Abstract
Advances in our molecular, clinical, and epidemiologic understanding of the risk and development of pancreatic cancer offer hope for preventing this disease, which is largely intractable once developed. This perspective on provocative, genetically engineered mouse-model work reported by Mohammed et al. (beginning on page XXX in this issue of the journal) examines the prospects for pancreatic-cancer chemoprevention with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs). Despite having limited value in advanced pancreatic cancer, EGFR TKIs show promise in the setting of early pancreatic carcinogenesis.
Exocrine pancreatic cancer remains a largely intractable malignancy. Despite some advances in the radiological assessment of pancreatic cancer resectability and improvements in surgical technique, the overall 5-year survival of all patients diagnosed with pancreatic cancer is still only 2%–3% (1). This poor survival persists despite extensive testing of chemotherapeutic agents and the integration of multiple modalities (primarily surgery, radiation therapy, and chemotherapy) into the management of patients with pancreatic cancer. The lack of progress against this malignancy is thought to be due to two elements inherent to its biology: 1) Insidious presentation due to the lack of specific symptoms and signs, often leading to an advanced stage at diagnosis, and 2) striking therapeutic resistance. The therapeutic resistance of pancreatic cancer is likely to be due to many factors, but includes the high frequency of KRAS-activating mutations (KRAS*) and the extensive stromal reaction engendered as the malignancy develops. This extensive stroma is thought to lead to poor delivery of chemotherapeutic agents to the malignant cells (2).
Despite lack of progress in the treatment of established pancreatic cancer, steady advances are being made in our knowledge of patients who are at risk for developing this disease. Our current understanding of the risk for developing invasive pancreatic cancer allows patients at an increased risk to be divided into three general groups: 1) Those individuals with known heritable risk factors such as germ-line mutations in cyclin-dependent kinase inhibitor 2A (CDKN2A), liver kinase B1 (LKB1), BRCA2, and PRSS1; refs. 3-6), or individuals with ≥2 first-degree family members diagnosed with pancreatic cancer (7); 2) patients with mucinous cystic neoplasms of the pancreas [Intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN); ref. 8); and 3) individuals with combinations of specific epidemiologic risk factors such as cigarette smoking, long-standing type II diabetes, and obesity (9, 10). So, although our ability to identify patients at risk of developing pancreatic cancer has improved, we have no interventions that can mitigate this risk other than partial or total pancreatectomy. Clearly, surgical resection is a radical intervention for patients whose lifetime risk of developing pancreatic cancer may be only elevated slightly over the baseline risk in the general population.
Like other epithelial cancers of the gastrointestinal tract, pancreatic cancer is thought to evolve through non-malignant precursor lesions termed pancreatic intraepithelial neoplasia (PanIN), and these lesions progress through states of increasing cytological atypia and dysplasia through the acquisition of increasing numbers of signature genetic alterations (11). The gatekeeper mutation for pancreatic cancer is KRAS*, with loss of tumor suppressor genes such as CDKN2A, p53, and Smad4/Dpc4 occurring very commonly as the PanIN lesions progress to carcinoma in situ and invasive pancreatic cancer. Recently, these pathological and genetic observations derived from patients have been confirmed using transgenic mouse models in which the early development and progression of pancreatic cancer can be recapitulated through the expression of KRAS* and accelerated by engineered loss of CDKN2A or p53 specifically in pancreatic epithelium (12-14).
In this issue of the journal, Mohammed et al. report their study employing the p48Cre/+ LSL-KRASG12D/+ transgenic mouse model of pancreatic cancer and demonstrate that the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) gefitinib prevents progression of PanINs to invasive pancreatic cancer (15). They argue that “these results have important implications for human pancreatic cancer chemoprevention.”
What is the evidence that examining such an intervention in patients at risk for pancreatic cancer is warranted? Qualitative protein expression data from human pancreatic cancer specimens have demonstrated that EGFR is frequently over-expressed. However, genetic analyses have failed to identify mutations, amplification, or activating translocations affecting EGFR, suggesting that (at least in the advanced-disease setting) inhibition of EGFR would be anticipated to have only limited clinical impact. This fact has been born out in prospective clinical trials that combined gemcitabine with the EGFR TKI erlotinib or the humanized monoclonal EGFR antibody cetuximab in patients with advanced pancreatic cancer (16, 17).
However, the study described by Mohammed et al. is provocative in that it suggests that targeting EGFR early in pancreatic carcinogenesis may be effective despite the limited value of this approach in advanced pancreatic cancer. So, are there data in addition to this study to suggest that gefitinib or other small-molecule EGFR TKIs represent a viable approach to pancreatic cancer chemoprevention? Right now the picture looks mixed. As pointed out above, in the advanced pancreatic cancer setting the impact of erlotinib is quite modest, and since we do not yet understand which pancreatic cancer patients are likely to benefit from erlotinib, the anticipated impact of small-molecule EGFR inhibitors in a chemopreventive setting may also be modest. Despite this, the Mohammed et al. study offers one hopeful experimental observation. That is, the effect of EGFR inhibition may be more significant early in pancreatic carcinogenesis. Reduction of PanIN-1 and -2 lesions was much more pronounced than was the effect on the more-advanced PanIN-3 histology. This observation should be confirmed in other studies and with genetically engineered animal models of pancreatic carcinogenesis that incorporate additional genetic changes; but it suggests that the therapeutic resistance conferred by KRAS* may require the acquisition of additional genetic changes. Thus, while the mechanism of such a differential effect is unknown, this observation could suggest that EGFR inhibition may be effective early in pancreatic carcinogenesis, yet ineffective later–an observation that would be consistent with the results of the Mohammed et al. study and the limited effect of erlotinib in advanced-disease clinical trials. Last, early results from a study of erlotinib in patients diagnosed with IPMN has demonstrated that at least one patient completely responded radiographically to this intervention [Steven Lipkin, personal communication, June 2010 ].
A general concern is whether the genetically engineered murine models that are completely dependent on a dominant oncogene (e.g., KRAS*) can inform us about a disease in humans that is much more genetically complex (18). Expression of KRAS* alone or expression of KRAS* coupled with loss of p53, CDKN2A, or other tumor suppressors leads to disease that evolves over months in mice and may not reproduce the full spectrum of genetic and biologic heterogeneity present in human pancreatic cancer, a disease that may take decades to develop. Of course, the answer to the question of the applicability of results generated in genetically engineered mouse models to humans ultimately depends not so much on how well the transgenic models recapitulate aspects of the biology of human pancreatic cancer, but whether these models can prove predictive for the identification of clinically useful interventions. Since the predictive track records of subcutaneous and orthotopic models of pancreatic cancer have been poor, there is considerable hope that the transgenic models that incorporate our increasing understanding of the driving genetic events in pancreatic carcinogenesis will provide the needed insights.
Despite the challenges inherent in modeling human cancers in mice, there is a clear need to identify novel approaches/agents for prevention and therapy. Thus far, the animal models suggest that there is likely to be an important convergence between targets for therapy and prevention, and the positive impact of an EGFR inhibitor early in pancreatic carcinogenesis may be different (and more powerful) than the same drug administered to patients with advanced pancreatic cancer (19). The reasons for this difference remain unknown, but with further progression of cancer there are a greater number of contributing molecular events, increasing biochemical complexity, cancer-cell heterogeneity, and extensive tumor-stromal interactions. All of these alterations may conspire to make advanced pancreatic cancer highly resistant, yet early in pancreatic carcinogenesis these changes are likely to be less fully developed, perhaps leading to drug vulnerabilities that are not encountered in the more advanced setting.
Our next hurdle will be to design clinical trials that can assess the ability of anti-cancer agents, administered early in pancreatic carcinogenesis, to have a beneficial clinical impact. Increasingly sophisticated transgenic animal models have also suggested that the sequence of mutational events can alter the morphology of pancreatic-cancer precursor lesions. For example, concomitant expression of KRASG12D and haploinsufficiency of the Smad4/Dpc4 tumor suppressor gene (KRASLSLG12D/+;Dpc4flox/+; p48Cre) give rise to mucinous cystic neoplasms that can then progress to invasive ductal adenocarcinoma through loss of heterozygosity of Dpc4 and mutation of either p53 or p16 (20). As indicated above, patients with mucinous cystic neoplasms of the pancreas have very similar genetics and are known through clinical studies to be at risk for invasive pancreatic cancer. Furthermore, because of the (over)utilization of computerized abdominal imaging, increasing numbers of patients are being diagnosed with previously unsuspected pancreatic cysts, some of which will be confirmed to be mucinous cystic neoplasms.
Thus, gefitinib or other rationally targeted agents should be studied in the KRASLSLG12D/+;Dpc4flox/+; p48Cre and KRASLSLG12D/+;Dpc4flox/flox; p48Cre transgenic models with the goal to then examine successful interventions in clinical trials of patients with newly identified mucinous cystic neoplasms (IPMN and MCN). As we learn more about how to effectively screen for pancreatic-cancer precursor lesions we will need to begin to examine the effects of medical interventions, reserving surgery for those patients with more advanced PanINs or progressing mucinous lesions or for those who do not respond to chemopreventive interventions. An enormous challenge will be to design these studies in a way that provides definitive evidence that the intervention inhibits pancreatic carcinogenesis without risking the development of invasive pancreatic cancer and to accomplish this with acceptable toxicity.
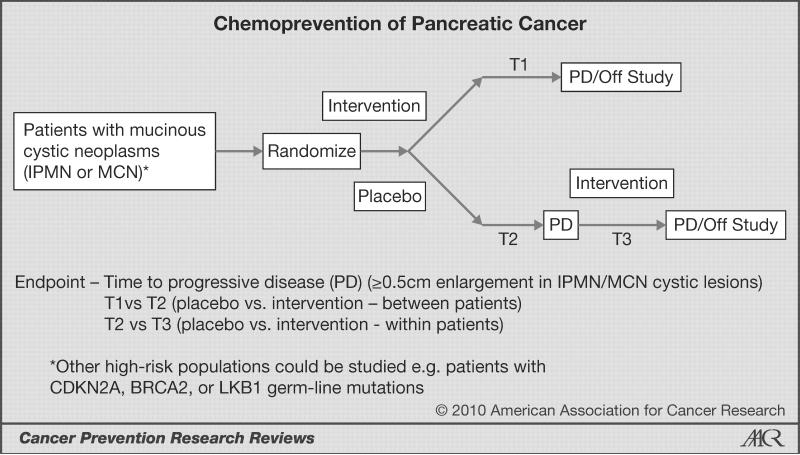
One approach to this problem is suggested in Fig. 1. As outlined in this figure, the primary goal would be to establish that the intervention increases the interval between entry into the trial and progression of the mucinous-lesion size or development of radiographic findings that have been recommended by consensus to necessitate surgical intervention [time interval 1 (T1); ref. 21). Also, the time interval to progression for patients initially randomized to placebo (T2) can be compared to the open-label intervention (T3) such that these patients can serve as their own control. Unfortunately, due to the fact that we are only now gathering experience and natural-history data on asymptomatic mucinous cystic lesions of the pancreas, there are many gaps in our knowledge. These questions include: 1) What is the rate of progression (enlargement) in MCNs and IPMNs over time? 2) Is the rate of progression the same for MCNs and IPMNs? 3) How much heterogeneity is there in the rate of progression for patients classified as MCN versus IPMN, and do these differences have a molecular basis? 4) Is the rate of change constant over time? 5) In the absence of development of worrisome signs indicating the need for surgery, what increase in cyst size defines progressive disease (0.5 cm is suggested in Fig. 1, but lesser or greater degrees of change could be considered)? 6) How reproducibly can we image small changes in cyst size, and what is the best imaging study to use [computed tomography (CT) versus magnetic resonance (MR)/MR cholangiopancreatography (MRCP) versus endoscopic ultrasound versus some combination of these modalities)? Other issues to consider include how extensive the initial evaluation would need to be to diagnose IPMN and MCN: 1) Should all newly identified cysts be aspirated to confirm the presence of mucin? 2) Are there cytological criteria that should be included in the diagnostic criteria (e.g., the presence of ovarian stroma in MCN)? 3) Should KRAS* testing be done on all patients? These and other eligibility and protocol-design issues would need to be decided on as an intervention trial was developed.
Fig. 1.
Concept for randomized phase-II trial of pancreatic-cancer chemoprevention in patients with mucinous cystic neoplasms. The primary endpoint is time to progressive disease (PD) defined radiographically (≥ 0.5 cm enlargement in the mucinous cyst) or time to radiographic findings that lead to a consensus recommendation for surgery. The relevant times are time to PD in the intervention arm (T1), time to PD in the placebo arm (T2), and time to PD after cross-over of patients initially randomized to placebo to open-label intervention (T3). Times of interest would be T1 versus T2 and T2 versus T3. IPMN, intraductal papillary mucinous neoplasm; MCN, mucinous cystic neoplasm; CDKN2A, cyclin-dependent kinase inhibitor 2A; LKB1, liver kinase B1.
In summary, whether or not gefitinib represents a reasonable intervention to inhibit pancreatic carcinogenesis, many important issues are raised by the provocative work of Mohammad et al. (15). It will be key to validate these observations with additional genetically engineered animal models, but as this study indicates, these models can be readily used to examine the impact of a variety of targeted interventions. It is hoped that we will discover that the models provide insights into interventions that can be rationally tested in well-designed clinical trials leading to prevention of this dreaded malignancy.
Acknowledgments
Grant Support Supported in part by the Lockton Endowment (CDL) and the SPORE in Pancreatic Cancer P20 CA101936 (JLA).
Footnotes
Disclosure of Potential Conflicts of Interest [XXXX]
References
- 1.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic Cancer. Lancet. 2004;363:1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 2.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–49. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 4.Bardeesy N, Aguirre AJ, Chu GC, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A. 2006;103:5947–52. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hezel AF, Gurumurthy S, Granot Z, et al. Pancreatic LKB1 deletion leads to acinar polarity defects and cystic neoplasms. Mol Cell Biol. 2008;28:2414–25. doi: 10.1128/MCB.01621-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebours V, Boutron-Ruault MC, Schnee M, et al. The natural history of hereditary pancreatitis: a national series. Gut. 2009 Jan;58(1):97–103. doi: 10.1136/gut.2008.149179. [DOI] [PubMed] [Google Scholar]
- 7.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–8. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 8.Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–26. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 9.Anderson K, Mack T, Silverman D. Cancer of the Pancreas. In: Schottenfeld D, Fraumeni J, editors. Cancer Epidemiology and Prevention. Second ed Oxford; New York: 2006. pp. 721–62. [Google Scholar]
- 10.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–6. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–72. [PubMed] [Google Scholar]
- 12.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 13.Aguirre AJ, Bardeesy N, Sinha M, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–26. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005 May;7(5):469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Mohammed A, Janakiram NB, Li Q, et al. EGFR inhibitor gefitinib prevents progression of pancreatic lesions to carcinoma in a conditional LSL-KrasG12D/+ transgenic mice model. Cancer Prev Res. 2010;3 doi: 10.1158/1940-6207.CAPR-10-0038. XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007 May 20;25(15):1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 17.Philip PA, Benedetti J, Corless CL, et al. Phase III Study Comparing Gemcitabine Plus Cetuximab Versus Gemcitabine in Patients With Advanced Pancreatic Adenocarcinoma: Southwest Oncology Group-Directed Intergroup Trial S0205. J Clin Oncol. 2010 Aug 1;28(22):3605–10. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008 Sep 26;321(5897):1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbruzzese JL, Lippman SM. The convergence of cancer prevention and therapy in early-phase clinical drug development. Cancer Cell. 2004 Oct;6(4):321–6. doi: 10.1016/j.ccr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Izeradjene K, Combs C, Best M, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell. 2007 Mar;11(3):229–43. doi: 10.1016/j.ccr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6(1-2):17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]