Abstract
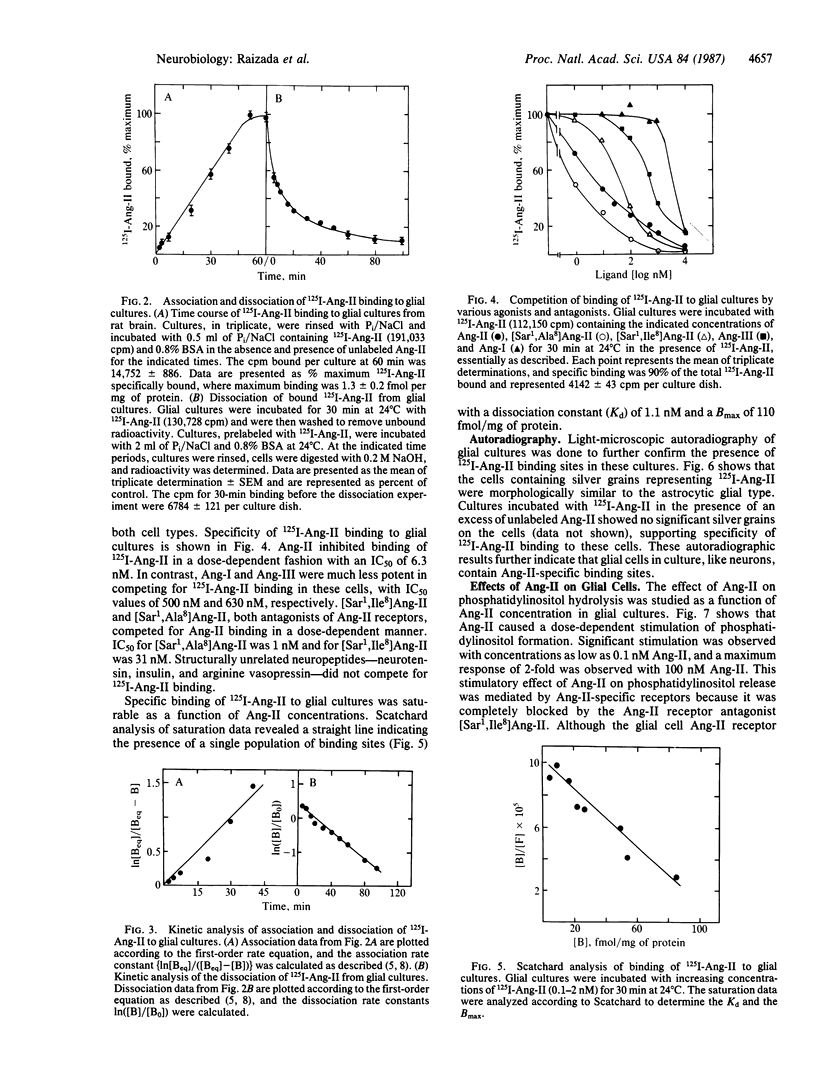
Angiotensin II (Ang-II) has profound effects on the brain. Receptors for Ang-II have been demonstrated on neurons, but no relationship between glial cells and Ang-II has been established. Glial cells (from the hypothalamus and brain stem of 1-day-old rat brains) in primary culture have been used to demonstrate the presence of specific Ang-II receptors. Binding of 125I-Ang-II to glial cultures was rapid, reversible, saturable, and specific for Ang-II. The rank order of potency of 125I-Ang-II binding was as follows: Ang-II = [sarcosine1,Ala8]Ang-II greater than [sarcosine1,Ile8]Ang-II much greater than Ang-III greater than Ang-I. Scatchard analysis revealed a homogeneous population of high-affinity (Kd = 1.1 nM) binding sites with a Bmax of 110 fmol/mg of protein. Light-microscopic autoradiography of 125I-Ang-II binding supported the kinetic data, documenting specific Ang-II receptors on the glial cells. Ang-II stimulated a dose-dependent hydrolysis of phosphatidylinositols in glial cells, an effect mediated by Ang-II receptors. However, Ang-II failed to influence [3H]norepinephrine uptake, and catecholamines failed to regulate Ang-II receptors, effects that occur in neurons. These observations demonstrate the presence of specific Ang-II receptors on the glial cells in primary cultures derived from normotensive rat brain. The receptors are kinetically similar to, but functionally distinct from, the neuronal Ang-II receptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke D. W., Boyd F. T., Jr, Kappy M. S., Raizada M. K. Insulin binds to specific receptors and stimulates 2-deoxy-D-glucose uptake in cultured glial cells from rat brain. J Biol Chem. 1984 Oct 10;259(19):11672–11675. [PubMed] [Google Scholar]
- Epstein A. N., Fitzsimons J. T., Rolls B. J. Drinking induced by injection of angiotensin into the rain of the rat. J Physiol. 1970 Sep;210(2):457–474. doi: 10.1113/jphysiol.1970.sp009220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein J. B., Gonzales R. A., Baker S. P., Sumners C., Crews F. T., Raizada M. K. Decreased alpha 1-adrenergic receptor-mediated inositide hydrolysis in neurons from hypertensive rat brain. Am J Physiol. 1986 Aug;251(2 Pt 1):C230–C237. doi: 10.1152/ajpcell.1986.251.2.C230. [DOI] [PubMed] [Google Scholar]
- Ganong W. F. The brain renin-angiotensin system. Annu Rev Physiol. 1984;46:17–31. doi: 10.1146/annurev.ph.46.030184.000313. [DOI] [PubMed] [Google Scholar]
- Ganten D., Hermann K., Bayer C., Unger T., Lang R. E. Angiotensin synthesis in the brain and increased turnover in hypertensive rats. Science. 1983 Aug 26;221(4613):869–871. doi: 10.1126/science.6879184. [DOI] [PubMed] [Google Scholar]
- Gonzales R. A., Feldstein J. B., Crews F. T., Raizada M. K. Receptor-mediated inositide hydrolysis is a neuronal response: comparison of primary neuronal and glial cultures. Brain Res. 1985 Oct 21;345(2):350–355. doi: 10.1016/0006-8993(85)91015-7. [DOI] [PubMed] [Google Scholar]
- Lind R. W., Swanson L. W., Ganten D. Angiotensin II immunoreactivity in the neural afferents and efferents of the subfornical organ of the rat. Brain Res. 1984 Nov 12;321(2):209–215. doi: 10.1016/0006-8993(84)90174-4. [DOI] [PubMed] [Google Scholar]
- Pelton E. W., 2nd, Kimelberg H. K., Shipherd S. V., Bourke R. S. Dopamine and norepinephrine uptake and metabolism by astroglial cells in culture. Life Sci. 1981 Apr 6;28(14):1655–1663. doi: 10.1016/0024-3205(81)90322-2. [DOI] [PubMed] [Google Scholar]
- Phillips M. I. Angiotensin in the brain. Neuroendocrinology. 1978;25(6):354–377. doi: 10.1159/000122756. [DOI] [PubMed] [Google Scholar]
- Raizada M. K., Muther T. F., Sumners C. Increased angiotensin II receptors in neuronal cultures from hypertensive rat brain. Am J Physiol. 1984 Nov;247(5 Pt 1):C364–C372. doi: 10.1152/ajpcell.1984.247.5.C364. [DOI] [PubMed] [Google Scholar]
- Raizada M. K., Phillips M. I., Gerndt J. S. Primary cultures from fetal rat brain incorporate [3H]-isoleucine and [3H]-valine into immunoprecipitable angiotensin II. Neuroendocrinology. 1983;36(1):64–67. doi: 10.1159/000123438. [DOI] [PubMed] [Google Scholar]
- Raizada M. K., Stenstrom B., Phillips M. I., Sumners C. Angiotensin II in neuronal cultures from brains of normotensive and hypertensive rats. Am J Physiol. 1984 Jul;247(1 Pt 1):C115–C119. doi: 10.1152/ajpcell.1984.247.1.C115. [DOI] [PubMed] [Google Scholar]
- Sirett N. E., McLean A. S., Bray J. J., Hubbard J. I. Distribution of angiotensin II receptors in rat brain. Brain Res. 1977 Feb 18;122(2):299–312. doi: 10.1016/0006-8993(77)90296-7. [DOI] [PubMed] [Google Scholar]
- Sumners C., Muther T. F., Raizada M. K. Altered norepinephrine uptake in neuronal cultures from spontaneously hypertensive rat brain. Am J Physiol. 1985 May;248(5 Pt 1):C488–C497. doi: 10.1152/ajpcell.1985.248.5.C488. [DOI] [PubMed] [Google Scholar]
- Sumners C., Phillips M. I., Raizada M. K. Angiotensin II stimulates changes in the norepinephrine content of primary cultures of rat brain. Neurosci Lett. 1983 Apr 29;36(3):305–309. doi: 10.1016/0304-3940(83)90017-4. [DOI] [PubMed] [Google Scholar]
- Sumners C., Raizada M. K. Angiotensin II stimulates norepinephrine uptake in hypothalamus-brain stem neuronal cultures. Am J Physiol. 1986 Feb;250(2 Pt 1):C236–C244. doi: 10.1152/ajpcell.1986.250.2.C236. [DOI] [PubMed] [Google Scholar]
- Sumners C., Raizada M. K. Catecholamine-angiotensin II receptor interaction in primary cultures of rat brain. Am J Physiol. 1984 May;246(5 Pt 1):C502–C509. doi: 10.1152/ajpcell.1984.246.5.C502. [DOI] [PubMed] [Google Scholar]
- Sumners C., Watkins L. L., Raizada M. K. Alpha 1-adrenergic receptor-mediated downregulation of angiotensin II receptors in neuronal cultures. J Neurochem. 1986 Oct;47(4):1117–1126. doi: 10.1111/j.1471-4159.1986.tb00729.x. [DOI] [PubMed] [Google Scholar]
- Wilson K. M., Sumners C., Hathaway S., Fregly M. J. Mineralocorticoids modulate central angiotensin II receptors in rats. Brain Res. 1986 Sep 10;382(1):87–96. doi: 10.1016/0006-8993(86)90114-9. [DOI] [PubMed] [Google Scholar]





