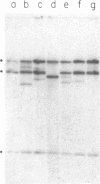
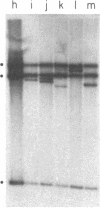
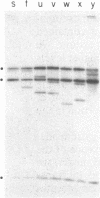
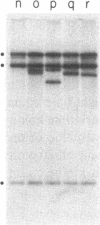
Abstract
Legionnaires disease is an acute respiratory disease that is often fatal for immunocompromised patients. The causative agent of this disease, Legionella pneumophila, is a Gram-negative bacterium that is present in a variety of aquatic environments. L. pneumophila is a facultative intracellular parasite; it grows within human phagocytic cells and eventually causes their destruction. In contrast to many other intracellular parasites, L. pneumophila is a Gram-negative bacterium that can be grown in standard microbiological culture medium. To determine the factors that enable this organism to enter, survive, and multiply within human mononuclear phagocytes, we chose bacteriophage Mu, a powerful genetic tool that transposes within the host cell genome, to generate insertion mutations and gene fusions in the Legionella genome. Certain derivatives of Mu are able to generate fusions between target genes and the lac operon from Escherichia coli. We have determined that although Mu is unable to attach to L. pneumophila or complete its life cycle within Legionella, it does transpose within the Legionella genome. Transposition was detected with a mini-Mu phage that carries the lac operon of E. coli.
Full text
PDF

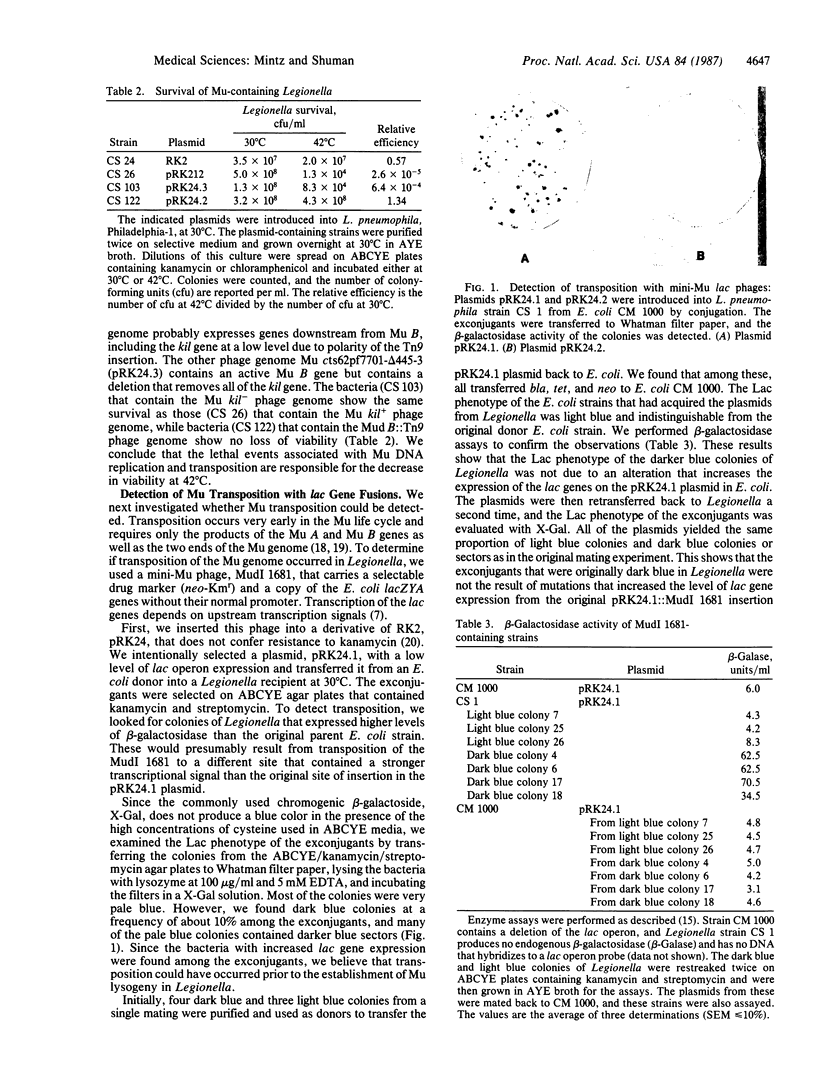


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. A., Howe M. M., Gross C. A. Mu dX, a derivative of Mu d1 (lac Apr) which makes stable lacZ fusions at high temperature. J Bacteriol. 1983 Nov;156(2):970–974. doi: 10.1128/jb.156.2.970-974.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I. Reversal of mutator phage Mu integration. J Mol Biol. 1975 Jul 25;96(1):87–99. doi: 10.1016/0022-2836(75)90183-7. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J. In vivo formation of gene fusions encoding hybrid beta-galactosidase proteins in one step with a transposable Mu-lac transducing phage. Proc Natl Acad Sci U S A. 1984 Jan;81(2):535–539. doi: 10.1073/pnas.81.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet L., Faelen M., Lefèbvre N., Résibois A., Toussaint A., van Gijsegem F. Genetic study of Mu transposition and Mu-mediated chromosomal rearrangements. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):355–363. doi: 10.1101/sqb.1981.045.01.049. [DOI] [PubMed] [Google Scholar]
- Dreyfus L. A., Iglewski B. H. Conjugation-mediated genetic exchange in Legionella pneumophila. J Bacteriol. 1985 Jan;161(1):80–84. doi: 10.1128/jb.161.1.80-84.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleberg N. C., Pearlman E., Eisenstein B. I. Legionella pneumophila surface antigens cloned and expressed in Escherichia coli are translocated to the host cell surface and interact with specific anti-Legionella antibodies. J Bacteriol. 1984 Oct;160(1):199–203. doi: 10.1128/jb.160.1.199-203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D., Meyer R., Miller D. S., Helinski D. R. Generation in vitro of deletions in the broad host range plasmid RK2 using phage Mu insertions and a restriction endonuclease. Gene. 1976;1(1):107–119. doi: 10.1016/0378-1119(76)90010-x. [DOI] [PubMed] [Google Scholar]
- Grundy F. J., Howe M. M. Involvement of the invertible G segment in bacteriophage mu tail fiber biosynthesis. Virology. 1984 Apr 30;134(2):296–317. doi: 10.1016/0042-6822(84)90299-x. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A. Phagocytosis of the Legionnaires' disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984 Jan;36(1):27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. C., Richmond M. H., Sykes R. B. Molecular characterization of the R factors implicated in the carbenicillin resistance of a sequence of Pseudomonas aeruginosa strains isolated from burns. Antimicrob Agents Chemother. 1973 Feb;3(2):279–288. doi: 10.1128/aac.3.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen M. G., Street E. D., Hoffman P. S. Broad-host-range plasmid pRK340 delivers Tn5 into the Legionella pneumophila chromosome. J Bacteriol. 1985 Jun;162(3):1332–1335. doi: 10.1128/jb.162.3.1332-1335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R., Figurski D., Helinski D. R. Physical and genetic studies with restriction endonucleases on the broad host-range plasmid RK2. Mol Gen Genet. 1977 Apr 29;152(3):129–135. doi: 10.1007/BF00268809. [DOI] [PubMed] [Google Scholar]
- Murooka Y., Takizawa N., Harada T. Introduction of bacteriophage Mu into bacteria of various genera and intergeneric gene transfer by RP4::Mu. J Bacteriol. 1981 Jan;145(1):358–368. doi: 10.1128/jb.145.1.358-368.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaki T., Bukhari A. I. Events following prophage Mu induction. J Bacteriol. 1975 May;122(2):437–442. doi: 10.1128/jb.122.2.437-442.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder W., van de Putte P. Genetic study of prophage excision with a temperature inducible mutant of Mu-1. Mol Gen Genet. 1974 May 21;130(2):99–104. doi: 10.1007/BF00269081. [DOI] [PubMed] [Google Scholar]
- van Vliet F., Silva B., van Montagu M., Schell J. Transfer of RP4::mu plasmids to Agrobacterium tumefaciens. Plasmid. 1978 Sep;1(4):446–455. doi: 10.1016/0147-619x(78)90003-3. [DOI] [PubMed] [Google Scholar]