Abstract
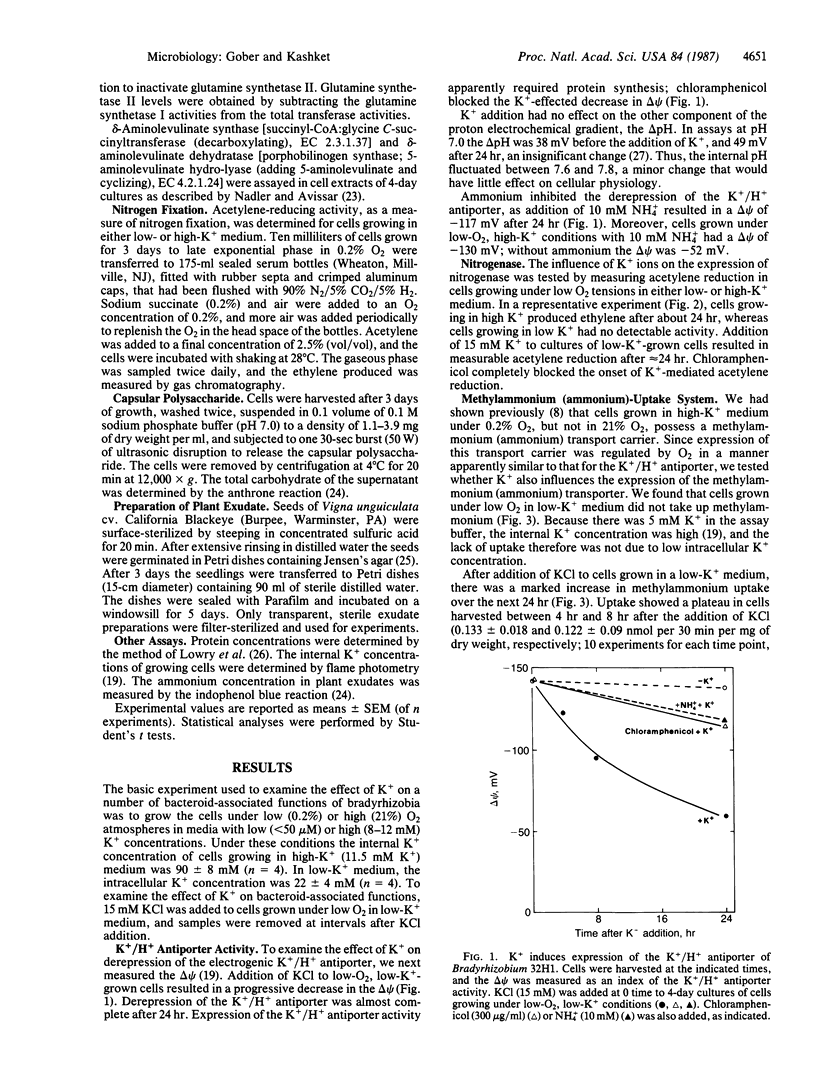
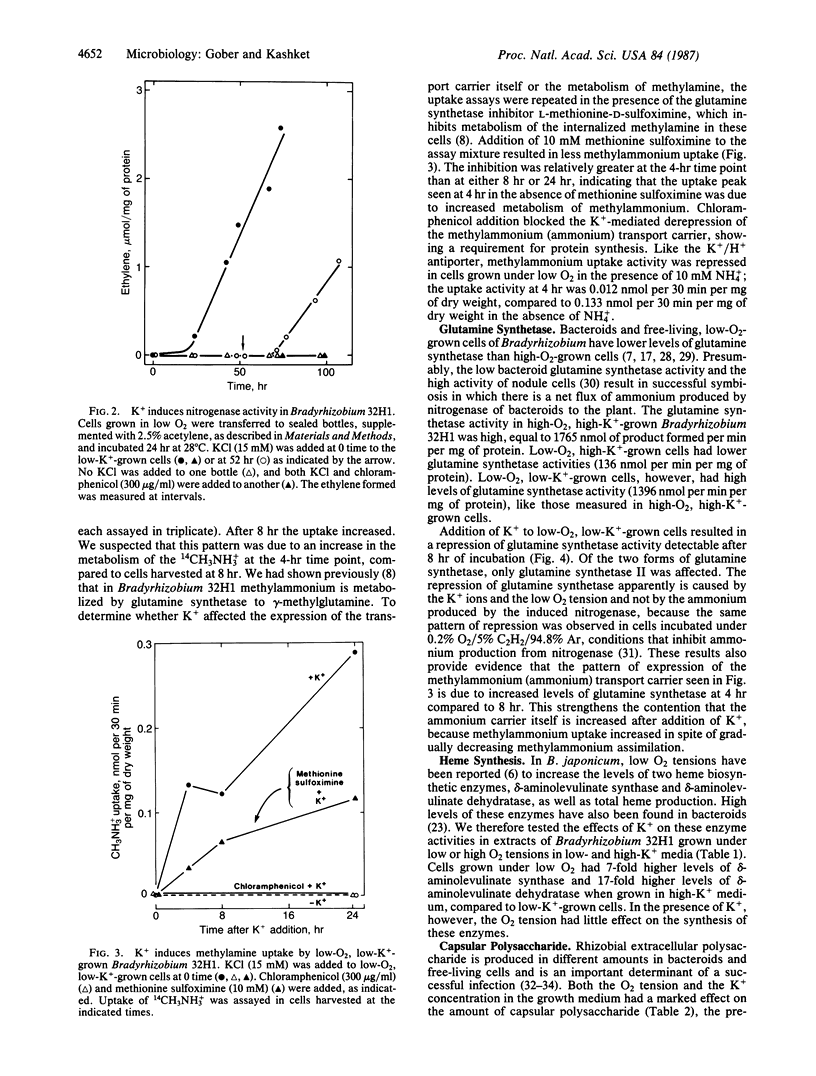
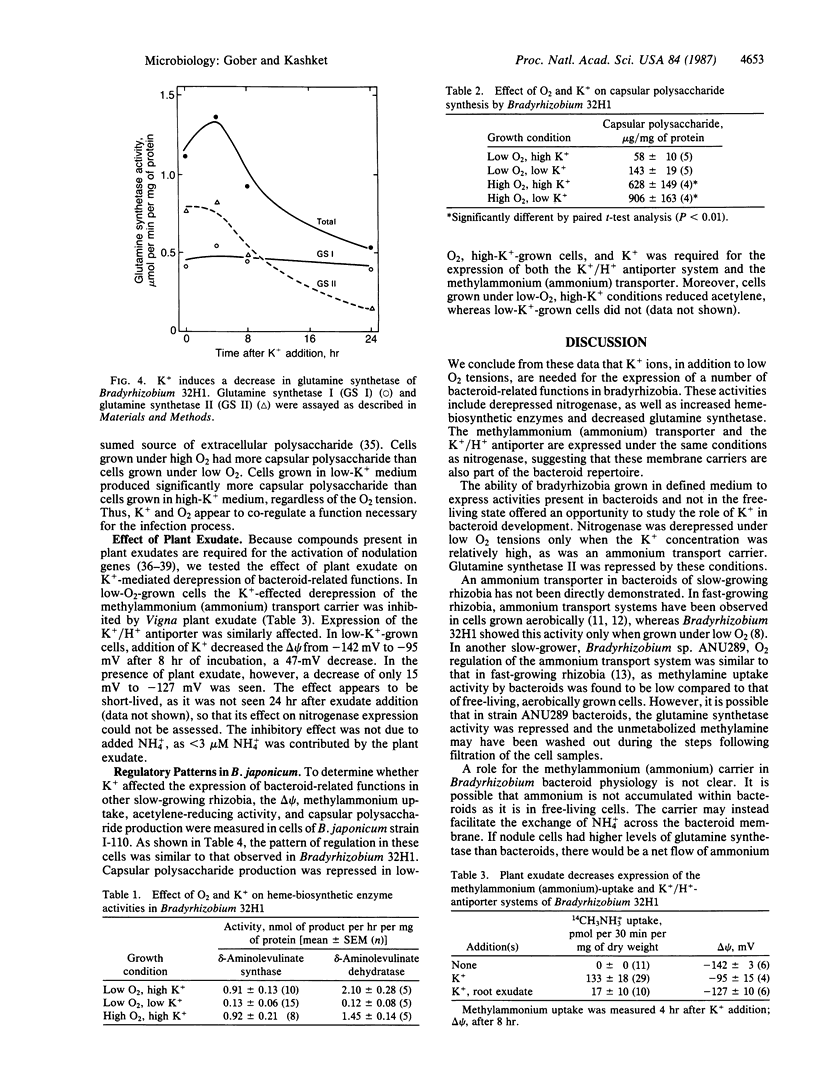
Cowpea Bradyrhizobium 32H1 cells, when grown under 0.2% O2, synthesize nitrogenase, as well as a methylammonium (ammonium) transport system and an electrogenic K+/H+ antiporter. This effect was seen in growth medium containing 8-12 mM K+ but not with 50 μM K+. Addition of K+ to cells growing under low O2 tensions in low-K+ medium led to various phenotypic properties associated with bacteroids, including the ability to reduce acetylene, induction of an ammonium transport carrier and the K+/H+ antiporter, and increased synthesis of two heme-biosynthetic enzymes, δ-aminolevulinate synthase and δ-aminolevulinate dehydratase. K+ addition caused the repression of glutamine synthetase and of capsular polysaccharide synthesis, functions related to the free-living state. A similar pattern of regulation was observed in Bradyrhizobium japonicum. In addition, K+-mediated depression in Bradyrhizobium 32H1 was inhibited by exudate of Vigna unguiculata, its host plant. We conclude that K+ ions, in addition to low O2 tension, are needed for the expression of several bacteroid-related functions in bradyrhizobia and thus are a major controlling influence in bacteroid development.
Keywords: nitrogen fixation, K+/H+ antiporter, ammonium uptake, glutamine synthetase, plant exudate
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar Y. J., Nadler K. D. Stimulation of tetrapyrrole formation in Rhizobium japonicum by restricted aeration. J Bacteriol. 1978 Sep;135(3):782–789. doi: 10.1128/jb.135.3.782-789.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R. A., Janssen K. A., Resnick A. D., Blumenberg M., Foor F., Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977 Feb;129(2):1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A. The genetic complexity of nitrogen fixation. The ninth Fleming lecture. J Gen Microbiol. 1984 Nov;130(11):2745–2755. doi: 10.1099/00221287-130-11-2745. [DOI] [PubMed] [Google Scholar]
- Finan T. M., Hirsch A. M., Leigh J. A., Johansen E., Kuldau G. A., Deegan S., Walker G. C., Signer E. R. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell. 1985 Apr;40(4):869–877. doi: 10.1016/0092-8674(85)90346-0. [DOI] [PubMed] [Google Scholar]
- Gober J. W., Kashket E. R. Effects of K+ on the proton motive force of Bradyrhizobium sp. strain 32H1. J Bacteriol. 1986 May;166(2):618–622. doi: 10.1128/jb.166.2.618-622.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gober J. W., Kashket E. R. H+/ATP stoichiometry of cowpea Rhizobium sp. strain 32H1 cells grown under nitrogen-fixing and nitrogen-nonfixing conditions. J Bacteriol. 1984 Oct;160(1):216–221. doi: 10.1128/jb.160.1.216-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gober J. W., Kashket E. R. Methylammonium uptake by Rhizobium sp. strain 32H1. J Bacteriol. 1983 Mar;153(3):1196–1201. doi: 10.1128/jb.153.3.1196-1201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
- Keister D. L. Acetylene reduction by pure cultures of Rhizobia. J Bacteriol. 1975 Sep;123(3):1265–1268. doi: 10.1128/jb.123.3.1265-1268.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuykendall L. D., Elkan G. H. Rhizobium japonicum derivatives differing in nitrogen-fixing efficiency and carbohydrate utilization. Appl Environ Microbiol. 1976 Oct;32(4):511–519. doi: 10.1128/aem.32.4.511-519.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R. A. Control of ammonium assimilation in Rhizobium 32H1. J Bacteriol. 1978 Jul;135(1):114–123. doi: 10.1128/jb.135.1.114-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R. A. Regulation of Rhizobium nitrogen fixation by the unadenylylated glutamine synthetase I system. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5817–5821. doi: 10.1073/pnas.77.10.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B. Genetic control of nitrogen assimilation in bacteria. Annu Rev Genet. 1982;16:135–168. doi: 10.1146/annurev.ge.16.120182.001031. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Composition of the Capsular and Extracellular Polysaccharides of Rhizobium japonicum: CHANGES WITH CULTURE AGE AND CORRELATIONS WITH BINDING OF SOYBEAN SEED LECTIN TO THE BACTERIA . Plant Physiol. 1980 Jul;66(1):158–163. doi: 10.1104/pp.66.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan J. T., Long S. R. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6609–6613. doi: 10.1073/pnas.82.19.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler K. D., Avissar Y. J. Heme Synthesis in Soybean Root Nodules: I. On the Role of Bacteroid delta-Aminolevulinic Acid Synthase and delta-Aminolevulinic Acid Dehydrase in the Synthesis of the Heme of Leghemoglobin. Plant Physiol. 1977 Sep;60(3):433–436. doi: 10.1104/pp.60.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gara F., Shanmugam K. T. Regulation of nitrogen fixation by Rhizobia. Export of fixed N2 as NH+4. Biochim Biophys Acta. 1976 Jul 21;437(2):313–321. doi: 10.1016/0304-4165(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Rao V. R., Darrow R. A., Keister D. L. Effect of oxygen tension on nitrogenase and on glutamine synthetases I and II in Rhizobium jaonicum 61A76. Biochem Biophys Res Commun. 1978 Mar 15;81(1):224–231. doi: 10.1016/0006-291x(78)91653-4. [DOI] [PubMed] [Google Scholar]
- Rossen L., Shearman C. A., Johnston A. W., Downie J. A. The nodD gene of Rhizobium leguminosarum is autoregulatory and in the presence of plant exudate induces the nodA,B,C genes. EMBO J. 1985 Dec 16;4(13A):3369–3373. doi: 10.1002/j.1460-2075.1985.tb04092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöllhorn R., Burris R. H. Acetylene as a competitive inhibitor of N-2 fixation. Proc Natl Acad Sci U S A. 1967 Jul;58(1):213–216. doi: 10.1073/pnas.58.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman C. A., Rossen L., Johnston A. W., Downie J. A. The Rhizobium leguminosarum nodulation gene nodF encodes a polypeptide similar to acyl-carrier protein and is regulated by nodD plus a factor in pea root exudate. EMBO J. 1986 Apr;5(4):647–652. doi: 10.1002/j.1460-2075.1986.tb04262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully R. E., Terry M. E. Decreased Exopolysaccharide Synthesis by Anaerobic and Symbiotic Cells of Bradyrhizobium japonicum. Plant Physiol. 1985 Oct;79(2):445–450. doi: 10.1104/pp.79.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbosch K. A., Noel K. D., Kaneko Y., Newcomb E. H. Nodule initiation elicited by noninfective mutants of Rhizobium phaseoli. J Bacteriol. 1985 Jun;162(3):950–959. doi: 10.1128/jb.162.3.950-959.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



