–8279. doi: 10.1158/0008-5472.CAN-06-1456. [DOI] [PubMed] [Google Scholar]
- 43.Callahan R, Smith GH. MMTV-induced mammary tumorigenesis: gene discovery, progression to malignancy and cellular pathways. Oncogene. 2000;19:992–1001. doi: 10.1038/sj.onc.1203276. [DOI] [PubMed] [Google Scholar]
- 44.Maglione JE, Moghanaki D, Young LJ, Manner CK, Ellies LG, Joseph SO, Nicholson B, Cardiff RD. MacLeod: Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res. 2001;61:8298–8305. [PubMed] [Google Scholar]
- 45.Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu TH, Chandramouli GV, Hunter KN, Alkharouf NW, Green JE, Liu ET. Global expression profiling identifies signatures of tumor virulence in MMTV-PyMT-transgenic mice: correlation to human disease. Cancer Res. 2004;64:5973–5981. doi: 10.1158/0008-5472.CAN-04-0242. [DOI] [PubMed] [Google Scholar]
- 47.Maglione JE, McGoldrick ET, Young LJ, Namba R, Gregg JP, Liu L, Moghanaki D, Ellies LD, Borowsky AD, Cardiff RD, MacLeod CL. Polyomavirus middle T-induced mammary intraepithelial neoplasia outgrowths: single origin, divergent evolution, and multiple outcomes. Mol Cancer Ther. 2004;3:941–953. [PubMed] [Google Scholar]
- 48.Berard Kl, Gaboury L, Landers M, De Repentigny Y, Houle B, Kothary R, Bradley WEC. Hyperplasia and tumours in lung, breast and other tissues in mice carrying a RARβ4-like transgene. EMBO J. 1994;13:5570–5580. doi: 10.1002/j.1460-2075.1994.tb06894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histology of primary breast cancers in 5.5 month old male (used as donors at 12 weeks of age when tumors are not yet palpable) mouse: A. Arrows point to tumor masses in right axilla and groin; B–D. H&E, primary lesions; E. lung metastasis. The variation of medullary, ductal and glandular histologic patterns of tumors is similar to the patterns seen in human breast cancer.
FISH for Y-chromosomes in a male (top) and female (bottom) in primary breast cancers in MMTV-PyMT mice. A,D. H&E; B,E. DAPI; E,F. FISH for Ychromosome. Approximately 80% of the cells of the male tumor are Y+; whereas none of the cells of the female tumor are Y+.
Grading of degree of glomerulerosclerosis. 0 Normal note prominent red cells in the glomeruli. + About 50% of glomeruli show moderate thickening of basement membrane. ++ 100% of glomeruli show mild to moderate thickening of basement membrane. +++ 100% of glomeruli have marked thickening of the basement membrane.
Fibrosarcoma. A. H & E; B,C. FISH for Y-chromosome. A. shows fibroblastic (top) and lipomatous (bottom) zones of a peri-renal fibrosarcoma from mouse #3; B. is FISH of the edge of the tumor which is heavily infiltrated with lymphocytes; C. is FISH from the densely fibroblastic zone of the tumor. The lymphocyte infiltrate in B contains over 90% Y+ nuclei. Over 80% of the nuclei in the fibroblastic zone of the tumor label for Y, but the labeling is less intense as the density of the nuclei is less than in the lymphocytic infiltrate.
Gel electrophoresis of RT-PCR products from mesenchymal stroma cells cultured from a primary breast cancer in MMTV-PyMT male mouse. Lanes 1 and 2 show that cultures of breast cancer epithelial cells contain both PyMT DNA and mRNA. Lane 3 shows that fibroblastic cultures contain PyMT DNA, but not mRNA.
A H & E; B FISH for Y in lymphoma in spleen of mouse 4. The lymphoma is a cleaved mixed cell lymphoma. The arrow in A points to a mitotic figures. The FISH in B shows Y− tumor cells infiltrated and surrounded by Y+ donor lymphocytes.


