Abstract
The reaction of cadmium nitrate and sodium nitrite in the presence of 1,10-phenanthroline yields the mixed nitrate–nitrite title complex, [Cd(NO2)1.75(NO3)0.25(C12H8N2)2]. The metal ion is bis-chelated by two N-heterocycles as well as by the nitrate/nitrite ions in a distorted dodecahedral CdN4O4 coordination environment. One nitrite group is ordered; the other is disordered with respect to a nitrate group (ratio 0.75:0.25) concerning the O atom that is not involved in bonding to the metal ion.
Related literature
For the crystal structure of [Cd(NO3)2(C12H8N2)2], see: Tadjarodi et al. (2001 ▶) and for the crystal structure of [Cd(NO2)2(C12H8N2)2], see: Abedini et al. (2005 ▶).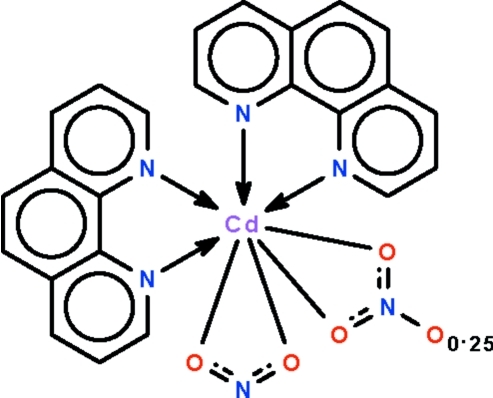
Experimental
Crystal data
[Cd(NO2)1.75(NO3)0.25(C12H8N2)2]
M r = 568.83
Triclinic,
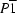
a = 9.1470 (4) Å
b = 10.1866 (4) Å
c = 13.0057 (6) Å
α = 76.953 (4)°
β = 77.270 (4)°
γ = 70.404 (4)°
V = 1098.27 (8) Å3
Z = 2
Mo Kα radiation
μ = 1.04 mm−1
T = 100 K
0.30 × 0.20 × 0.10 mm
Data collection
Agilent Technologies SuperNova Dual diffractometer with an Atlas detector
Absorption correction: multi-scan (CrysAlis PRO; Agilent Technologies, 2010 ▶) T min = 0.745, T max = 0.903
8702 measured reflections
4852 independent reflections
4256 reflections with I > 2σ(I)
R int = 0.032
Refinement
R[F 2 > 2σ(F 2)] = 0.033
wR(F 2) = 0.073
S = 1.02
4852 reflections
325 parameters
H-atom parameters constrained
Δρmax = 0.49 e Å−3
Δρmin = −0.69 e Å−3
Data collection: CrysAlis PRO (Agilent Technologies, 2010 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811002431/wm2452sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811002431/wm2452Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| Cd1—O3 | 2.355 (2) |
| Cd1—N6 | 2.390 (2) |
| Cd1—N4 | 2.393 (2) |
| Cd1—N3 | 2.418 (2) |
| Cd1—O1 | 2.4547 (19) |
| Cd1—O4 | 2.503 (2) |
| Cd1—O2 | 2.5041 (19) |
| Cd1—N5 | 2.510 (2) |
Acknowledgments
We thank Shahid Beheshti University and the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
We had previously reported the structure of the 1,10-phenanthroline adduct of cadmium nitrate. In the corresponding structure, the cadmium ion, situated on a twofold rotation axis, shows eightfold coordination, which is somewhat less common (Tadjarodi et al., 2001). The compound is conveniently synthesized by the direct addition of 1,10-phenanthroline to a cadmium nitrate solution. In a similar reaction, but when nitrite ions present, a mixed nitrate/nitrite compound is obtained.
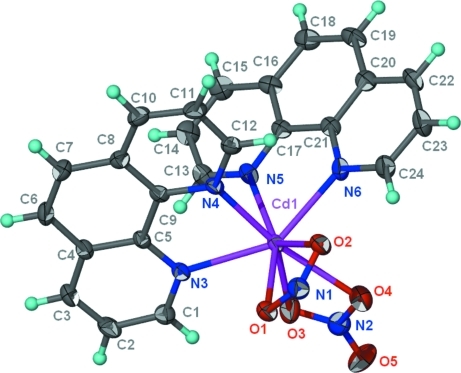
In the title compound, Cd(NO2)1.75(NO3)0.25(C12H8N2)2 (Scheme I), the metal ion also exists in an eight-coordinate distorted dodecahedral CdN4O4 geometry (Fig. 1). The metal ion is bis-chelated by two N-heterocycles as well as by the nitrate/nitrite ions. The molecule lies on a general position, and one nitrite group is disordered with respect to a nitrate group (ratio 0.75:0.25).
Experimental
Cadmium nitrate (1 mmol), sodium nitrite (1 mmol) and 1,10-phenanthroline (1 mmol) were loaded into a convection tube. The tube was filled with dry methanol and kept at 333 K. Colorless crystals were collected from the side arm of the tube after several days.
Refinement
Carbon-bound H-atoms were placed in calculated positions [C—H 0.95 Å, Uiso(H) 1.2Ueq(C)] and were included in the refinement in the riding model approximation.
The structure, when refined as a dinitrite, had a high remaining peak approximately 1.2 Å away from one of the two N atoms of the nitrite groups. This site was allow to refine as an O atom of a disordered nitrate group. As the occupancy refined to nearly 1/4, its occupancy was eventually fixed as 0.25.
Figures
Fig. 1.
Thermal ellipsoid plot (Barbour, 2001) of Cd(NO2)1.75(NO3)0.25(C12H8N2)2 at the 70% probability level; hydrogen atoms are drawn as spheres of arbitrary radius.
Crystal data
| [Cd(NO2)1.75(NO3)0.25(C12H8N2)2] | Z = 2 |
| Mr = 568.83 | F(000) = 568 |
| Triclinic, P1 | Dx = 1.720 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.1470 (4) Å | Cell parameters from 4883 reflections |
| b = 10.1866 (4) Å | θ = 2.4–29.3° |
| c = 13.0057 (6) Å | µ = 1.04 mm−1 |
| α = 76.953 (4)° | T = 100 K |
| β = 77.270 (4)° | Irregular, colorless |
| γ = 70.404 (4)° | 0.30 × 0.20 × 0.10 mm |
| V = 1098.27 (8) Å3 |
Data collection
| Agilent Technologies SuperNova Dual diffractometer with an Atlas detector | 4852 independent reflections |
| Radiation source: SuperNova X-ray Source | 4256 reflections with I > 2σ(I) |
| Mirror | Rint = 0.032 |
| Detector resolution: 10.4041 pixels mm-1 | θmax = 27.5°, θmin = 2.4° |
| ω scans | h = −10→11 |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent Technologies, 2010) | k = −13→11 |
| Tmin = 0.745, Tmax = 0.903 | l = −16→12 |
| 8702 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.033 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.073 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0283P)2 + 0.1342P] where P = (Fo2 + 2Fc2)/3 |
| 4852 reflections | (Δ/σ)max = 0.001 |
| 325 parameters | Δρmax = 0.49 e Å−3 |
| 0 restraints | Δρmin = −0.69 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Cd1 | 0.55052 (2) | 0.243966 (18) | 0.241633 (15) | 0.01614 (7) | |
| N1 | 0.7446 (3) | −0.0427 (2) | 0.3117 (2) | 0.0230 (5) | |
| N2 | 0.8042 (3) | 0.3662 (3) | 0.1701 (2) | 0.0297 (6) | |
| N3 | 0.4863 (3) | 0.2130 (2) | 0.07978 (17) | 0.0169 (5) | |
| N4 | 0.3528 (3) | 0.1267 (2) | 0.28117 (17) | 0.0154 (5) | |
| N5 | 0.3074 (3) | 0.4518 (2) | 0.22889 (17) | 0.0178 (5) | |
| N6 | 0.4512 (3) | 0.3368 (2) | 0.40496 (18) | 0.0192 (5) | |
| O1 | 0.7414 (2) | 0.01355 (19) | 0.21528 (15) | 0.0232 (4) | |
| O2 | 0.6588 (2) | 0.0353 (2) | 0.37712 (15) | 0.0244 (5) | |
| O3 | 0.6973 (3) | 0.3783 (2) | 0.11795 (17) | 0.0324 (5) | |
| O4 | 0.7905 (3) | 0.2957 (2) | 0.26214 (17) | 0.0311 (5) | |
| O5 | 0.9091 (12) | 0.4103 (10) | 0.1371 (8) | 0.042 (2) | 0.25 |
| C1 | 0.5549 (4) | 0.2514 (3) | −0.0180 (2) | 0.0207 (6) | |
| H1 | 0.6401 | 0.2882 | −0.0259 | 0.025* | |
| C2 | 0.5079 (4) | 0.2403 (3) | −0.1101 (2) | 0.0245 (7) | |
| H2 | 0.5599 | 0.2697 | −0.1788 | 0.029* | |
| C3 | 0.3864 (4) | 0.1866 (3) | −0.1000 (2) | 0.0214 (6) | |
| H3 | 0.3534 | 0.1778 | −0.1617 | 0.026* | |
| C4 | 0.3102 (3) | 0.1446 (3) | 0.0025 (2) | 0.0172 (6) | |
| C5 | 0.3661 (3) | 0.1595 (2) | 0.0909 (2) | 0.0150 (5) | |
| C6 | 0.1825 (3) | 0.0874 (3) | 0.0203 (2) | 0.0212 (6) | |
| H6 | 0.1455 | 0.0768 | −0.0392 | 0.025* | |
| C7 | 0.1132 (4) | 0.0480 (3) | 0.1201 (2) | 0.0220 (6) | |
| H7 | 0.0260 | 0.0133 | 0.1297 | 0.026* | |
| C8 | 0.1691 (3) | 0.0577 (3) | 0.2120 (2) | 0.0181 (6) | |
| C9 | 0.2942 (3) | 0.1139 (2) | 0.1978 (2) | 0.0151 (6) | |
| C10 | 0.1059 (4) | 0.0116 (3) | 0.3171 (2) | 0.0231 (6) | |
| H10 | 0.0202 | −0.0261 | 0.3305 | 0.028* | |
| C11 | 0.1695 (3) | 0.0216 (3) | 0.4007 (2) | 0.0224 (6) | |
| H11 | 0.1302 | −0.0116 | 0.4722 | 0.027* | |
| C12 | 0.2919 (3) | 0.0807 (3) | 0.3790 (2) | 0.0193 (6) | |
| H12 | 0.3337 | 0.0884 | 0.4374 | 0.023* | |
| C13 | 0.2348 (4) | 0.5050 (3) | 0.1442 (2) | 0.0208 (6) | |
| H13 | 0.2824 | 0.4683 | 0.0798 | 0.025* | |
| C14 | 0.0912 (4) | 0.6131 (3) | 0.1453 (2) | 0.0242 (7) | |
| H14 | 0.0419 | 0.6469 | 0.0833 | 0.029* | |
| C15 | 0.0235 (4) | 0.6689 (3) | 0.2363 (2) | 0.0246 (7) | |
| H15 | −0.0732 | 0.7429 | 0.2381 | 0.029* | |
| C16 | 0.0972 (3) | 0.6166 (3) | 0.3281 (2) | 0.0195 (6) | |
| C17 | 0.2391 (3) | 0.5055 (3) | 0.3202 (2) | 0.0171 (6) | |
| C18 | 0.0342 (4) | 0.6698 (3) | 0.4263 (2) | 0.0240 (7) | |
| H18 | −0.0612 | 0.7451 | 0.4309 | 0.029* | |
| C19 | 0.1074 (4) | 0.6156 (3) | 0.5130 (2) | 0.0240 (7) | |
| H19 | 0.0644 | 0.6547 | 0.5768 | 0.029* | |
| C20 | 0.2491 (4) | 0.4999 (3) | 0.5094 (2) | 0.0209 (6) | |
| C21 | 0.3163 (3) | 0.4449 (3) | 0.4137 (2) | 0.0175 (6) | |
| C22 | 0.3269 (4) | 0.4374 (3) | 0.5986 (2) | 0.0242 (7) | |
| H22 | 0.2855 | 0.4706 | 0.6648 | 0.029* | |
| C23 | 0.4628 (4) | 0.3281 (3) | 0.5883 (2) | 0.0269 (7) | |
| H23 | 0.5165 | 0.2837 | 0.6477 | 0.032* | |
| C24 | 0.5216 (4) | 0.2824 (3) | 0.4898 (2) | 0.0233 (6) | |
| H24 | 0.6177 | 0.2080 | 0.4836 | 0.028* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cd1 | 0.01776 (13) | 0.01855 (11) | 0.01235 (11) | −0.00468 (8) | −0.00285 (8) | −0.00377 (8) |
| N1 | 0.0219 (14) | 0.0227 (12) | 0.0237 (14) | −0.0033 (10) | −0.0042 (11) | −0.0069 (11) |
| N2 | 0.0284 (17) | 0.0363 (15) | 0.0304 (15) | −0.0163 (13) | 0.0004 (13) | −0.0124 (12) |
| N3 | 0.0190 (13) | 0.0177 (11) | 0.0145 (12) | −0.0060 (9) | −0.0005 (10) | −0.0050 (9) |
| N4 | 0.0149 (13) | 0.0173 (11) | 0.0116 (11) | −0.0024 (9) | −0.0016 (10) | −0.0021 (9) |
| N5 | 0.0247 (14) | 0.0156 (11) | 0.0137 (12) | −0.0073 (10) | −0.0027 (10) | −0.0021 (9) |
| N6 | 0.0219 (14) | 0.0186 (11) | 0.0189 (12) | −0.0043 (10) | −0.0082 (11) | −0.0043 (10) |
| O1 | 0.0218 (12) | 0.0298 (10) | 0.0171 (11) | −0.0051 (9) | −0.0027 (9) | −0.0063 (9) |
| O2 | 0.0224 (12) | 0.0309 (11) | 0.0177 (11) | −0.0022 (9) | −0.0037 (9) | −0.0081 (9) |
| O3 | 0.0370 (15) | 0.0430 (13) | 0.0277 (12) | −0.0244 (11) | −0.0071 (11) | −0.0057 (10) |
| O4 | 0.0328 (14) | 0.0357 (12) | 0.0275 (13) | −0.0096 (10) | −0.0054 (11) | −0.0110 (10) |
| O5 | 0.031 (6) | 0.053 (6) | 0.054 (6) | −0.035 (5) | 0.001 (5) | −0.009 (5) |
| C1 | 0.0216 (17) | 0.0242 (14) | 0.0180 (15) | −0.0102 (12) | −0.0011 (12) | −0.0035 (12) |
| C2 | 0.0307 (19) | 0.0293 (15) | 0.0130 (14) | −0.0130 (13) | 0.0029 (13) | −0.0027 (12) |
| C3 | 0.0274 (18) | 0.0234 (14) | 0.0154 (14) | −0.0083 (12) | −0.0044 (13) | −0.0056 (12) |
| C4 | 0.0192 (16) | 0.0164 (13) | 0.0161 (14) | −0.0030 (11) | −0.0045 (12) | −0.0048 (11) |
| C5 | 0.0160 (15) | 0.0132 (12) | 0.0140 (13) | −0.0003 (10) | −0.0034 (11) | −0.0043 (10) |
| C6 | 0.0227 (17) | 0.0243 (14) | 0.0201 (15) | −0.0068 (12) | −0.0091 (13) | −0.0056 (12) |
| C7 | 0.0182 (16) | 0.0262 (14) | 0.0248 (16) | −0.0086 (12) | −0.0045 (13) | −0.0062 (13) |
| C8 | 0.0148 (15) | 0.0194 (13) | 0.0188 (14) | −0.0032 (11) | −0.0024 (12) | −0.0038 (11) |
| C9 | 0.0148 (15) | 0.0119 (12) | 0.0143 (13) | 0.0020 (10) | −0.0024 (11) | −0.0026 (10) |
| C10 | 0.0166 (16) | 0.0298 (15) | 0.0225 (16) | −0.0090 (12) | −0.0013 (13) | −0.0021 (13) |
| C11 | 0.0176 (16) | 0.0305 (15) | 0.0147 (14) | −0.0076 (12) | 0.0008 (12) | 0.0021 (12) |
| C12 | 0.0162 (15) | 0.0235 (14) | 0.0151 (14) | −0.0032 (11) | −0.0011 (12) | −0.0027 (11) |
| C13 | 0.0279 (18) | 0.0167 (13) | 0.0167 (14) | −0.0053 (12) | −0.0049 (13) | −0.0012 (11) |
| C14 | 0.0268 (18) | 0.0201 (14) | 0.0212 (16) | −0.0029 (12) | −0.0088 (14) | 0.0038 (12) |
| C15 | 0.0252 (18) | 0.0171 (13) | 0.0278 (17) | −0.0012 (12) | −0.0071 (14) | −0.0015 (12) |
| C16 | 0.0213 (17) | 0.0152 (13) | 0.0188 (15) | −0.0046 (11) | 0.0010 (12) | −0.0020 (11) |
| C17 | 0.0195 (16) | 0.0157 (13) | 0.0165 (14) | −0.0073 (11) | 0.0004 (12) | −0.0032 (11) |
| C18 | 0.0243 (17) | 0.0176 (13) | 0.0275 (17) | −0.0053 (12) | 0.0013 (14) | −0.0053 (12) |
| C19 | 0.0297 (18) | 0.0203 (14) | 0.0213 (16) | −0.0088 (13) | 0.0046 (13) | −0.0088 (12) |
| C20 | 0.0231 (17) | 0.0207 (14) | 0.0205 (15) | −0.0101 (12) | −0.0005 (13) | −0.0038 (12) |
| C21 | 0.0214 (16) | 0.0161 (13) | 0.0155 (14) | −0.0086 (11) | −0.0007 (12) | −0.0014 (11) |
| C22 | 0.0327 (19) | 0.0289 (15) | 0.0147 (14) | −0.0134 (14) | 0.0005 (13) | −0.0087 (12) |
| C23 | 0.035 (2) | 0.0279 (15) | 0.0201 (16) | −0.0068 (14) | −0.0116 (14) | −0.0051 (13) |
| C24 | 0.0240 (17) | 0.0238 (14) | 0.0226 (16) | −0.0036 (12) | −0.0077 (13) | −0.0064 (12) |
Geometric parameters (Å, °)
| Cd1—O3 | 2.355 (2) | C6—H6 | 0.9500 |
| Cd1—N6 | 2.390 (2) | C7—C8 | 1.434 (4) |
| Cd1—N4 | 2.393 (2) | C7—H7 | 0.9500 |
| Cd1—N3 | 2.418 (2) | C8—C10 | 1.403 (4) |
| Cd1—O1 | 2.4547 (19) | C8—C9 | 1.403 (4) |
| Cd1—O4 | 2.503 (2) | C10—C11 | 1.377 (4) |
| Cd1—O2 | 2.5041 (19) | C10—H10 | 0.9500 |
| Cd1—N5 | 2.510 (2) | C11—C12 | 1.391 (4) |
| N1—O2 | 1.250 (3) | C11—H11 | 0.9500 |
| N1—O1 | 1.258 (3) | C12—H12 | 0.9500 |
| N2—O5 | 1.151 (9) | C13—C14 | 1.403 (4) |
| N2—O4 | 1.254 (3) | C13—H13 | 0.9500 |
| N2—O3 | 1.267 (3) | C14—C15 | 1.362 (4) |
| N3—C1 | 1.323 (3) | C14—H14 | 0.9500 |
| N3—C5 | 1.349 (3) | C15—C16 | 1.410 (4) |
| N4—C12 | 1.320 (3) | C15—H15 | 0.9500 |
| N4—C9 | 1.359 (3) | C16—C17 | 1.409 (4) |
| N5—C13 | 1.326 (3) | C16—C18 | 1.428 (4) |
| N5—C17 | 1.356 (3) | C17—C21 | 1.451 (4) |
| N6—C24 | 1.319 (3) | C18—C19 | 1.351 (4) |
| N6—C21 | 1.353 (4) | C18—H18 | 0.9500 |
| C1—C2 | 1.398 (4) | C19—C20 | 1.430 (4) |
| C1—H1 | 0.9500 | C19—H19 | 0.9500 |
| C2—C3 | 1.365 (4) | C20—C22 | 1.409 (4) |
| C2—H2 | 0.9500 | C20—C21 | 1.410 (4) |
| C3—C4 | 1.406 (4) | C22—C23 | 1.367 (4) |
| C3—H3 | 0.9500 | C22—H22 | 0.9500 |
| C4—C5 | 1.414 (3) | C23—C24 | 1.395 (4) |
| C4—C6 | 1.426 (4) | C23—H23 | 0.9500 |
| C5—C9 | 1.447 (4) | C24—H24 | 0.9500 |
| C6—C7 | 1.350 (4) | ||
| O3—Cd1—N6 | 111.58 (7) | N3—C5—C4 | 122.6 (2) |
| O3—Cd1—N4 | 149.81 (7) | N3—C5—C9 | 118.3 (2) |
| N6—Cd1—N4 | 90.18 (8) | C4—C5—C9 | 119.1 (2) |
| O3—Cd1—N3 | 81.88 (7) | C7—C6—C4 | 121.2 (2) |
| N6—Cd1—N3 | 145.83 (8) | C7—C6—H6 | 119.4 |
| N4—Cd1—N3 | 68.85 (7) | C4—C6—H6 | 119.4 |
| O3—Cd1—O1 | 95.42 (7) | C6—C7—C8 | 121.0 (3) |
| N6—Cd1—O1 | 127.58 (7) | C6—C7—H7 | 119.5 |
| N4—Cd1—O1 | 86.59 (7) | C8—C7—H7 | 119.5 |
| N3—Cd1—O1 | 79.44 (7) | C10—C8—C9 | 117.5 (2) |
| O3—Cd1—O4 | 51.44 (7) | C10—C8—C7 | 123.1 (3) |
| N6—Cd1—O4 | 81.44 (8) | C9—C8—C7 | 119.4 (3) |
| N4—Cd1—O4 | 157.42 (7) | N4—C9—C8 | 122.6 (2) |
| N3—Cd1—O4 | 127.41 (8) | N4—C9—C5 | 117.8 (2) |
| O1—Cd1—O4 | 82.14 (7) | C8—C9—C5 | 119.6 (2) |
| O3—Cd1—O2 | 125.56 (7) | C11—C10—C8 | 119.2 (3) |
| N6—Cd1—O2 | 77.83 (7) | C11—C10—H10 | 120.4 |
| N4—Cd1—O2 | 78.09 (7) | C8—C10—H10 | 120.4 |
| N3—Cd1—O2 | 120.90 (6) | C10—C11—C12 | 119.3 (3) |
| O1—Cd1—O2 | 50.33 (6) | C10—C11—H11 | 120.4 |
| O4—Cd1—O2 | 79.66 (7) | C12—C11—H11 | 120.4 |
| O3—Cd1—N5 | 89.71 (8) | N4—C12—C11 | 123.0 (3) |
| N6—Cd1—N5 | 67.54 (7) | N4—C12—H12 | 118.5 |
| N4—Cd1—N5 | 79.26 (7) | C11—C12—H12 | 118.5 |
| N3—Cd1—N5 | 81.80 (7) | N5—C13—C14 | 123.1 (3) |
| O1—Cd1—N5 | 159.63 (7) | N5—C13—H13 | 118.4 |
| O4—Cd1—N5 | 115.94 (7) | C14—C13—H13 | 118.4 |
| O2—Cd1—N5 | 138.17 (7) | C15—C14—C13 | 119.1 (3) |
| O2—N1—O1 | 114.4 (2) | C15—C14—H14 | 120.5 |
| O5—N2—O4 | 121.3 (5) | C13—C14—H14 | 120.5 |
| O5—N2—O3 | 124.7 (6) | C14—C15—C16 | 119.8 (3) |
| O4—N2—O3 | 113.9 (2) | C14—C15—H15 | 120.1 |
| C1—N3—C5 | 118.4 (2) | C16—C15—H15 | 120.1 |
| C1—N3—Cd1 | 124.62 (18) | C17—C16—C15 | 117.0 (3) |
| C5—N3—Cd1 | 116.90 (17) | C17—C16—C18 | 119.7 (3) |
| C12—N4—C9 | 118.3 (2) | C15—C16—C18 | 123.2 (3) |
| C12—N4—Cd1 | 123.87 (17) | N5—C17—C16 | 122.9 (2) |
| C9—N4—Cd1 | 117.69 (17) | N5—C17—C21 | 118.1 (2) |
| C13—N5—C17 | 118.0 (2) | C16—C17—C21 | 119.0 (3) |
| C13—N5—Cd1 | 125.85 (19) | C19—C18—C16 | 121.5 (3) |
| C17—N5—Cd1 | 115.91 (17) | C19—C18—H18 | 119.3 |
| C24—N6—C21 | 118.0 (3) | C16—C18—H18 | 119.3 |
| C24—N6—Cd1 | 121.76 (19) | C18—C19—C20 | 120.5 (3) |
| C21—N6—Cd1 | 120.25 (17) | C18—C19—H19 | 119.7 |
| N1—O1—Cd1 | 98.71 (15) | C20—C19—H19 | 119.7 |
| N1—O2—Cd1 | 96.51 (15) | C22—C20—C21 | 117.6 (3) |
| N2—O3—Cd1 | 100.74 (17) | C22—C20—C19 | 122.5 (3) |
| N2—O4—Cd1 | 93.91 (16) | C21—C20—C19 | 119.9 (3) |
| N3—C1—C2 | 123.1 (3) | N6—C21—C20 | 122.6 (2) |
| N3—C1—H1 | 118.5 | N6—C21—C17 | 118.0 (2) |
| C2—C1—H1 | 118.5 | C20—C21—C17 | 119.3 (3) |
| C3—C2—C1 | 119.1 (3) | C23—C22—C20 | 119.0 (3) |
| C3—C2—H2 | 120.4 | C23—C22—H22 | 120.5 |
| C1—C2—H2 | 120.4 | C20—C22—H22 | 120.5 |
| C2—C3—C4 | 119.6 (2) | C22—C23—C24 | 119.3 (3) |
| C2—C3—H3 | 120.2 | C22—C23—H23 | 120.4 |
| C4—C3—H3 | 120.2 | C24—C23—H23 | 120.4 |
| C3—C4—C5 | 117.2 (2) | N6—C24—C23 | 123.5 (3) |
| C3—C4—C6 | 123.3 (2) | N6—C24—H24 | 118.2 |
| C5—C4—C6 | 119.5 (3) | C23—C24—H24 | 118.2 |
| O3—Cd1—N3—C1 | 9.7 (2) | O3—N2—O4—Cd1 | 1.6 (2) |
| N6—Cd1—N3—C1 | 126.5 (2) | O3—Cd1—O4—N2 | −0.98 (16) |
| N4—Cd1—N3—C1 | −177.9 (2) | N6—Cd1—O4—N2 | −127.53 (17) |
| O1—Cd1—N3—C1 | −87.4 (2) | N4—Cd1—O4—N2 | 163.19 (18) |
| O4—Cd1—N3—C1 | −16.0 (2) | N3—Cd1—O4—N2 | 32.21 (19) |
| O2—Cd1—N3—C1 | −117.2 (2) | O1—Cd1—O4—N2 | 102.42 (17) |
| N5—Cd1—N3—C1 | 100.5 (2) | O2—Cd1—O4—N2 | 153.38 (17) |
| O3—Cd1—N3—C5 | −166.68 (19) | N5—Cd1—O4—N2 | −67.72 (18) |
| N6—Cd1—N3—C5 | −49.9 (2) | C5—N3—C1—C2 | 0.6 (4) |
| N4—Cd1—N3—C5 | 5.81 (17) | Cd1—N3—C1—C2 | −175.7 (2) |
| O1—Cd1—N3—C5 | 96.22 (18) | N3—C1—C2—C3 | −0.4 (4) |
| O4—Cd1—N3—C5 | 167.70 (16) | C1—C2—C3—C4 | 0.4 (4) |
| O2—Cd1—N3—C5 | 66.5 (2) | C2—C3—C4—C5 | −0.5 (4) |
| N5—Cd1—N3—C5 | −75.80 (18) | C2—C3—C4—C6 | −179.9 (3) |
| O3—Cd1—N4—C12 | −167.40 (19) | C1—N3—C5—C4 | −0.7 (4) |
| N6—Cd1—N4—C12 | −30.0 (2) | Cd1—N3—C5—C4 | 175.90 (19) |
| N3—Cd1—N4—C12 | 177.7 (2) | C1—N3—C5—C9 | 178.3 (2) |
| O1—Cd1—N4—C12 | 97.7 (2) | Cd1—N3—C5—C9 | −5.2 (3) |
| O4—Cd1—N4—C12 | 37.7 (3) | C3—C4—C5—N3 | 0.7 (4) |
| O2—Cd1—N4—C12 | 47.6 (2) | C6—C4—C5—N3 | −179.9 (2) |
| N5—Cd1—N4—C12 | −97.0 (2) | C3—C4—C5—C9 | −178.3 (2) |
| O3—Cd1—N4—C9 | 8.8 (3) | C6—C4—C5—C9 | 1.2 (4) |
| N6—Cd1—N4—C9 | 146.27 (18) | C3—C4—C6—C7 | 179.8 (3) |
| N3—Cd1—N4—C9 | −6.09 (17) | C5—C4—C6—C7 | 0.4 (4) |
| O1—Cd1—N4—C9 | −86.08 (18) | C4—C6—C7—C8 | −2.2 (4) |
| O4—Cd1—N4—C9 | −146.08 (19) | C6—C7—C8—C10 | −176.7 (3) |
| O2—Cd1—N4—C9 | −136.21 (19) | C6—C7—C8—C9 | 2.3 (4) |
| N5—Cd1—N4—C9 | 79.19 (18) | C12—N4—C9—C8 | 1.8 (4) |
| O3—Cd1—N5—C13 | 68.5 (2) | Cd1—N4—C9—C8 | −174.59 (18) |
| N6—Cd1—N5—C13 | −177.9 (2) | C12—N4—C9—C5 | −177.6 (2) |
| N4—Cd1—N5—C13 | −83.2 (2) | Cd1—N4—C9—C5 | 6.0 (3) |
| N3—Cd1—N5—C13 | −13.3 (2) | C10—C8—C9—N4 | −1.0 (4) |
| O1—Cd1—N5—C13 | −36.4 (3) | C7—C8—C9—N4 | 179.8 (2) |
| O4—Cd1—N5—C13 | 114.5 (2) | C10—C8—C9—C5 | 178.4 (2) |
| O2—Cd1—N5—C13 | −141.39 (19) | C7—C8—C9—C5 | −0.7 (4) |
| O3—Cd1—N5—C17 | −117.09 (18) | N3—C5—C9—N4 | −0.5 (3) |
| N6—Cd1—N5—C17 | −3.51 (17) | C4—C5—C9—N4 | 178.5 (2) |
| N4—Cd1—N5—C17 | 91.18 (19) | N3—C5—C9—C8 | −179.9 (2) |
| N3—Cd1—N5—C17 | 161.08 (19) | C4—C5—C9—C8 | −1.0 (4) |
| O1—Cd1—N5—C17 | 138.0 (2) | C9—C8—C10—C11 | −0.8 (4) |
| O4—Cd1—N5—C17 | −71.16 (19) | C7—C8—C10—C11 | 178.3 (3) |
| O2—Cd1—N5—C17 | 33.0 (2) | C8—C10—C11—C12 | 1.8 (4) |
| O3—Cd1—N6—C24 | −99.0 (2) | C9—N4—C12—C11 | −0.8 (4) |
| N4—Cd1—N6—C24 | 102.5 (2) | Cd1—N4—C12—C11 | 175.4 (2) |
| N3—Cd1—N6—C24 | 152.85 (19) | C10—C11—C12—N4 | −1.0 (4) |
| O1—Cd1—N6—C24 | 16.6 (2) | C17—N5—C13—C14 | −0.4 (4) |
| O4—Cd1—N6—C24 | −56.5 (2) | Cd1—N5—C13—C14 | 173.9 (2) |
| O2—Cd1—N6—C24 | 24.7 (2) | N5—C13—C14—C15 | 1.4 (4) |
| N5—Cd1—N6—C24 | −179.2 (2) | C13—C14—C15—C16 | −0.7 (4) |
| O3—Cd1—N6—C21 | 83.4 (2) | C14—C15—C16—C17 | −0.9 (4) |
| N4—Cd1—N6—C21 | −75.1 (2) | C14—C15—C16—C18 | 179.7 (3) |
| N3—Cd1—N6—C21 | −24.8 (3) | C13—N5—C17—C16 | −1.4 (4) |
| O1—Cd1—N6—C21 | −160.97 (17) | Cd1—N5—C17—C16 | −176.21 (19) |
| O4—Cd1—N6—C21 | 125.9 (2) | C13—N5—C17—C21 | 178.5 (2) |
| O2—Cd1—N6—C21 | −152.9 (2) | Cd1—N5—C17—C21 | 3.7 (3) |
| N5—Cd1—N6—C21 | 3.16 (18) | C15—C16—C17—N5 | 2.0 (4) |
| O2—N1—O1—Cd1 | −1.1 (2) | C18—C16—C17—N5 | −178.5 (2) |
| O3—Cd1—O1—N1 | 133.52 (16) | C15—C16—C17—C21 | −177.9 (2) |
| N6—Cd1—O1—N1 | 10.90 (19) | C18—C16—C17—C21 | 1.6 (4) |
| N4—Cd1—O1—N1 | −76.70 (16) | C17—C16—C18—C19 | −0.3 (4) |
| N3—Cd1—O1—N1 | −145.80 (16) | C15—C16—C18—C19 | 179.2 (3) |
| O4—Cd1—O1—N1 | 83.69 (16) | C16—C18—C19—C20 | −1.6 (4) |
| O2—Cd1—O1—N1 | 0.64 (14) | C18—C19—C20—C22 | −178.1 (3) |
| N5—Cd1—O1—N1 | −122.5 (2) | C18—C19—C20—C21 | 2.2 (4) |
| O1—N1—O2—Cd1 | 1.1 (2) | C24—N6—C21—C20 | −0.4 (4) |
| O3—Cd1—O2—N1 | −64.39 (17) | Cd1—N6—C21—C20 | 177.34 (19) |
| N6—Cd1—O2—N1 | −172.34 (17) | C24—N6—C21—C17 | 179.7 (2) |
| N4—Cd1—O2—N1 | 94.86 (16) | Cd1—N6—C21—C17 | −2.6 (3) |
| N3—Cd1—O2—N1 | 38.65 (18) | C22—C20—C21—N6 | −0.5 (4) |
| O1—Cd1—O2—N1 | −0.64 (14) | C19—C20—C21—N6 | 179.1 (2) |
| O4—Cd1—O2—N1 | −88.97 (16) | C22—C20—C21—C17 | 179.4 (2) |
| N5—Cd1—O2—N1 | 153.45 (15) | C19—C20—C21—C17 | −0.9 (4) |
| O5—N2—O3—Cd1 | 175.7 (6) | N5—C17—C21—N6 | −0.9 (4) |
| O4—N2—O3—Cd1 | −1.7 (3) | C16—C17—C21—N6 | 179.0 (2) |
| N6—Cd1—O3—N2 | 59.66 (19) | N5—C17—C21—C20 | 179.1 (2) |
| N4—Cd1—O3—N2 | −167.00 (16) | C16—C17—C21—C20 | −1.0 (4) |
| N3—Cd1—O3—N2 | −152.96 (18) | C21—C20—C22—C23 | 0.4 (4) |
| O1—Cd1—O3—N2 | −74.47 (18) | C19—C20—C22—C23 | −179.3 (3) |
| O4—Cd1—O3—N2 | 0.99 (16) | C20—C22—C23—C24 | 0.6 (4) |
| O2—Cd1—O3—N2 | −30.6 (2) | C21—N6—C24—C23 | 1.5 (4) |
| N5—Cd1—O3—N2 | 125.28 (18) | Cd1—N6—C24—C23 | −176.2 (2) |
| O5—N2—O4—Cd1 | −175.9 (6) | C22—C23—C24—N6 | −1.6 (5) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: WM2452).
References
- Abedini, J., Morsali, A. & Kempe, R. (2005). J. Coord. Chem. 58, 1161–1167.
- Agilent Technologies (2010). CrysAlis PRO Agilent Technologies, Yarnton, England.
- Barbour, L. J. (2001). J. Supramol. Chem. 1, 189–191.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Tadjarodi, A., Taeb, A. & Ng, S. W. (2001). Main Group Met. Chem. 24, 805–806.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811002431/wm2452sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811002431/wm2452Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report