Abstract
Objective
To improve the accuracy of genotype prediction and guide genetic testing in patients with muscle channelopathies we applied and refined specialised electrophysiological exercise test parameters.
Methods
We studied 56 genetically confirmed patients and 65 controls using needle electromyography, the long exercise test, and short exercise tests at room temperature, after cooling, and rewarming.
Results
Concordant amplitude-and-area decrements were more reliable than amplitude-only measurements when interpreting patterns of change during the short exercise tests. Concordant amplitude-and-area pattern I and pattern II decrements of >20% were 100% specific for PMC and MC respectively. When decrements at room temperature and after cooling were <20%, a repeat short exercise test after rewarming was useful in patients with myotonia congenita. Area measurements and rewarming distinguished true temperature sensitivity from amplitude reduction due to cold-induced slowing of muscle fibre conduction. In patients with negative short exercise tests, symptomatic eye closure myotonia predicted sodium channel myotonia over myotonia congenita. Distinctive ‘tornado-shaped’ neuromyotonia-like discharges may be seen in patients with paramyotonia congenita. In the long exercise test, area decrements from pre-exercise baseline were more sensitive than amplitude decrements-from-maximum-CMAP in patients with Andersen-Tawil syndrome. Possible ethnic differences in the normative data of the long exercise test argue for the use of appropriate ethnically-matched controls.
Interpretation
Concordant CMAP amplitude-and-area decrements of >20% allow more reliable interpretation of the short exercise tests and aid accurate DNA-based diagnosis. In patients with negative exercise tests, specific clinical features are helpful in differentiating sodium from chloride channel myotonia. A modified algorithm is suggested..
Introduction
The skeletal muscle channelopathies can be divided into those presenting with myotonia (non-dystrophic myotonias), and those associated with episodes of weakness (the periodic paralyses). In the former, channel dysfunction causes mild depolarisation of the muscle membrane, leading to spontaneous self-sustained trains of muscle fibre action potentials (myotonic discharges). In the latter, the degree of sarcolemmal depolarisation intermittently becomes of sufficient severity as to render the muscle fibre electrically inexcitable, leading to paralysis. The clinical spectrum ranges from pure myotonia, to an overlap of myotonia and intermittent weakness, to periodic paralysis without electrical myotonia; all forms can be seen with mutations of the sodium channel (Nav 1.4, SCN4A), whereas mutations in other channels cause a more limited phenotype.
The non-dystrophic myotonias include myotonia congenita (MC, ClC-1, CLCN1), paramyotonia congenita (PMC, SCN4A), and the sodium channel myotonias (SCM, SCN4A). The periodic paralyses are typically classified as hypokalaemic (CACNA1S or SCN4A mutations) or hyperkalaemic (SCN4A). Andersen-Tawil syndrome is a rarer form of periodic paralysis associated with cardiac arrhythmias and skeletal anomalies due to mutations of an inwardly rectifying potassium channel (KIR2.1, KCNJ2).
Specific patterns of response in electrophysiological exercise tests broadly correlate with the underlying genotype, thus providing a guide to genetic analysis1,2,3,4. As these exercise tests become established as part of routine neurophysiological testing, the need for standardisation and identification of the most useful parameters, has become apparent. The parameter measured in the long exercise test has varied in the literature, and includes decrement expressed as a percentage of the highest compound muscle action potential (CMAP) during exercise5, or of the highest CMAP during-or-after exercise3, or of the highest CMAP after exercise (CMAPs not recorded during exercise)6, or as a percentage of baseline7,8,1. The diagnostic significance of amplitude or area increments during exercise5,3, or of area decrements during the rest period3, remains uncertain. The duration of testing following exercise has varied from 30–60 minutes5,7,9,3,10, and uncertainty remains as to the minimum duration for which CMAPs should be monitored for optimum sensitivity.
In the short exercise (SE) and cooling tests, immediate post-exercise amplitude decrements of >40% are clearly indicative of chloride channel myotonia, whereas decrements of 10–40% are often difficult to interpret1,2,4. It has been suggested that 10 Hz repetitive stimulation at room temperature may be superior to SE testing for the identification of recessive MC4. Clarification of the most diagnostically useful parameters, and means of optimising the sensitivity and specificity of these tests, as well as identification of technical factors which may introduce error when monitoring CMAPs over extended periods would improve current practice.
Patients and Methods
Patients
All patients included in the study had confirmed ion channel mutations (Table 1).
Table 1.
Characteristics of Patients and Control subjects
| Subjects | Age (years) | ||||||
|---|---|---|---|---|---|---|---|
| Clinical Phenotype | Gene | Mutation | Total | Men | Women | Mean | Range |
| Controls | - | - | 65 | 27 | 38 | 37 | 18–67 |
| Patients | |||||||
| Myotonia Congenita (MC) | CLCN1 | * | 18 | 12 | 6 | 44 | 20–70 |
| Paramyotonia Congenita (PMC) | SCN4A | T1313M (n=6) Q270K (n=1) |
7 | 5 | 2 | 46 | 19–70 |
| Sodium Channel Myotonia (SCM) | SCN4A | V1589M (n=5) V1293I (n=2) G1306A (n=3) L128P |
11 | 6 | 5 | 40 | 14–66 |
| Myotonia and PP | SCN4A | T704A | 1 | 1 | 24 | ||
| Calcium Channel PP (HypoPP) | CACNA1S | R528H (n=2) R1239H (n=2) |
4 | 3 | 1 | 32 | 19–40 |
| Sodium Channel PP (HyperPP) | SCN4A | M1592V (n=2) T704M (n=2) |
4 | 1 | 3 | 51 | 30–66 |
| Andersen-Tawil Syndrome (ATS) | KCNJ2 | Y68D (n=3) R82Q (n=2) R312C (n=2) R218W (n=2) L217P R67W |
11 | 7 | 4 | 43 | 17–60 |
| Whole patient group | 56 | 35 | 21 | 43 | 14–70 | ||
AR (n=11): c.180+3 A>T and G190R (2 patients); G285E and M485V; H369P and E624fs; homozygous c.180+3 A>T; homozygous for intronic sequence variant c.1930+6 T>G; intronic sequence variant c.1167-10 T>C; R105C, F167L and E624fs; V327I and R894X, 180+3A>T and 774+1G>A, G285E. AD (n=7): A313V (2 patients); G230E (3 patients); E624fs; F167L
Myotonia Patients
Eighteen patients (16 families) had chloride channel mutations, mean age 44 years (range 20–70). They were classified as autosomal dominant (AD) or autosomal recessive (AR) based on a combination of the family history and the results of genetic analysis. In all, the clinical myotonia became less marked with repetition, consistent with the ‘warm up’ phenomenon. Transient weakness on initiating activity was seen in 3 patients with AR MC. Seven patients (6 families) had paramyotonia congenita (PMC), mean age 46 years (range 19–70). All had paradoxical myotonia and cold-induced weakness. There were 11 patients (9 families) with sodium channel myotonia (SCM), mean age 40 years (range 14–66). Most patients demonstrated a ‘warm-up’ phenomenon, but some patients had a combination of warm-up and paradoxical myotonia, particularly affecting eye closure. None had cold-induced weakness. One patient with a T704A SCN4A mutation had an ‘overlap syndrome’ with prominent symptomatic myotonia and 3 episodes of paralysis in his lifetime.
Periodic Paralysis Patients
Four patients had hyperkalaemic periodic paralysis (mean age 51 years, range 30–66), and 4 had calcium channel hypokalaemic periodic paralysis (mean age 32 years, range 19–40). There were eleven patients (6 families) with Andersen-Tawil syndrome (mean age 43 years, range 17–60); all had attacks of periodic paralysis, some had prolonged QT intervals, and most had typical morphological changes.
Unaffected Controls (n=65)
There were 32 asymptomatic volunteers, 11 men and 21 women (mean age 37 years, range 19–65). Unaffected controls (n=33, 16 men, 17 women; mean age 38 years, range 18–67) were individuals referred for exercise tests for diagnostic evaluation, but who were found following comprehensive clinical and genetic assessment to not have any evidence of neuromuscular disease. These were patients referred to the department as part of a national referral scheme for suspected muscle channelopathies. Many of these patients had had previous negative investigations at their local centres and were referred specifically for expert clinical evaluation and for more extensive neurophysiological and genetic testing. In this group the main presenting complaints were fluctuating fatigue or weakness (n= 18), cramps and/or muscle spasms and/or myalgia, often with fatigue (n= 11), stiffness with or without fatigue (n= 4). Their final diagnoses included ‘non-specific symptoms’ (n=29), cataplexy (n=1), paroxysmal exercise-induced dyskinesia (n=1), hyperventilation-induced carpo-pedal spasm (n=1), and sleep paralysis (n=1).
The two control groups were initially analysed separately. There was no difference in data from the two groups except when comparing the long exercise test in selected patients based on ethnicity. The data, with the exception of the long exercise data, were therefore combined for comparison with the patient groups.
The genetic diagnosis was not known to the examiner or patient at the time of the neurophysiological examination in 15/18 MC, 11/11 SCM, and 3/7 PMC patients. Similarly, the genetic diagnosis was not established in 4/4 HypoPP, and 4/4 HyperPP patients. The ATS patients were all genetically confirmed prior to neurophysiological testing. None of the out-patient controls had an established diagnosis at the time of testing; the neurophysiologist was effectively blinded as to whether these subjects would eventually turn out to be patients or controls. Written informed consent was obtained from all study patients and normal volunteers.
Methods
Short exercise tests (SETs) were performed as described by Streib and colleagues11–13 and Fournier et al1. Cooling was performed on the contralateral hand2. To look for cold-induced changes persisting after rewarming (as in PMC14), we also performed SETs after rewarming; CMAPs after rewarming were plotted as a percentage of the original pre-cooling baseline. The long exercise test was performed as described by McManis et al5. CMAPs were recorded pre-exercise, during the 5 minutes of exercise, at 1 minute intervals for the first 6 minutes of rest, and then at 2 minute intervals for 40–50 minutes. Repetitive stimulation of the ulnar nerve at 10 Hz for 5 seconds was performed at room temperature as described by Michel et al.4 The effect of technical factors on CMAP size was also investigated (supplementary text).
Statistical Analysis
CMAP amplitude (negative peak), and area (negative peak area) were expressed as a percentage of the baseline values. The values were plotted as means ± standard errors of the means. The normal range was defined as mean ± 2 standard deviations. Upper limits for decrements were expressed as mean + 2 standard deviations. Decrements were expressed amplitude-only, area-only, or concordant amplitude-and-area (CAA) decrements, defined as decrements occurring in both amplitude and area measurements simultaneously. Because of the relatively small numbers of patients in each group, the unequal variance unpaired t-test (Welch test) was used for comparing patient and control groups, and to compare the long exercise test in the African-Caribbean and non-African-Caribbean controls.
Results
Needle Electromyography – Unusual Myotonic discharges in patients with PMC
Typical ‘waxing and waning’ myotonic discharges were present in all patients with MC, PMC and SCM. Widespread profuse myotonic discharges were typical in the SCM and PMC patients; occasional patients with AD MC had relatively scanty discharges in some muscles despite clear clinical myotonia.
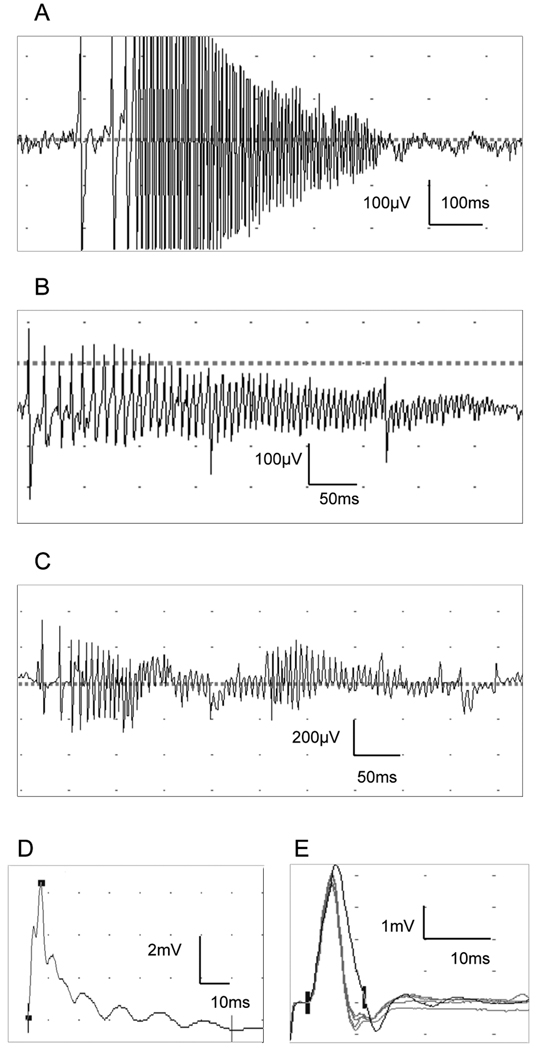
In addition to typical myotonic discharges, unusual low amplitude (100–600µV) high frequency ‘musical’ 150–250 Hz discharges were seen in all 7 patients with PMC. These were most prominent in the larger limb muscles (biceps, vastus medialis, tibialis anterior). In contrast to typical myotonic discharges, with the aural characteristics of higher or lower pitched growls or intermittent revs on a motorcycle (i.e. the frequency and amplitude tended to gradually increase and decrease), these discharges had a more bursting (rather than gradual) onset, best appreciated on the loudspeaker, and were generally of higher frequency, with a musical component. When occurring repeatedly in close succession, they were reminiscent of ‘dolphin sounds’. In addition, higher amplitude bursting, rapidly-decrementing, ‘tornado-shaped’ discharges, resembling neuromyotonic discharges were occasionally seen (Figure 1). When these were of high frequency, there was a ‘pinging’ sound on the loudspeaker resembling a sustained musical tone. We did not see these discharges in patients with SCM or MC..
Figure 1.
Myotonic discharges were prominent in the patient with ‘overlap syndrome’, and in 2/4 patients with hyperkalaemic periodic paralysis (both M1592V). In one of the latter, there were associated myopathic features. None of the patients with hypokalaemic periodic paralysis had myotonic discharges; one (R1239H) had myopathic changes.
Short and Long Exercise Tests
Importance of Concordant Amplitude-and-Area (CAA) CMAP changes
The normative data for the SET at room temperature, after cooling and after rewarming are shown in Table 2 and Supplementary Figure 1. The mean change in the baseline CMAP after cooling compared with the pre-cooling baseline was a decrement of 3% ±3.8 (mean ± SE of mean) for amplitude, and an increment of 15% ±3.7 for area. As described by Fournier et al2, there was a change in CMAP shape with cooling, with an increase in CMAP duration, probably related to non-uniform slowing of muscle fibre conduction velocities.
Table 2.
Short Exercise Test – Unaffected Controls. Percentage change from baseline CMAP seen during the SET at room temperature, after cooling and after rewarming.
| SET at RT (N=51) | SET after cooling (N=43) | SET after rewarming * (N=40) | |
|---|---|---|---|
| AMPLITUDE | |||
| Mean | +4.4 | +3.3 | +2 |
| Range | −28 to +27.3 | −57.5 to +83.7 | −44.7 to +53.4 |
| ( mean ± 2SD) | −11.4 to +20.2 | −30.7 to +37.3 | −21.4 to +25.4 |
| AREA | |||
| Mean | +4.3 | +9.3 | +4 |
| Range | −24.4 to +31.9 | −42.5 to +79.5 | −39.6 to +73.6 |
| ( mean ± 2SD) | −12.5 to +21.1 | −27.1 to +45.7 | −26.6 to +34.6 |
|
Maximum Concordant
Amplitude-and-Area decrement |
17.7% | 13.7% | 18.5% |
|
Recommended ULN for
concordant decrement |
20% | 20% | 20% |
Expressed as % of original (pre-cooling) baseline
Although our normative data are consistent with published results1,2, the range of change for amplitude-only, or area-only, was wide, particularly with exercise in the cold. However, the maximum CAA decrements during the short exercise test at room temperature, after cooling and after rewarming did not exceed 19% (Table 2); we considered CAA decrements of >20% (i.e. simultaneous decrements >20% in amplitude and in area) as abnormal.
If areas had not been plotted, the amplitude changes with cooling in some controls may have been misinterpreted as being indicative of cold-sensitivity. Unlike patients with PMC12, in controls, the amplitude and areas recovered with rewarming (Supplementary Figure 1B).
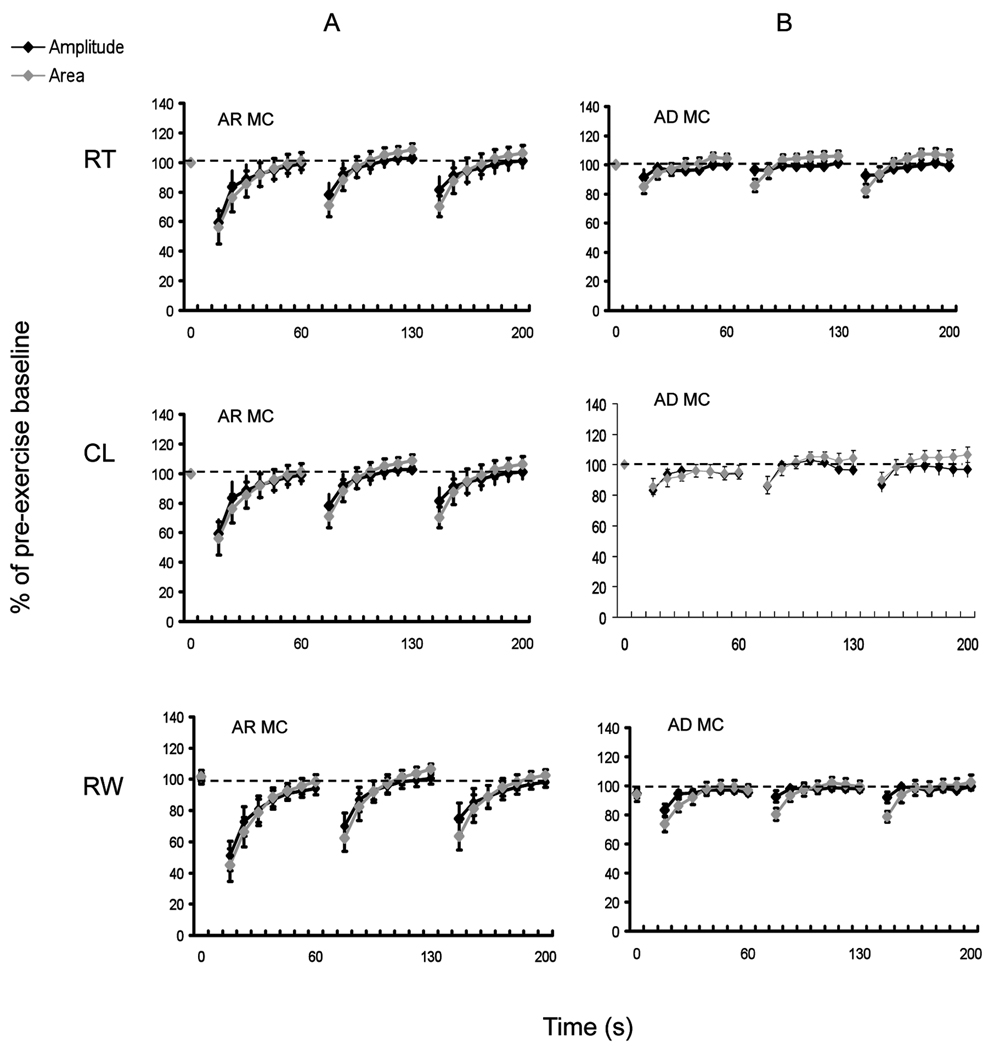
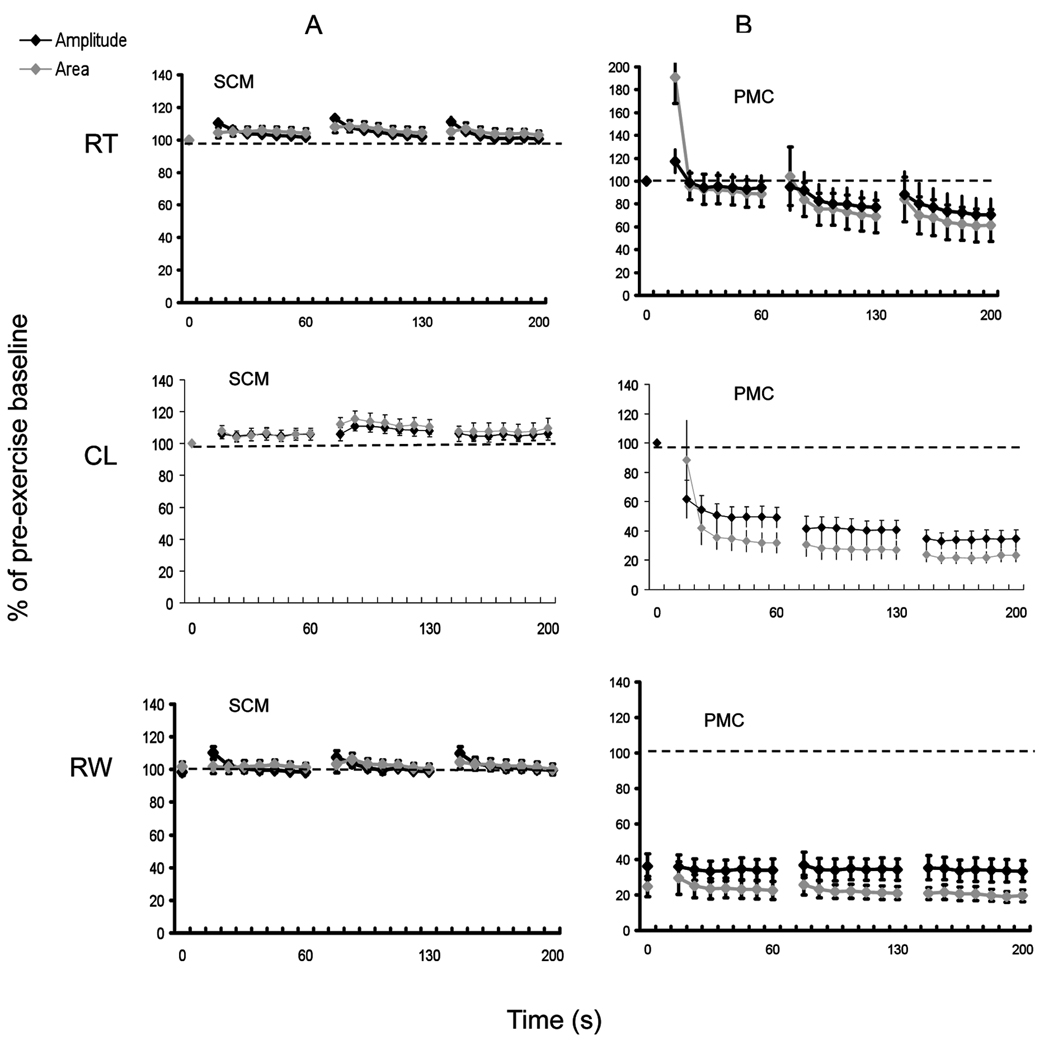
SE Test at Room Temperature, after Cooling, and Rewarming - Myotonia patients
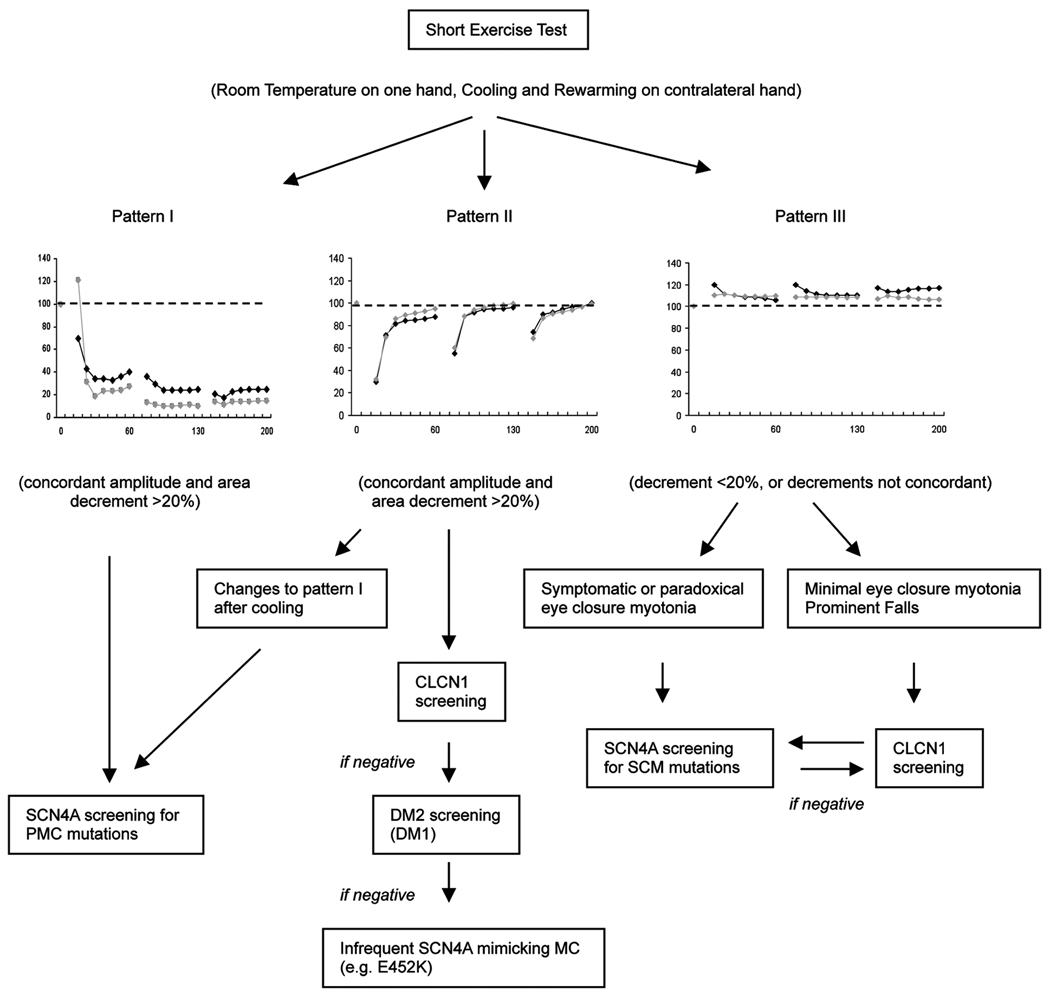
The results of the SETs at room temperature, after cooling and rewarming in patients with MC (AR and AD), SCM and PMC are shown in Figures 2 and 3, and illustrate the typical patterns of CMAP amplitude change (patterns I-III) previously described for these conditions1,2, 13,14. Decrement at room temperature was seen in 3/7 PMC patients; all had T1313M mutations. In the other 4 PMC patients, including one patient with a Q270K mutation, decrement was only seen after cooling.
Figure 2.
Figure 3.
As previously described1, we saw prominent post-exercise myotonic potentials (PEMPs) in PMC patients at room temperature and after cooling (Figure 1), reflected by a large increase in area immediately after exercise (Figure 3).
In PMC, the amplitude decrements were typically maximal after the 3rd SE trial, and were −12 % ±20% (p=0.2) at room temperature, −65.4% ±6% (p<0.001) after cooling, and −64.3% ±7% (p<0.001) after rewarming. Area decrements were similar and concordant. Pattern I CAA decrements of >20% had 100% sensitivity and 100% specificity for PMC in our cohort. If pattern I amplitude-only decrements were used, sensitivity was unchanged, but specificity was reduced to 88%.
The maximum amplitude change in AR MC, which typically occurred following the first exercise trial, was −39% ±7% (p<0.001) at room temperature, −42 % ±5% (p <0.001) after cooling, and −40% ±8% (p<0.001) after rewarming. Area decrements were similar and concordant (Figure 2). For AD MC, the maximum amplitude change was −8% ±4% (p=0.01) at room temperature, −13% ±5% (p=0.001) after cooling, and −17% ±5% (p=0.001) after rewarming.
The addition of cooling and rewarming to the routine SE protocol at room temperature was associated with a positive test in an additional 3 MC patients (17%); in 2 of those patients a positive test was only seen after rewarming. Pattern II CAA decrements of >20% had 72% sensitivity and 100% specificity for MC; using pattern II amplitude-only decrements improved sensitivity to78% but reduced the specificity to 93%.
There were no significant CAA decrements in the patients with SCM, where the mean amplitude change was +10% ±2%, + 6% ±3%, and +10% ±4% at room temperature, after cooling, and after rewarming, respectively.
Combining SETs with Symptomatic Eye Closure myotonia allows better differentiation between SCM and MC
A proportion of MC patients do not show significant decrements on the SETs1,2. In order to differentiate SCM from MC patients with negative SETs, we explored combining the results with specific selected clinical features (Table 3). Symptomatic eye closure myotonia, defined as myotonia causing discomfort, or interfering with daily activities, was more common in patients with SCM, as was the presence of variable paradoxical eye closure myotonia on clinical examination. In addition, 4/11 (36%) patients with SCM complained of myotonia of the extraocular muscles, causing transient diplopia. These patients were less likely than those with MC to have severe generalised myotonia associated with ‘locking’ or falls. When using a negative SET as the sole criterion for suspecting SCM in our series, the sensitivity was 100%, but the specificity was only 72% because of the relatively large number of MC patients (5/18) with negative SETs. The addition of ‘symptomatic eye closure myotonia’ to a negative SET improved the specificity for SCM to 94%, with a slightly reduced sensitivity of 82%.
Table 3.
Comparison of selected clinical characteristics in the Chloride and Sodium Channel Myotonias
| Chloride channel myotonia (n=18) |
Sodium Channel myotonia (n=11) |
|
|---|---|---|
| Eye closure myotonia | ||
| Present (% of whole group) |
56 | 91 |
| Warm-up phenomenon (% of those with EC myotonia) |
80 | 60* |
| Paradoxical myotonia (% of those with EC myotonia) |
20 | 70* |
| Clinically symptomatic **
(% of whole group) |
17 | 82 |
| Falls*** | ||
| Present (%) | 78 | 27 |
| Mild (%) | 33 | 18 |
| Moderate (%) | 22 | 0 |
| Severe (%) | 22 | 1 |
EC = eye closure
Some sodium channel myotonia patients had a combination of paradoxical eye closure myotonia and warm-up
The patient considers the eye closure myotonia uncomfortable or interfering with daily activities e.g. driving, walking in the cold.
Mild: Infrequent falls with minor or no injuries; Moderate: fairly frequent falls, occasional injuries; Severe: ‘locking’ with trauma to head or face
Unusual SET patterns in Patients with Myotonia
One patient with PMC (T1313M) showed an immediate decrement during SE at room temperature reminiscent of the pattern seen in MC (Supplementary Figure 2). SET changes mimicking MC at room temperature were previously described in two PMC patients with Q270K mutations and this pattern was thought to be mutation specific2.
One patient with AD MC had an apparent ‘reversed’ pattern of decrement, with the maximum decrement being after the third SE trial (Supplementary Figure 2). In another 3 patients, the decrement was maximal after the 2nd trial. Although as a group, patients with MC showed a maximum decrement after the 1st SE trial, these findings would caution against the use of only a single SE trial4 when assessing patients with myotonia.
SET in Patients with Periodic Paralysis
Progressive increment during SE at room temperature has previously been described in T704M hyperkalaemic periodic paralysis1. We did not see this in our two patients with T704M mutations, nor in the two other patients (M1592V) with hyperkalaemic periodic paralysis. The maximum amplitude change was +12% ±9% (mean ±SE) room temperature, +12 % ±15% after cooling, and −17% ±11% after rewarming. The amplitude change with rewarming was not concordant (area change +12% ±13%).
The SET was also within normal limits in the patients with hypokalaemic periodic paralysis; the maximum amplitude change was +12% ±3% at room temperature, −9% ±7% after cooling, and +11% ±4% after rewarming. The amplitude change during cooling was not concordant (area change +59% ±29%).
In patients with Andersen-Tawil Syndrome (n=11), the SET at room temperature showed a mild immediate post-exercise increment in amplitude (+18% ±3%, p<0.01), and area (+12% ±4%, p<0.01) (Supplementary Figure 3).
10 Hz repetitive stimulation
We compared 10 Hz repetitive stimulation for 5 seconds at room temperature, with the results of the SE test at room temperature, after cooling, and rewarming, in 8 patients with MC (6 AR, 2 AD), 2 patients with SCM, and 3 patients with PMC. In our series of AR and AD MC, 10 Hz repetitive stimulation did not add any information, with 3 patients who showed a positive SET (patients 6–8, Table 4), not showing >20% decrement with 10 Hz repetitive stimulation.
Table 4.
Comparison of 10 Hz Repetitive stimulation for 5 seconds at Room Temperature against the short exercise and cooling tests.
| Maximum amplitude decrement (% of baseline) | |||||||
|---|---|---|---|---|---|---|---|
| NDM classification |
Patient | Mutation | AR / AD | 10 Hz repetitive stimulation |
SET at Room Temperature |
SET after Cooling |
SET after Rewarming |
| MC | |||||||
| Pt 1 | c.180+3 A>T and G190R | AR | 80 | 72 | 70 | 87 | |
| Pt 2 | c.180+3 A>T and G190R | AR | 82 | 44 | 66 | 80 | |
| Pt 3 | Homozygous for intronic sequence variant c.1930+6 T>G |
AR | 94 | 68 | 59 | 62 | |
| Pt 4 | R105C, F167L and E624fs | AR | 9 | 24* | 24* | No decrement | |
| Pt 5 | 180+3A>T and 774+1G>A | AR | 25 | 38 | 41 | 18 | |
| Pt 6 | G285E** | AR | No decrement | 31 | 20 | 6 | |
| Pt 7 | E624fs | AD | No decrement | 8 | 12 | 28 | |
| Pt 8 | G230E | AD | 8 | 27 | 45 | 30 | |
| SCM | |||||||
| Pt 9 | G1306A | AD | No decrement | No decrement | 10 | 8 | |
| Pt 10 | V1589M | AD | No decrement | No decrement | 10 | No decrement | |
| PMC | |||||||
| Pt11 | T1313M | AD | No decrement | 82 | 48 | 65 | |
| Pr 12 | T1313M | AD | No decrement | No decrement | 56 | 75 | |
| Pt 13 | T1313M | AD | No decrement | 73 | 81 | 83 | |
MC: myotonia congenita; SCM: sodium channel myotonia; PMC: paramyotonia congenita; Pt: patient; AR: autosomal recessive; AD: autosomal dominant
Area decrements did not exceed 20%, therefore SETs were negative.
The G285E is considered to be a recessive mutation based on family and functional studies. This patient has had the entire coding sequence of the CLCN1 gene (encompassing consensus spice sites) analysed along with rearrangement analysis to detect exonic deletion/duplications, and only the G285E mutation was detected. We cannot exclude mutations in regulatory sequences elsewhere within the gene.
Long Exercise Test - Possible Ethnic differences
Based on the recommended upper limit of 40% amplitude decrement in the long exercise test5, three of our asymptomatic normal controls had an abnormal decrement. Two (1 man, 1 woman, aged 33 and 35 years) were from Ghana, and one (woman, aged 34 years) from Jamaica. We therefore compared the data in our African-Caribbean controls (all were asymptomatic normal controls) against our non-African-Caribbean controls (Supplementary Figure 4)( Supplementary Tables 1 and 2).
In our non-African-Caribbean group (n=42), the upper limit (mean+2SD) for amplitude decrement from maximum CMAP during exercise (mean=24.1%), and from the maximum CMAP during-or-after-exercise (mean=26%), was 40% for both parameters, and is consistent with previously published data3, and the recommended upper limit suggested by McManis et al5. In the African-Caribbean controls (n=10), the corresponding value was 54% (mean=31.4%, p=0.04). The upper limit of normal (mean+2SD) for amplitude decrement from baseline was 28% in the non-African Caribbean controls (mean=11.6%), and 50% in the African-Caribbean controls (mean=21.6%, p=0.03). The difference between the groups was less marked for area change, and did not reach significance for decrement from maximum (p=0.34), or decrement from baseline (p=0.08). There was a difference in the area increment during exercise (p=0.01), of uncertain diagnostic relevance.
As all our patients were non-African-Caribbean, when interpreting the long exercise test decrements in our patients, we used the data from the non-African-Caribbean controls (Table 5).
Table 5.
Long Exercise Test : Suggested Normal Upper Limits for Decrement for Non-African Caribbean individuals
| ULN | |
|---|---|
| Ethnic Group | Non-African-Caribbean |
|
Amplitude Change |
|
| Change during rest after exercise | |
| Maximum Decrement (during rest after exercise) as % of maximum during exercise | 40 |
| Maximum Decrement (during rest after exercise) as % of maximum during- or-after exercise | 40 |
| Maximum Decrement (during rest after exercise) as % of baseline | 30 |
| Change during rest after exercise |
Area Change |
| Maximum Decrement (during rest after exercise) as % of maximum during exercise | 60 |
| Maximum Decrement (during rest after exercise) as % of maximum during- or-after exercise | 60 |
| Maximum Decrement (during rest after exercise) as % of baseline | 40 |
See Supplementary Tables 1 and 2 for data comparing African-Caribbean and non-African-Caribbean controls.
Long Exercise Test – Myotonia patients
Positive long exercise tests have occasionally been reported in MC and PMC3. In our series, we found an abnormal decrement in 2/18 MC patients; both had AR MC. The maximum decrement occurred during the 5 minutes of exercise instead of during the rest period. In AR MC patients in whom the decrement did not exceed 40%, the pattern was similar (Supplementary Figure 5A), and therefore the pattern of change could not be confused with that seen in patients with periodic paralysis. In 6/7 patients with PMC, there was an amplitude decrement of >40% during the long exercise test. However, the pattern of decrement was the reverse of that typically seen in periodic paralysis, with progressive decrement occurring during exercise, and slow recovery in the rest period (Supplementary Figure 5B). The long exercise test was within normal limits in the patients with SCM.
Long Exercise Test - Periodic Paralysis patients
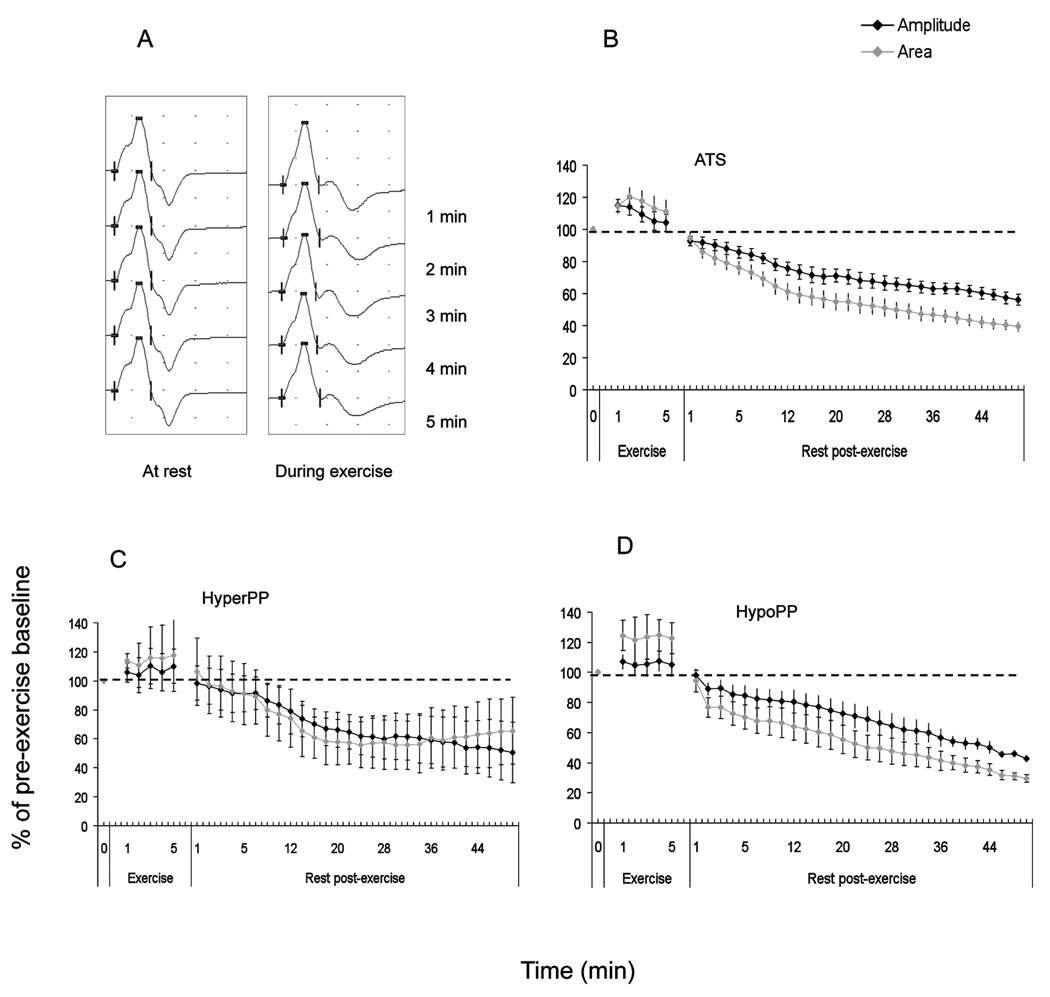
In our patients with Andersen-Tawil syndrome (n=11), 9 patients (82%) had a positive long exercise test based on an amplitude decrement of >40% from maximum during-or-after exercise, 10 patients (90%) had an abnormal amplitude decrement of >30% from baseline, and all 11 patients (100%) had an abnormal area decrement of >40% from baseline. This suggests area decrements from baseline may be a more sensitive parameter in ATS; the specificity was 95% in non-African-Caribbeans. The mean increment during exercise was 17% (range 7–38) and was not significantly different from controls. In the patients who had an amplitude decrement of >40% from maximum, the mean rest time taken to achieve this decrement was 24 minutes, but the range was wide (4–50 minutes). Small afterpotentials were seen during the 5 minutes of exercise in one patient (Figure 4A and B).
Figure 4.
All four patients with hyperkalaemic periodic paralysis had an amplitude decrement from maximum CMAP during-or-after exercise of >40%. The mean time to reach an abnormal decrement was 22 minutes (range 6–38). Only 3 had an abnormal amplitude or area decrement from baseline. All four patients with calcium channel (hypokalaemic) periodic paralysis had a positive long exercise test (on amplitude decrements from maximum as well as from baseline). The mean time taken to reach an abnormal decrement was 30 minutes (range 10–38). Unlike other groups, we did not see an abnormal increment in amplitude or area during exercise in our patients with hyperkalaemic5 or hypokalaemic periodic paralysis3 (Figure 4C and D).
Discussion
The current recommendations for the upper limit of normal for amplitude decrement in the short exercise test at room temperature is 10%1. There are currently no clear recommended upper limits of normal for amplitude or area decrements after cooling. Amplitude-only, or area-only, decrements of >10% were associated with a high proportion of false positives in our controls, as well as 2 patients with SCM (Table 6A). The proportion of false positives was slightly lower if we used amplitude-only or area-only decrements of >20% (Table 6B), but it was only by using CAA decrements of >20%, that we increased the specificity of the SETs to 100%. CAA decrements were particularly useful when interpreting SET changes after cooling. Despite prolonged cooling (>7 minutes) and achieving a surface temperature of ≤14°C2, some individuals showed progressive change in CMAP duration which affected the CMAP amplitude but not the area. In our series, pattern I CAA decrements of >20% after cooling were specific for PMC. Amplitude-only decrements had a lower specificity and increased the likelihood of misdiagnosis of a cold-sensitive channelopathy. If areas are not monitored, repeating the SET after rewarming is helpful for separating PMC patients from controls.
Table 6.
Amplitude-only or Area-only decrements versus Concordant Amplitude-and-Area decrements on the Short Exercise Tests at room temperature, after cooling, and after rewarming.
| A. Decrements of >10% | |||
|---|---|---|---|
| Amplitude decrement >10% N (%) |
Area decrement >10% N (%) |
CAA decrement >10% N (%) |
|
| Asymptomatic Normal Controls (n=32) | 10 (31) | 13 (41) | 4 (13) |
| Combined Asymptomatic and out-patient control group (n=65) |
20 (31) | 34 (52) | 9 (14) |
| Sodium Channel myotonia (n=11) | 2 (18) | 6 (54) | 1 (10) |
| B. Decrements of >20% | |||
|---|---|---|---|
| Amplitude decrement >20% N (%) |
Area decrement >20% N (%) |
CAA decrement >20% N (%) |
|
| Asymptomatic Normal Controls (n=32) | 6(19) | 5 (17) | 0 |
| Combined Asymptomatic and out-patient control group (n=65) |
8 (12) | 10 (15) | 0 |
| Sodium Channel myotonia (n=11) | 1 (10) | 1 (10) | 0 |
CAA decrement: Concordant amplitude-and-area decrement
Although pattern II amplitude-only changes have been described in patients with SCM2, in our cohort, pattern II CAA decrements of >20% were specific (100%) for MC; we saw only mild non-concordant decrements in our SCM patients. Although in general, AR MC patients had larger pattern II decrements, the occasional patient (R105C, F167L and E624fs) had negative SETs, and another (V327I and R894X) showed a positive SET only after rewarming. In our series, 10 Hz repetitive stimulation for 5 seconds did not provide additional information compared with the SETs. In the group of myotonia patients with normal SETs, considering a few specific clinical features such as eye closure myotonia may further improve the accuracy of referrals for genetic testing. We suggest a modified algorithm based on that proposed by Fournier et al2, incorporating our observations and more recently published data15, when using the SET to guide genetic analysis in patients with myotonia (Figure 5).
Figure 5.
In the long exercise tests, the range of time taken to achieve an abnormal result in our patients with periodic paralysis suggests that CMAPs should be monitored for a rest period of at least 50 minutes; it may be argued a longer period of monitoring may be required in sodium channel hypokalaemic periodic paralysis patients (not included in this series) who may show a late decrement1. Amplitude decrements of >40% from maximum CMAP during-or-after exercise showed a 100% sensitivity in our small cohort of patients with hypokaleamic and hyperkalaemic periodic paralysis. Amplitude or area decrements from baseline were less sensitive in the hyperkalaemic group. Area decrements from baseline were more sensitive in our ATS patients. Although the sensitivity of the long exercise test was high in our series, it is well recognised that the test may at times be negative in genetically confirmed patients, presumably because the degree to which the sarcolemmal membrane is depolarised in the interictal state is influenced by a variety of factors including diet, exercise, hormonal factors, stress, and cold16. In one of our patients with hyperkalaemic periodic paralysis (T704M), the long exercise test was initially positive (83% amplitude decrement) when she was on no medication, but became negative after she was treated with oral salbutamol, and remained negative 2 years later when she was taking acetazolamide; raising the question as to whether medication should be omitted prior to exercise testing. One ATS patient with an abnormal decrement had a normal study when this was repeated 5 years later without change in her medication. Although >40% amplitude decrement appears specific in the non-African-Caribbean population, the specificity falls from 100% to 94% if we include controls of African-Caribbean origin. The larger decrement from maximum CMAP in the long exercise test in the African-Caribbean group was an unexpected finding. A larger study designed to address this issue would help to clarify whether the upper limit for decrement should be different in this group. However, although we cannot be certain that our observations would be borne out in a larger study, the differences in the normal range for CK in this ethnic group compared with non-African-Caribbeans suggests that there may indeed be some ethnic differences in muscle physiology, and until further studies are done, we suggest that caution be exercised when interpreting mildly abnormal decrements in the long exercise test in African-Caribbean individuals. Although uncommon, periodic paralysis has been described in this population17,18. We did not find increments during the long or short exercise tests to be helpful diagnostically.
Supplementary Material
Acknowledgements
The authors are members of The Consortium for Clinical Investigation of Neurologic Channelopathies (CINCH) funded by the National Institutes of Health, [5 U54 NS059065-05S2(NINDS/ORD) and R13 NS057995]. In the UK this work was supported by the Brain Research Trust, a Medical Research Council Centre grant [G0601943] and by the National Center for Research Resources [5U54 RR019498-05]. Part of this work was undertaken at University College London Hospitals/University College London, which received a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme.
The authors wish to thank Professor Hugh Bostock for his helpful comments and suggestions on the manuscript.
References
- 1.Fournier E, Arzel M, Sternberg D, et al. Electromyography guides toward subgroups of mutations in muscle channelopathies. Ann Neurol. 2004;56:650–661. doi: 10.1002/ana.20241. [DOI] [PubMed] [Google Scholar]
- 2.Fournier E, Viala K, Gervais H, et al. Cold extends electromyography distinction between ion channel mutations causing myotonia. Ann Neurol. 2006;60:356–365. doi: 10.1002/ana.20905. [DOI] [PubMed] [Google Scholar]
- 3.Kuntzer T, Flocard F, Vial C, et al. Exercise test in muscle channelopathies and other muscle disorders. Muscle Nerve. 2000;23:1089–1094. doi: 10.1002/1097-4598(200007)23:7<1089::aid-mus12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Michel P, Sternberg D, Jeannet PY, et al. Comparative efficacy of repetitive nerve stimulation, exercise, and cold in differentiating myotonic disorders. Muscle Nerve. 2007;36:643–650. doi: 10.1002/mus.20856. [DOI] [PubMed] [Google Scholar]
- 5.McManis PG, Lambert EH, Daube JR. The exercise test in periodic paralysis. Muscle Nerve. 1986;9:704–710. doi: 10.1002/mus.880090805. [DOI] [PubMed] [Google Scholar]
- 6.Tengan CH, Antunes AC, Gabbai AA, et al. The exercise test as a monitor of disease status in hypokalaemic periodic paralysis. J Neurol Neurosurg Psychiatry. 2004;75:497–499. doi: 10.1136/jnnp.2003.013870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arimura Y, Arimura K, Suwazono S, et al. Predictive value of the prolonged exercise test in hypokalemic paralytic attack. Muscle Nerve. 1995;18:472–474. doi: 10.1002/mus.880180418. [DOI] [PubMed] [Google Scholar]
- 8.Arimura K, Arimura Y, Ng AR, et al. Muscle membrane excitability after exercise in thyrotoxic periodic paralysis and thyrotoxicosis without periodic paralysis. Muscle Nerve. 2007;36:784–788. doi: 10.1002/mus.20865. [DOI] [PubMed] [Google Scholar]
- 9.Katz JS, Wolfe GI, Iannaccone S, et al. The exercise test in Andersen syndrome. Arch Neurol. 1999;56:352–356. doi: 10.1001/archneur.56.3.352. [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, Zhang Y, Liu ZL, et al. Exercise test on the patients with normokalaemic periodic paralysis from a Chinese family with a mutation in the SCN4A gene. Chin Med J (Engl ) 2008;121:1915–1919. [PubMed] [Google Scholar]
- 11.Streib EW. AAEE minimonograph #27: differential diagnosis of myotonic syndromes. Muscle Nerve. 1987;10:603–615. doi: 10.1002/mus.880100704. [DOI] [PubMed] [Google Scholar]
- 12.Streib EW. Evoked response testing in myotonic syndromes. Muscle Nerve. 1984;7:590–592. doi: 10.1002/mus.880070709. [DOI] [PubMed] [Google Scholar]
- 13.Streib EW, Sun SF, Yarkowsky T. Transient paresis in myotonia syndromes: a simplified electrophysiologic approach. Muscle Nerve. 1982;5:719–723. [Google Scholar]
- 14.Streib EW, Sun SF, Hanson M. Paramyotonia congenita: clinical and electrophysiologic studies. Electromyogr Clin Neurophysiol. 1983;23:315–325. [PubMed] [Google Scholar]
- 15.Dupre N, Chrestian N, Bouchard JP, et al. Clinical, electrophysiologic, and genetic study of non-dystrophic myotonia in French-Canadians. Neuromuscul Disord. 2009;19:330–334. doi: 10.1016/j.nmd.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Miller TM, Dias da Silva MR, Miller HA, et al. Correlating phenotype and genotype in the periodic paralyses. Neurology. 2004;63:1647–1655. doi: 10.1212/01.wnl.0000143383.91137.00. [DOI] [PubMed] [Google Scholar]
- 17.Corbett VA, Nuttall FQ. Familial hypokalemic periodic paralysis in blacks. Ann Intern Med. 1975;83:63–65. doi: 10.7326/0003-4819-83-1-63. [DOI] [PubMed] [Google Scholar]
- 18.Kilpatrick RE, Seiler-Smith S, Levine SN. Thyrotoxic hypokalemic periodic paralysis: report of four cases in black American males. Thyroid. 1994;4:441–445. doi: 10.1089/thy.1994.4.441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.