Abstract
Processes of vestibular compensation mediate recovery of many aspects of vestibular dysfunction following unilateral vestibular injury. The VOR in response to high-frequency, high-acceleration head movements, however, retains an enduring asymmetry. Head movements that are inhibitory with respect to semicircular canals on the intact side lead to a diminished VOR whereas head movements that are excitatory for semicircular canals on the intact side lead to a VOR that returns close to normal. We review our work directed toward understanding the processes of VOR compensation to high-frequency, high-acceleration head movements and the related topic of adaptation to changes in the visual requirements for a compensatory VOR. Our work has shown that the processes of both compensation and adaptation to these stimuli can be described by a mathematical model with inputs from tonic and phasic components. We have further shown that the dynamics of regular afferents have close resemblance to the tonic pathway whereas the dynamics of irregular afferents match those of the phasic pathway.
Keywords: regular afferent, irregular afferent, labyrinthectomy, vestibular, semicircular canals, mathematical mode, high acceleration head movement, vestibular hypofunction, retinal slip error
Introduction
Loss of function in one labyrinth occurs in a variety of different vestibular disorders. Vestibular neuritis [3], Meniere’s disease [5], and vestibular schwannoma [24] are all conditions that can lead to unilateral vestibular hypofunction. This dysfunction in a labyrinth may be partial or complete, fluctuant or constant, progressive or recovering. Surgical removal of function in one labyrinth as occurs with labyrinthectomy or vestibular neurectomy for Meniere’s disease or removal of a vestibular schwannoma leads to permanent and irreversible loss of vestibular function on the operated side. Mechanisms of vestibular compensation are involved in making the adjustments in central processes required to restore the accuracy and symmetry of pathways and reflexes that are dependent upon information from the labyrinths [11,28].
Initial studies of the horizontal angular VOR following unilateral loss of vestibular function emphasized the decrease in spontaneous nystagmus that occurs with time after the lesion and the restoration in symmetry of the VOR evoked by low frequency rotations with lower peak velocity [31,14,18,26]. Asymmetries in the form of a bias velocity noted in the VOR evoked by sinusoidal rotations with a peak velocity > 150°/s and a clipping of eye-velocity response to steps of head velocity with an amplitude > 150°/s towards the lesioned side provided an indication of the side of vestibular hypofunction. These asymmetries in responses to low frequency rotations with relatively high peak velocity can, however, be relatively subtle [31, 26].
Halmagyi and colleagues identified a marked asymmetry in the horizontal VOR evoked by high-frequency, high-acceleration head movements [12,13]. Responses for head rotations that were excitatory with respect to the horizontal canal on the lesioned side were of low amplitude whereas those for rapid head movements that were excitatory with respect to the horizontal canal on the intact side were often indistinguishable from normal responses. The same principles were noted in responses to head movements in the planes of the vertical canals [1,9,4]. These findings were robust and did not change appreciably over time. The “head thrust test” provides a valuable method to detect vestibular hypofunction and to identify the specific semicircular canals that are affected. On clinical examination we tend to focus on the deficient VOR elicited by head movements that are excitatory with respect to semicircular canals on the lesioned side and the compensatory saccadic eye movement that brings the eyes back onto target. Perhaps of even greater physiologic interest are the mechanisms that underlie recovery of responses to rotations that are excitatory for semicircular canals on the intact side to near-normal levels. There are at least 4 questions that arise from rapid head movement testing after loss of function in one labyrinth:
What are the mechanisms that underlie the remarkable recovery of contralesional responses?
What are the processes that are responsible for the failure of ipsilesional responses to improve?
How do the processes of vestibular compensation relate to the processes that mediate adaptation to changes in the visual requirements for a compensatory VOR?
What are the neural signals that are important to driving these processes of vestibular compensation?
We have conducted a series of investigations of the horizontal VOR in squirrel monkeys over the range of frequencies and accelerations that are important for eliciting the head thrust asymmetry identified by Halmagyi et al. [13]. We investigated normal responses [25] as well as the VOR following unilateral plugging of the 3 semicircular canals [19], unilateral labyrinthectomy [20], and spectacle-induced adaptation in animals with normal vestibular function [6,7]. Our findings provided evidence in support of a mathematical model of the VOR consisting of inputs from tonic and phasic components. We further showed that the dynamics of the tonic component bear close resemblance to those of regular afferents whereas the dynamics of the phasic component have many features in common with irregular afferents.
Horizontal VOR evoked by high-frequency, high-acceleration head movements
Normal Responses
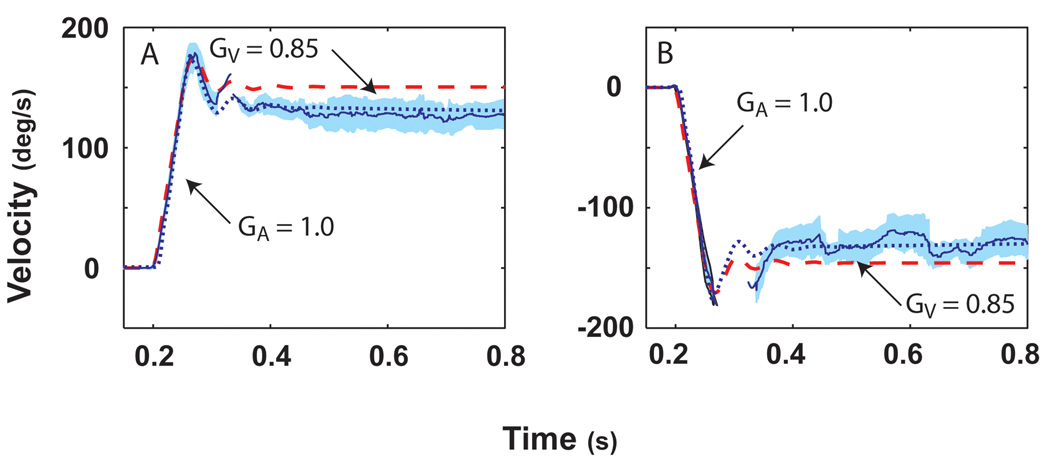
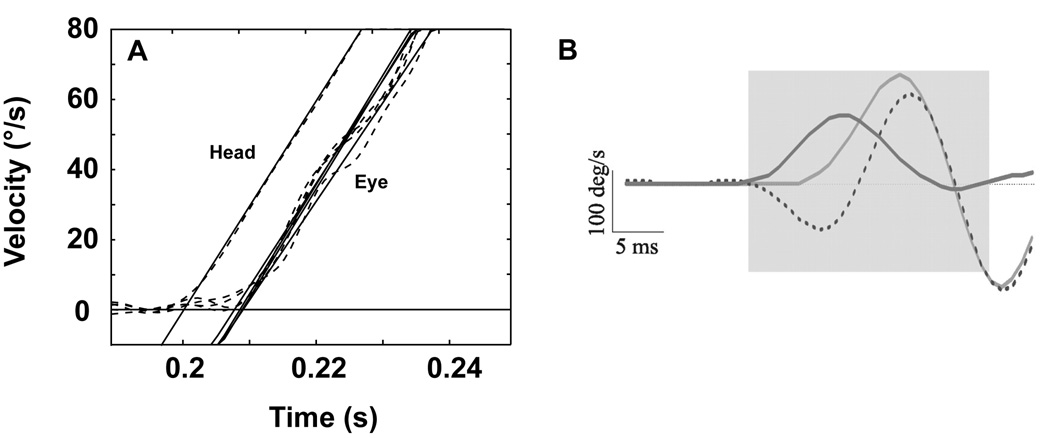
Impulses of acceleration (3000°/s2 to a plateau velocity of 150°/s) in squirrel monkeys elicited a robust VOR [25]. Figure 1 presents average data from repeated trials for leftward and rightward rotations in darkness. Two parameters can be defined: an acceleration gain (GA) that is defined as eye velocity / head velocity during the first 20–40 ms of the initial acceleration and a velocity gain (GV) that is defined as eye velocity / head velocity evaluated at 100–300 ms after the plateau head velocity had been reached for each trial. GA was consistently greater than GV, a finding that has also been observed in humans in responses to comparable stimuli [8]. The latency of the VOR measured in squirrel monkeys from responses to these stimuli was 7 ms (Figure 2A). In a study performed in macaques with acceleration stimuli reaching 20,000°/s2 [17], the VOR latency was calculated as 5–6 ms (Figure 2B). The findings provide compelling evidence of the efficacy of the VOR in maintaining the stability of images on the retina during these rapid head movements.
Figure 1.
Average of 30 stimulus repetitions for the horizontal eye and head velocity of responses to leftward (A) and rightward (B) accelerations at 3,000°/s2 reaching a peak velocity of 150°/s in a squirrel monkey after the fast phases were removed. Head velocity is indicated by the dashed red line and eye velocity by the solid blue line. One standard deviation is indicated by the light blue shading around this line. In this and all other figures, head velocity was inverted to facilitate direct comparison to eye velocity. Simulations were performed using our bilateral mathematical model described in Figure 4 of this paper. The acceleration gain of the VOR, GA, was measured for each trial as the ratio of the slope of a line through the eye velocity to the slope of a line through the head velocity during 20 – 40 ms into the acceleration. The velocity gain of the VOR, GV, was measured from the ratio of the mean eye and head velocity evaluated at 100–300 ms after the plateau head velocity had been reached for each trial. This figure was adapted with permission from [25].
Figure 2.
The latency of onset of eye movement calculated from rapid head rotations in the squirrel monkey (A) and the rhesus monkey (B). Panel A shows the VOR latency calculated from comparison of the intercept on the time axis for linear fits to head and eye velocities for 4 stimulus repetitions at 3000°/s2. The eye begins to move at ~7 ms. This panel was adapted with permission from [25]. Panel B shows the gaze (dotted), eye (light grey), and head-velocity (dark grey) responses following very short head perturbations. Each average trace consists of a minimum 20 trials. Following the onset of head rotation, the eye begins to counter-rotate after a latency of ~5–6 ms. This panel was adapted with permission from [17]. No anti-compensatory eye movements were ever observed in the interval immediately following onset of head rotation.
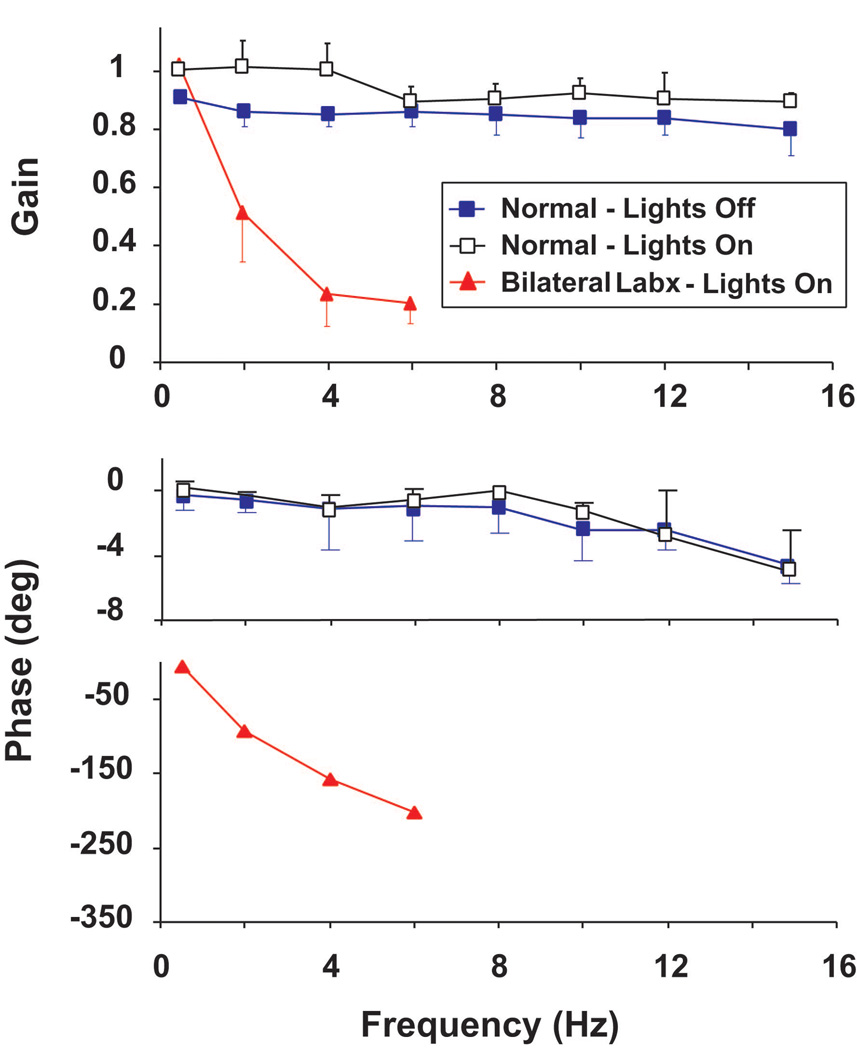
The VOR in response to sinusoidal rotations (0.5 – 15 Hz, peak velocity = 20°/s) was studied in darkness and in light [25]. Figure 3 shows a plot of gain and phase vs. frequency for averaged data from 5 squirrel monkeys. The average gain across all frequencies measured 0.83 ± 0.06 and did not differ across frequencies or animals (p > 0.2). Phase measured −1.4 ± 0.8° for frequencies up to and including 12 Hz. Phase at 15 Hz measured −5.0 ± 0.9°. Gains measured in light were greater than those measured in darkness at 0.5, 2, and 4 Hz (p < 0.05 at each frequency) but did not differ from those measured in darkness frequencies >4 Hz (p > 0.8 at each frequency). Also included in Figure 3 are responses to rotation in light in an animal after bilateral labyrinthectomy. No vestibular response was observed in the dark in this animal. Responses in the light had a gain identical to that in animals with intact vestibular function for rotations at 0.5 Hz, ± 20°/s, declined in gain at higher rotational frequencies, and had an increasing phase lag. The findings indicate that vestibular function is required for stabilization of images during head movements in responses to mid- and high-frequency rotations.
Figure 3.
The average gain and phase plots of responses to 0.5- to 15-Hz rotations given in darkness and in light, ±20°/s for 5 squirrel monkeys. Also included are data from one animal rotated in the light after bilateral labyrinthectomy. In this and all other figures for gain and phase data, error bars show ±1 SD. Error bars for the phase data in the animal tested after bilateral labyrinthectomy are not visible because of their small size relative to the scale of the phase. This figure was adapted with permission from [25].
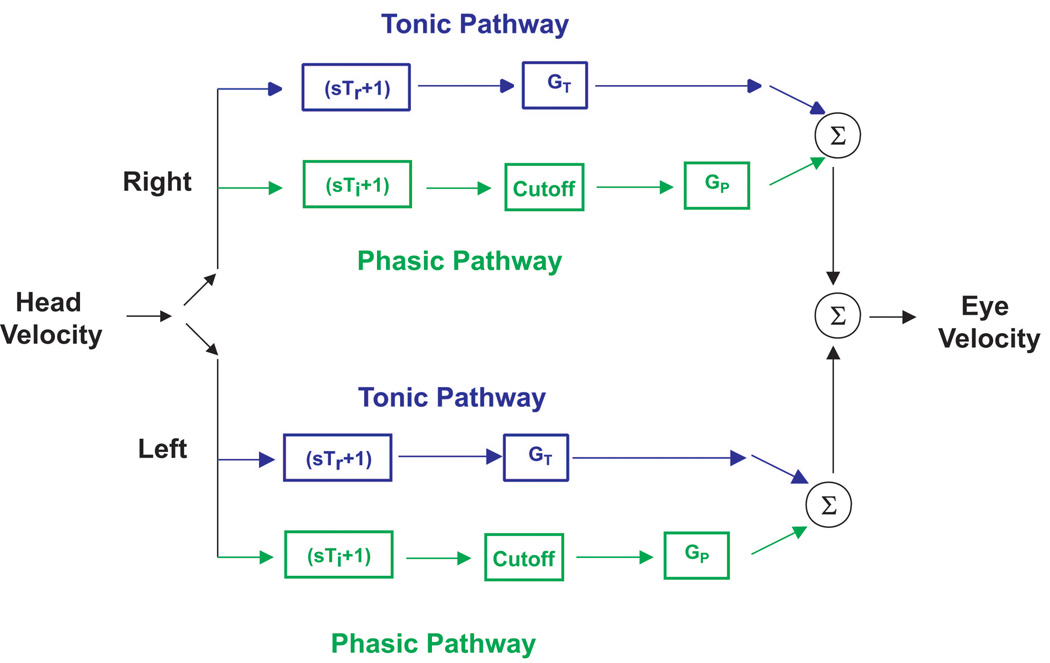
These findings were incorporated into a model of the VOR consisting of tonic and phasic pathways (Figure 4). Anatomically, we conjecture that these two pathways are a representation of a range of parallel frequency-tuned channels. Evidence of this tuning has been observed for vestibular-nerve afferents, secondary vestibular neurons and motor neurons (for review, [29]). In this paper we focus specifically on the distribution of afferent dynamics as being sufficient to explain the model. The tonic pathway conveys a signal corresponding to head velocity over a broad range of rotational frequencies and velocities and has linear response properties (eye velocity scales in proportion to head velocity). This pathway is represented by a transfer function that describes regular afferent responses (sTr + 1). The phasic pathway has dynamic properties that make it sensitive to angular acceleration (as well as velocity) as the frequency of rotation increases. The phasic pathway is represented by a transfer function that describes irregular afferent responses (sTi + 1). A cutoff block in the phasic pathway represents the inhibitory cutoff that occurs for neurons conveying signals in this pathway (irregular afferents are highly susceptible to inhibitory cutoff). Both pathways have separate gain elements, GT and GP. GP is nonlinear to reflect the rise in gain with head velocity (proportional to the cube of head velocity) at higher frequencies in squirrel monkeys. Studies of the horizontal VOR evoked by comparable rotations in macaques and humans have provided evidence for similar pathway-specific responses, but the increase in the magnitude of the phasic signal with the cube of head velocity has been noted to be less prominent than observed in squirrel monkeys [17,27].
Figure 4.
Schematic diagram of a bilateral model of the VOR derived from behavioral data obtained in squirrel monkeys and macaques. During simulations of a unilateral labyrinthectomy the input from the lesioned side is removed. A tonic pathway (represented by a transfer function that resembles regular afferent dynamics, Tr = 6.5 × 10−3 s) and a phasic pathway (represented by a transfer function that resembles irregular afferent dynamics, Ti = 0.05 s) transduce head velocity. A cutoff block in the phasic pathway represents the inhibitory cutoff that occurs for neuronal elements in this pathway (irregular afferents are highly susceptible to inhibitory cutoff). Both pathways have separate gain elements GT and GP. GP is nonlinear (represented by a cubic term) in squirrel monkeys but is linear in macaques. The values of these gain elements increase after a unilateral labyrinthectomy in order to represent the increase in gain due to compensation. However, GP (x2.5) is more modifiable than GT (x1.6). In addition, a first-order band-pass filter, represented by a single pole (TP) and a single zero (TZ), is added to the tonic pathway (not shown in the figure). This low-pass filter accounts for the observation that low frequency responses adapt better than high frequency responses in this pathway [20]. Similar changes in these pathways occur in response to adaptation to magnifying spectacles [6] and in response to vergence-induced changes in the VOR [21].
Responses after unilateral vestibular lesions (labyrinthectomy and canal plugging)
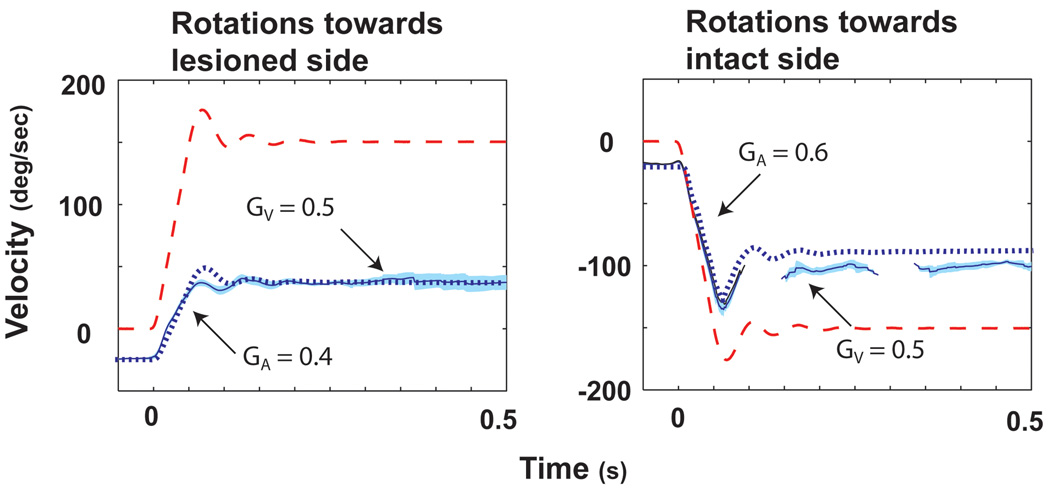
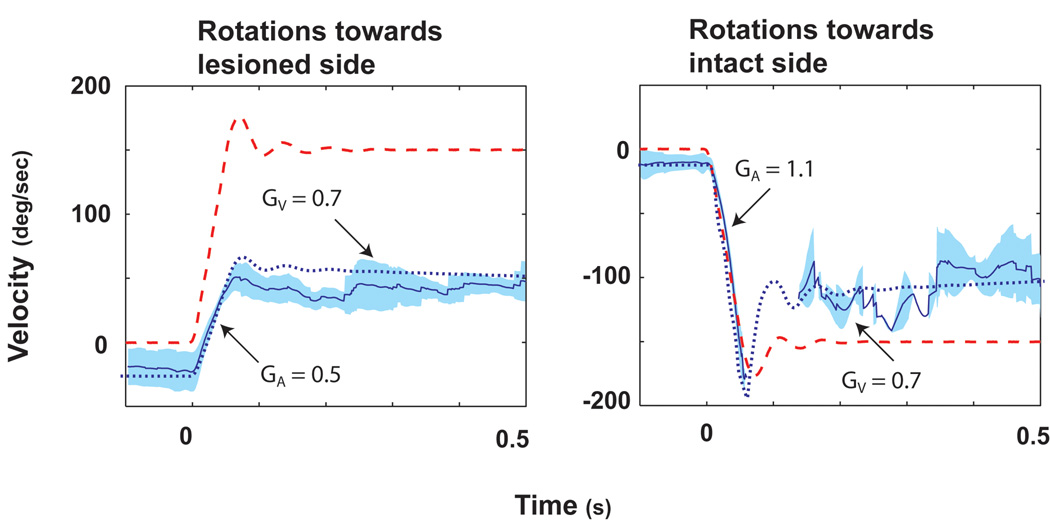
Unilateral labyrinthectomy resulted in a spontaneous nystagmus (slow phase components towards the lesioned side) and in a marked reduction in VOR responses [20]. The method we used to calculate the gain of the VOR (eye velocity / head velocity) was independent of spontaneous nystagmus. When studied at 3 days after labyrinthectomy while animals were kept in darkness, GA for rotations towards the intact side (contralesional rotations) measured in 4 squirrel monkeys was 0.67 ± 0.12. GA for rotations toward the lesioned side (ipsilesional rotations) measured 0.39 ± 0.04 (p < 0.02). Representative data from one animal are shown in Fig. 5. Within 18–24 h after return to light, GA for contralesional rotations increased to 0.87 ± 0.08, whereas there was only a slight increase for ipsilesional rotations to 0.41 ± 0.06. GA for contralesional rotations increased further to 1.04 ± 0.07 day 10 after the lesion, but there were very minimal changes in GA for ipsilesional rotations. Responses measured 10 days after the unilateral labyrinthectomy are shown in Fig. 6. The data in Fig. 6 were obtained from the same animal as in Fig. 5. Thus, the GA for contralesional rotations recovers to near normal values when animals are permitted to move freely in the light. The values of GV increased acutely after animals were brought into light but remained relatively constant thereafter. There was no asymmetry in values of GV during any of the testing sessions after labyrinthectomy.
Figure 5.
The average eye and head velocity responses for ispi- (A) and contralesional (B) rotations on day 1 (while the animal was still in darkness) after a unilateral labyrinthectomy in a squirrel monkey. Head velocity is indicated by the dashed red line and eye velocity by the solid blue line. One standard deviation is indicated by the light blue shading around this line. The dotted blue line is the simulated eye response calculated from our bilateral model. Simulated responses were obtained by removing the input from one side of the model representing normal responses. This figure was adapted with permission from [20].
Figure 6.
The average eye and head velocity responses for ispi- (A) and contralesional (B) rotations on day 10 (the animal had been returned to light on day 4) after a unilateral labyrinthectomy. Data in Fig. 5 and Fig. 6 were from the same squirrel monkey. Head velocity is indicated by the dashed red line and eye velocity by the solid blue line. One standard deviation is indicated by the light blue shading around this line. The dotted blue line is the simulated eye response calculated from our bilateral model. Changes in gain due to compensation were simulated by increasing the gain of GP by x2.5 and GT by x1.6. In addition, a first-order band-pass filter, represented by a single pole (0.02 s) and a single zero (0.01 s) were added to the tonic pathway. This figure was adapted with permission from [20].
Recovery of the sinusoidal responses showed low-pass filter characteristics in that, once animals were returned to light, lower frequencies recovered better than higher frequencies for rotations with a peak velocity of 20°/s. There was evidence of a nonlinear increase in responses for higher velocities at higher frequencies.
The model in Fig. 4 can account for the changes in the VOR after unilateral labyrinthectomy. Removal of the input from the lesioned side causes a reduction in gain of approximately ½ and produces the modest asymmetry shown in Fig 5. These changes result from the relatively small contribution of the phasic pathway to responses in normal animals. Selective modification of the phasic pathway (to a gain that is 2.5X the normal gain) and the tonic pathway (to a gain that is 1.6X the normal gain) on the intact side results in the return of GA for contralesional rotations to normal values, little improvement in GA for ipsilesional rotations (due to inhibitory cutoff), and symmetric but incomplete recovery of GV (because responses at the velocity plateau only have contributions from the tonic pathway). In addition, a first-order band-pass filter, represented by a single pole (TP) and a single zero (TZ), is added to the tonic pathway. This low-pass filter accounts for the observation that even at low velocities the low frequency responses adapt better than high frequency responses in this pathway [20].
Similar processes of VOR compensation were noted in responses after unilateral plugging of the 3 semicircular canals [19]. Unilateral canal plugging preserves resting discharge activity in vestibular-nerve afferents so the findings indicate that the asymmetries in GA for contralesional in comparison to ipsilesional rotations is not dependent upon elimination of the tonic afferent activity in the ipsilateral vestibular nerve. The time course of recovery of GA for contralesional rotations was faster after canal plugging than after labyrinthectomy, and there was a larger increase in GV after canal plugging for both directions of rotation.
Responses after spectacle-induced adaptation in animals with normal vestibular function
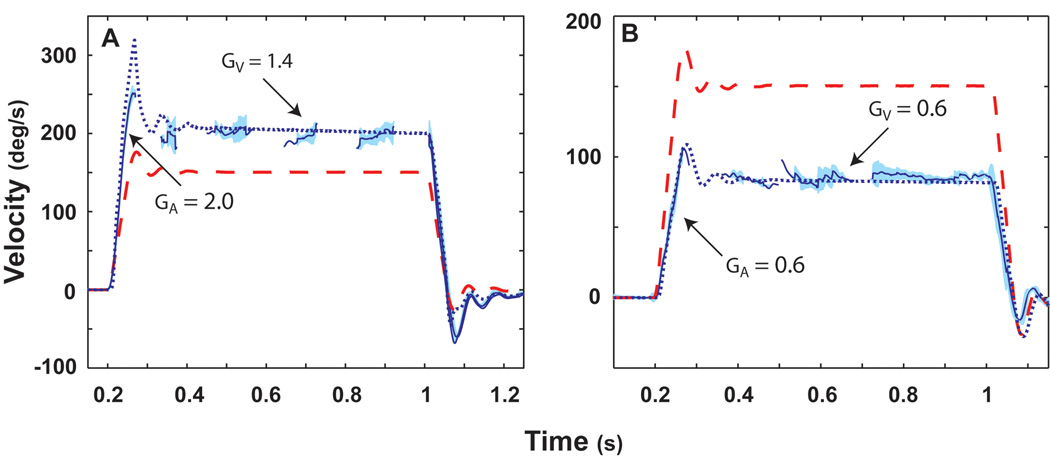
The VOR adapts to changes in the visual requirements for a compensatory response. We studied adaptation of the squirrel monkey horizontal VOR induced by magnifying (X 2.2) or minimizing (X 0.45) spectacles [6]. Animals wore the spectacles continuously for 5–7 days and the VOR was then tested in darkness using the rotational stimuli we have previously described for testing normal VOR and the VOR after unilateral lesions. The VOR evoked by impulses of acceleration following adaptation with magnifying spectacles demonstrated increased gain during both the acceleration and constant velocity periods of the stimulus (Fig. 7A). Similarly, the VOR gain during both components of the stimulus was reduced following adaptation to the minimizing spectacles (Fig. 7B).
Figure 7.
Averaged responses to 3,000°/s2 accelerations to a peak velocity of 150°/s following adaptation to ×2.2 spectacles (A), and following adaptation to ×0.45 spectacles (B). Fast phases were removed prior to averaging the responses. Head velocity is indicated by the dashed red line and eye velocity by the solid blue line. One standard deviation is indicated by the light blue shading around this line. The dotted blue line is the simulated eye response calculated from our bilateral model. Changes in gain due to magnifying spectacles were simulated by increasing the gain of GP by x2.5 and GT by x1.7. In addition, a first-order band-pass filter, represented by a single pole (0.02 s) and a single zero (0.01 s) were added to the tonic pathway. Changes in gain due to minimizing spectacles were simulated by decreasing the gain of GP by × 0 and GT by × 0.6. In addition, a first-order band-pass filter, represented by a single pole (0.02 s) and a single zero (0.03 s) were added to the tonic pathway. This high-pass filter accounts for the observation that low-frequency responses adapt better than high-frequency responses in the tonic pathway. This figure was adapted with permission from [6].
For the four monkeys that were adapted with the magnifying spectacles, the mean GA during 3,000°/s2 to 150°/s acceleration impulses was 1.05 ± 0.08 (mean ± SD) preadaptation and 1.96 ± 0.16 postadaptation (p < 0.01). For the same animals, the mean GV prior to adaptation was 0.93 ± 0.04, and postadaptation the mean GV was 1.36 ± 0.08 (p < 0.01). While both GA and GV increased with adaptation, there was a significant difference in the relative increases of the two components of the response. The acceleration gain increased by a factor of 1.92, while the velocity gain only increased by a factor of 1.46 (p < 0.01). These findings are in agreement with the adaptation of contralesional responses to the same rotational stimuli after unilateral vestibular lesions (labyrinthectomy and canal plugging). The response during the acceleration is more modifiable than the response at the constant-velocity plateau. The changes in the parameters used to simulate adaptation to magnifying spectacles are virtually identical to changes in the parameters used to simulate recovery after labyrinthectomy. We hypothesize that this is because adaptation and compensation in both experimental paradigms require the VOR pathways to approximately double their output.
For the four monkeys that were adapted to the minimizing spectacles, the gain during the acceleration component of the 3,000°/s2 to 150°/s acceleration impulses decreased from 1.04 ± 0.05 preadaptation to 0.61 ± 0.08 postadaptation (p < 0.01). For the same animals, GV prior to adaptation was 0.94 ± 0.03, and the postadaptation GV was 0.59 ± 0.09 (p < 0.01). There was no difference between GA and GV following adaptation to the minimizing spectacles. Unlike the asymmetric increases in GA and GV following adaptation with the magnifying spectacles, the monkeys displayed similar decreases in both GA and GV (43% and 35%, respectively). These findings indicate that the phasic component of the response is more effective at increasing the gain of the VOR (as during adaptation to magnifying spectacles) than it is in decreasing the gain.
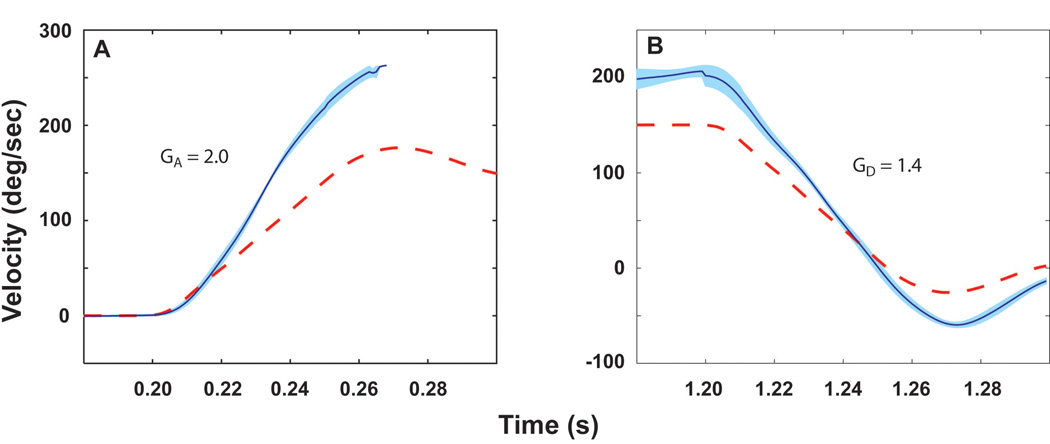
Further evidence of the greater enhancement of the phasic when compared to the tonic pathway is provided by the observation that the gain during the acceleration (on-ramp) portion of the stimulus (GA) was greater than during the deceleration (off-ramp) portion of the stimulus (GD) following adaptation to magnifying spectacles. Prior to adaptation there was no difference in the gain values between the acceleration and deceleration ramps. However, after adaptation to the magnifying spectacles, there was a significant asymmetry to the response with the acceleration gain being greater than the deceleration gain (Fig. 8). In contrast to the difference between GA and GD postadaptation, there was no difference between GD and GV postadaptation. This asymmetric response to acceleration and deceleration impulses with identical stimulus profiles can also be explained by a greater enhancement of the phasic pathway. The dynamics of the phasic pathway coupled with its inhibitory cutoff result in GD and GV being controlled principally by inputs from the tonic pathway.
Figure 8.
Averaged responses to 3,000°/s2 to 150°/s acceleration (A) and deceleration (B) impulses following adaptation to × 2.2 spectacles in squirrel monkeys. The deceleration impulse followed a 0.9–1.1 s constant velocity rotation. This figure was adapted with permission from [6].
The model used to account for the findings in Clendaniel et al. [6] is fundamentally the same as that in Fig. 4. For simulation of changes following spectacle- induced adaptation, the transfer function added to the tonic pathway becomes a high-pass filter during minimization to reflect the observation that gains decrease more at low frequencies than at high frequencies. (see Appendix to Clendaniel et al. [6] for details). As was the case for the responses following unilateral labyrinthectomy and unilateral canal plugging, the model accounts for the postadaptation responses because the phasic pathway is more modifiable than is the tonic pathway.
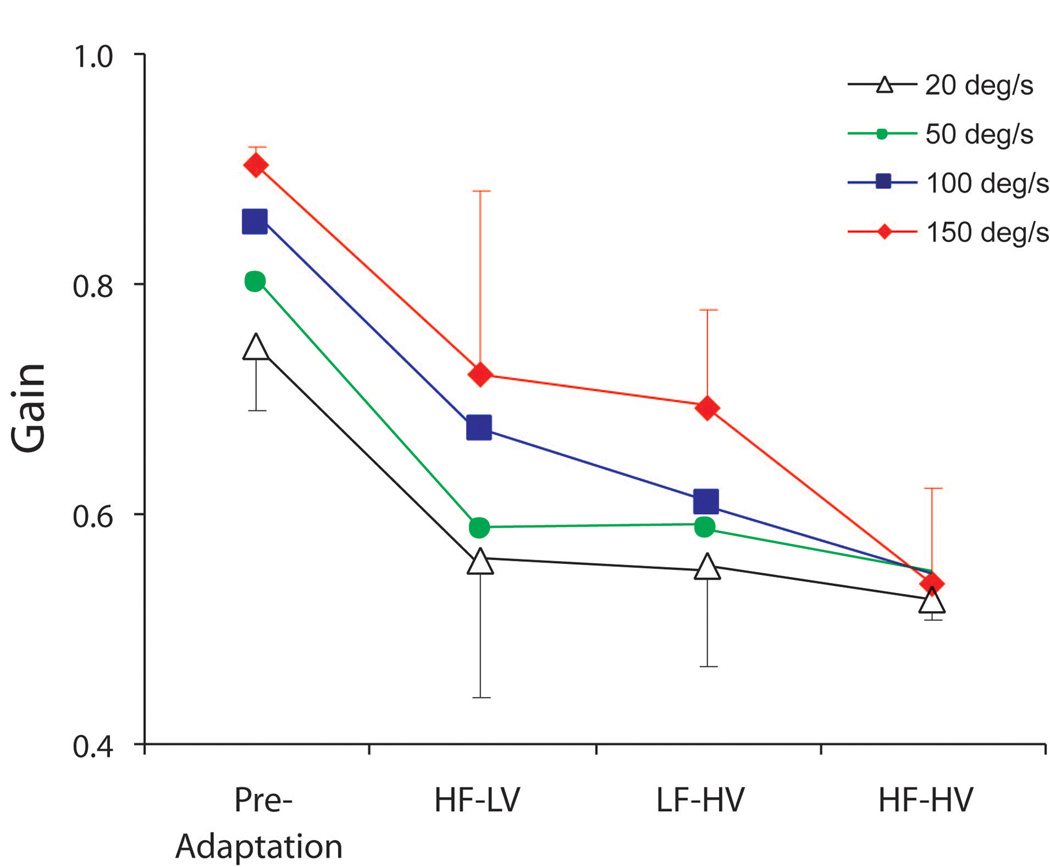
Findings from short-term adaptation studies provide further evidence in support of the selective modification of tonic and phasic pathways [7]. Squirrel monkeys with normal vestibular function wore minimizing (X0.45) spectacles while being rotated with different sum-of-sines stimuli. Three combinations of rotational frequencies and velocities were used: low frequencies - high velocities; high frequencies - low velocities; and high frequencies – high velocities. Animals were then tested in darkness after 4 h of adaptation to each stimulus condition. Fig. 9 shows the VOR gain for rotations at 4 Hz at differing peak velocities of rotation. The VOR gain was lower following adaptation to each stimulus condition. However, the velocity-dependent rise in gain (attributable to the nonlinearity in the phasic pathway) was only attenuated for the adaptation condition that included high frequencies and high velocities. These findings provide direct evidence that exposure to stimuli that elicit appreciable responses from the phasic pathway is needed in order modify responses that are mediated by this pathway.
Figure 9.
Pre- and postadaptation gains to 4 Hz sinusoidal rotations with peak velocities of 20, 50, 100, and 150°/s. HF-LV, gains after the high-frequency  low-velocity adaptation paradigm; LF-HV, gains after the low-frequency
low-velocity adaptation paradigm; LF-HV, gains after the low-frequency high-velocity paradigm; and HF-HV, gains after the high-frequency
high-velocity paradigm; and HF-HV, gains after the high-frequency high-velocity adaptation paradigm. In only the HF-HV paradigm do we see changes in gain associated with the tonic and phasic pathways. This figure was adapted with permission from [7].
high-velocity adaptation paradigm. In only the HF-HV paradigm do we see changes in gain associated with the tonic and phasic pathways. This figure was adapted with permission from [7].
Studies of VOR adaptation prior to Clendaniel et al. [6] would, on first inspection, appear to lead to different conclusions regarding the characteristics of the more modifiable signals. Lisberger and Pavelko [23] measured a dynamic index (DI) defined as the ratio of maximum eye velocity to steady-state eye velocity during a transient change in head velocity that had the following characteristics: 600°/s2 acceleration to a peak velocity of 30°/s. They reported that, although the peak and steady-state eye velocities increased following adaptation to the magnifying spectacles, there was a decrease in the DI. Conversely, while the peak and steady-state eye velocities decreased following adaptation to the minimizing spectacles, there was an increase in the DI. There was a greater peak eye velocity relative to the steady-state eye velocity after adaptation to the minimizing spectacles. This increased DI following adaptation to the minimizing spectacles was thought to be due the presence of a nonmodifiable component in the VOR pathway.
Clendaniel et al. [6] measured the DI for the 3,000°/s2 to 60°/s stimulus, and the results differed from the responses reported by Lisberger and Pavelko [23]. There was no change in the DI postadaptation to either the magnifying or minimizing spectacles. There were several differences between Clendaniel et al. [6] and Lisberger and Pavelko [23]. First, the rotational stimuli used in the two studies were different. Clendaniel et al. [6] used accelerations of 3,000°/s2, to a peak velocity of 60°/s. The acceleration impulses used in the earlier studies were significantly lower (600°/s2 to a peak velocity of 30°/s) and would not have been expected to evoke an appreciable response from the phasic pathway. Recruitment of the phasic pathway could account for the lack of decrease in the DI seen following magnification in the study of Clendaniel et al. [6]. In other words, Clendaniel et al. [7] showed that the phasic pathway is only modifiable if you rotate the animal at sufficiently high frequencies and velocities.
Clendaniel et al. [6] also showed that there was not a fixed latency to the beginning of the adapted response as had previously been suggested by Lisberger [22]. The absence of a fixed latency would appear to be in conflict with the prediction of models that include fixed modifiable and nonmodifiable components. The latency that is measured is dependent on both the dynamics of the stimulus and the frequency-dependent nature of the processes that mediate adaptation.
Neural signals that mediate processes of vestibular compensation
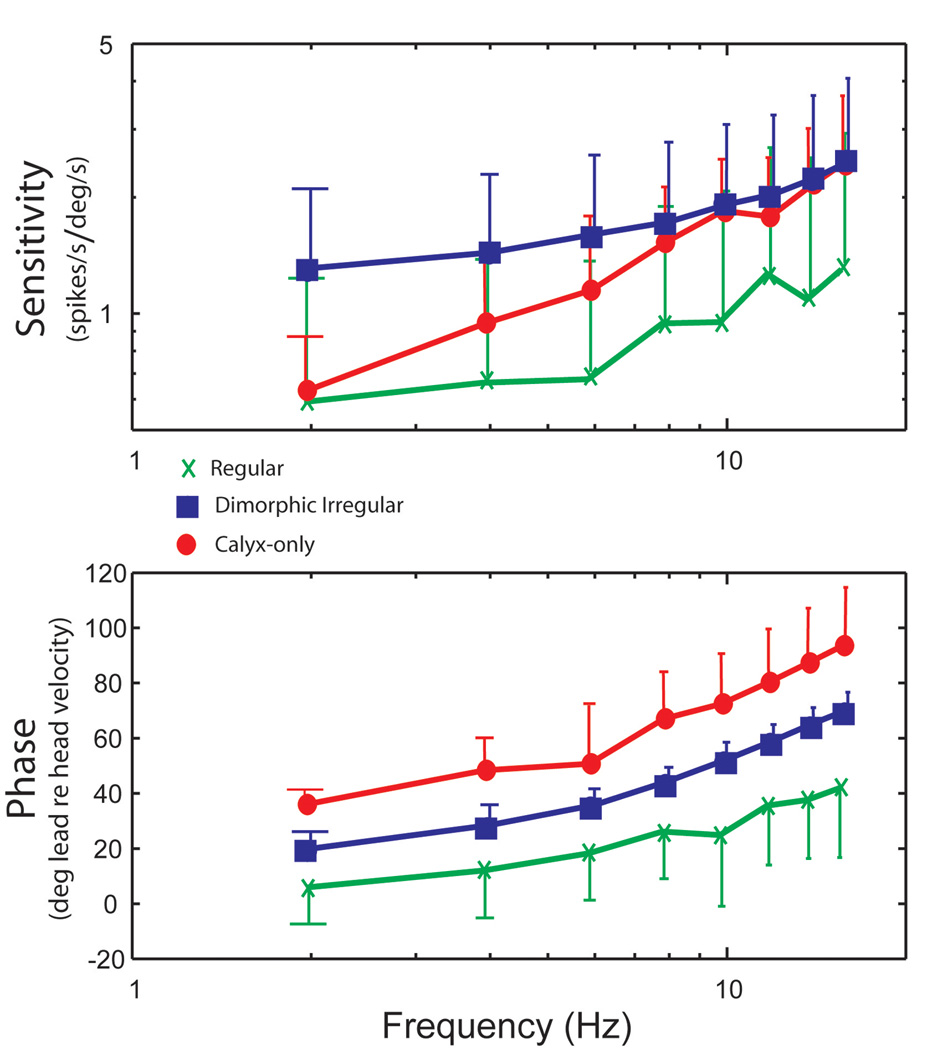
Our initial studies of the neural signals that are involved in processes of vestibular compensation began with analyses of the responses of vestibular-nerve afferents innervating the semicircular canals to a range of rotational stimuli similar to that used in our studies of the VOR. A sensitivity and phase plot describing the dynamics of vestibular afferents in chinchillas is shown in Fig. 10. The transfer function describing the response dynamics of regularly firing afferents closely resembles the transfer function used in the tonic pathway in Fig. 4. Whereas, the transfer function describing the rotational responses of irregular afferents has close resemblance to the transfer function used in the phasic pathway of the VOR. We found that the vast majority of regular afferents had a modulation in their firing rate with minimal distortion and no cutoff or saturation over a broad range of rotational stimuli [16]. Irregular afferents, in contrast, exhibit a significant amount of cutoff when rotated in the inhibitory direction. Irregular afferents can be divided into dimorphic and calyx-only groups based upon their distinctive discharge properties at 2 Hz [2]. The calyx-only afferents innervate exclusively Type I hair cells in the central zone of the cristae ampullaris whereas the dimorphic afferents innervate Type I hair cells with calyx endings and Type II hair cells with bouton endings in regions of the sensory epithelium closer to the central than to the peripheral zone. Calyx-only irregular afferents have low sensitivities, when compared to dimorphic irregular afferents, in responses to rotations at 2 Hz. The sensitivity of both groups of irregular afferents increased with increasing frequency [15]. This increase was the most pronounced for the calyx-only afferents. At higher rotational frequencies, these calyx-only irregular afferents have an approximately 90 deg phase lead with respect to head velocity which means that their responses are closely related to acceleration. Because calyx-only afferents respond to head acceleration at high-frequencies, we believe it is likely that they make a contribution to the responses of the phasic pathway.
Figure 10.
Average sensitivities and phases of regular afferents (n = 14,  ), high-gain irregular afferents (n = 9,
), high-gain irregular afferents (n = 9,  ), and low-gain irregular afferents (n = 7,
), and low-gain irregular afferents (n = 7,  ) measured from sinusoidal rotations. Top: sensitivity (spikes/s per deg/s); bottom: phase (deg lead relative to head velocity). Error bars represent mean gain ± 1 SD. Only one bar is shown for clarity. Note that the average responses of low-gain irregular afferents rise to match those of high-gain irregular afferents at high frequencies. This figure was adapted with permission from [15].
) measured from sinusoidal rotations. Top: sensitivity (spikes/s per deg/s); bottom: phase (deg lead relative to head velocity). Error bars represent mean gain ± 1 SD. Only one bar is shown for clarity. Note that the average responses of low-gain irregular afferents rise to match those of high-gain irregular afferents at high frequencies. This figure was adapted with permission from [15].
Conclusions
One of the key insights arising from our bilateral model of the VOR is that the mechanism underlying the gain asymmetry in response to rapid head movements after a unilateral vestibular lesion involves inhibitory cutoff in processes that mediate compensation. This inhibitory cutoff is occurring principally in the phasic components of the response. These phasic signals make little contribution to the normal VOR, but have a critically important role in compensation after a lesion. The findings from studies in which animals were kept in darkness after labyrinthectomy indicate that excitatory and inhibitory inputs to the tonic component are relatively symmetric before compensation begins and, by inference, under normal conditions. The neurons that provide inputs to phasic pathways are the most sensitive to inhibitory cutoff and it is these phasic responses that are most modifiable. A retinal slip error signal appears to be required for the compensation processes because GA and GV are symmetrically decreased when animals are kept in darkness following unilateral labyrinthectomy. The increase in GA occurs relatively rapidly (within 1 day) when animals move about their environment in light. Greater modification of the phasic in comparison to the tonic component of the response is also seen after adaptation to magnifying spectacles. The properties of the phasic pathway resemble those of irregular afferents whereas those of the tonic pathway resemble those of regular afferents.
Our findings from experiments that involve spectacle-induced adaptation provide further support for the conclusion that phasic inputs are more modifiable than tonic inputs to the VOR pathways. Two questions arise from these findings concerning the limited adaptive capability of the tonic pathway: 1) What are the mechanisms responsible for its limited adaptation? 2) Are there specific stimulus conditions or paradigms that could improve its adaptation? Our preliminary data indicate that repeated rotations towards the side of the lesion can be effective in decreasing asymmetries after unilateral labyrinthectomy [30].
The next article in this series of reviews [10] provides further information on studies of neural mechanisms that underlie vestibular compensation. Recordings from vestibular-nerve afferents on the intact side after unilateral labyrinthectomy showed an increase in the proportion of irregular afferents and a decrease in the proportion of regular afferents. This change in afferent distribution could contribute to the accentuated contribution of the phasic pathway after the lesion. Future studies focused on the dynamics of central vestibular neurons after unilateral labyrinthectomy are likely to provide greater insight into the mechanisms of compensation.
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders Grants R01 DC-02390.
Reference List
- 1.Aw ST, Halmagyi GM, Haslwanter T, Curthoys IS, Yavor RA, Todd MJ. Three-dimensional vector analysis of the human vestibulo-ocular reflex in response to high acceleration head rotations II. Responses in subjects with unilateral vestibular loss and selective semicircular canal occlusion. J Neurophysiol. 1996;76:4021–4030. doi: 10.1152/jn.1996.76.6.4021. [DOI] [PubMed] [Google Scholar]
- 2.Baird RA, Desmadryl G, Fernandez C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol. 1988;60:182–203. doi: 10.1152/jn.1988.60.1.182. [DOI] [PubMed] [Google Scholar]
- 3.Baloh RH. Clinical practice. Vestibular neuritis. N Engl J Med. 2003;348:1027–1032. doi: 10.1056/NEJMcp021154. [DOI] [PubMed] [Google Scholar]
- 4.Carey JP, Migliaccio AA, Minor LB. Semicircular canal function before and after surgery for superior canal dehiscence. Otol. Neurotol. 2007;28:356–364. doi: 10.1097/01.mao.0000253284.40995.d8. [DOI] [PubMed] [Google Scholar]
- 5.Carey JP, Minor LB, Peng GC, Della Santina CC, Cremer PD, Haslwanter T. Changes in the three-dimensional angular vestibulo-ocular reflex following intratympanic gentamicin for Meniere's disease. J Assoc Res Otolaryngol. 2002;3:430–443. doi: 10.1007/s101620010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clendaniel RA, Lasker DM, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. IV. Responses after spectacle-induced adaptation. J Neurophysiol. 2001;86:1594–1611. doi: 10.1152/jn.2001.86.4.1594. [DOI] [PubMed] [Google Scholar]
- 7.Clendaniel RA, Lasker DM, Minor LB. Differential adaptation of the linear and nonlinear components of the horizontal vestibuloocular reflex in squirrel monkeys. J. Neurophysiol. 2002;88:3534–3540. doi: 10.1152/jn.00404.2002. [DOI] [PubMed] [Google Scholar]
- 8.Collewijn H, Smeets JB. Early components of the human vestibulo-ocular response to head rotation: latency and gain. J Neurophysiol. 2000;84:376–389. doi: 10.1152/jn.2000.84.1.376. [DOI] [PubMed] [Google Scholar]
- 9.Cremer PD, Halmagyi GM, Aw ST, Curthoys IS, McGarvie LA, Todd MJ, Black RA, Hannigan IP. Semicircular canal plane head impulses detect absent function of individual semicircular canals. Brain. 1998;121:699–716. doi: 10.1093/brain/121.4.699. [DOI] [PubMed] [Google Scholar]
- 10.Cullen KE, Sadeghi SG, Beraneck M, Minor LB. Neural substrates underlying vestibular compensation: Contributions of peripheral versus central processing. J Vestib Res. 2010 doi: 10.3233/VES-2009-0357. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curthoys IS, Halmagyi GM. Vestibular compensation: A review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J Vestib Res. 1995;5:67–107. [PubMed] [Google Scholar]
- 12.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 13.Halmagyi GM, Curthoys IS, Cremer PD, Henderson CJ, Todd MJ, Staples MJ, D'Cruz DM. The human horizontal vestibulo-ocular reflex in response to high-acceleration stimulation before and after unilateral vestibular neurectomy. Exp Brain Res. 1990;81:479–490. doi: 10.1007/BF02423496. [DOI] [PubMed] [Google Scholar]
- 14.Honrubia V, Jenkins HA, Baloh R, Yee RD, Lau CGY. Vestibulo-ocular reflexes in peripheral labyrinthine lesions: I. Unilateral dysfunction. Am J Otolaryngol. 1984;5:15–26. doi: 10.1016/s0196-0709(84)80016-2. [DOI] [PubMed] [Google Scholar]
- 15.Hullar TE, Della Santina CC, Hirvonen TP, Lasker DM, Carey JP, Minor LB. Responses of Irregularly Discharging Chinchilla Semicircular Canal Vestibular-Nerve Afferents During High-Frequency Head Rotations. J Neurophysiol. 2005:2777–2786. doi: 10.1152/jn.01002.2004. [DOI] [PubMed] [Google Scholar]
- 16.Hullar TE, Minor LB. High-frequency dynamics of regularly discharging canal afferents provide a linear signal for angular vestibuloocular reflexes. J. Neurophysiol. 1999;82:2000–2005. doi: 10.1152/jn.1999.82.4.2000. [DOI] [PubMed] [Google Scholar]
- 17.Huterer M, Cullen KE. Vestibuloocular reflex dynamics during high-frequency and high- acceleration rotations of the head on body in rhesus monkey. J Neurophysiol. 2002;88:13–28. doi: 10.1152/jn.2002.88.1.13. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins HA. Long-term adaptive changes of the vestibul-ocular reflex in patients following acoustic neuroma surgery. Laryngoscope. 1985;95:1224–1234. doi: 10.1288/00005537-198510000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Lasker DM, Backous DD, Lysakowski A, Davis GL, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. II. Responses after canal plugging. J Neurophysiol. 1999;82:1271–1285. doi: 10.1152/jn.1999.82.3.1271. [DOI] [PubMed] [Google Scholar]
- 20.Lasker DM, Hullar TE, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. III. Responses after labyrinthectomy. J Neurophysiol. 2000;83:2482–2496. doi: 10.1152/jn.2000.83.5.2482. [DOI] [PubMed] [Google Scholar]
- 21.Lasker DM, Ramat S, Carey JP, Minor LB. Vergence-mediated modulation of the human horizontal angular VOR provides evidence of pathway-specific changes in VOR dynamics. Ann NY Acad Sci. 2002;956:324–337. doi: 10.1111/j.1749-6632.2002.tb02831.x. [DOI] [PubMed] [Google Scholar]
- 22.Lisberger SG. The latency of pathways containing the site of motor learning in the monkey vestibulo-ocular reflex. Science. 1984;225:74–76. doi: 10.1126/science.6610214. [DOI] [PubMed] [Google Scholar]
- 23.Lisberger SG, Pavelko TA. Vestibular signals carried by pathways subserving plasticity of the vestibulo-ocular reflex in monkeys. J Neurosci. 1986;6:346–354. doi: 10.1523/JNEUROSCI.06-02-00346.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minor LB, Haslwanter T, Straumann D, Zee DS. Hyperventilation-induced nystagmus in patients with vestibular schwannoma. Neurology. 1999;53:2158–2168. doi: 10.1212/wnl.53.9.2158. [DOI] [PubMed] [Google Scholar]
- 25.Minor LB, Lasker DM, Backous DD, Hullar TE. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. I. Normal responses. J Neurophysiol. 1999;82:1254–1270. doi: 10.1152/jn.1999.82.3.1254. [DOI] [PubMed] [Google Scholar]
- 26.Paige GD. Nonlinearity and asymmetry in the human vestibulo-ocular reflex. Acta Otolaryngol (Stockh) 1989;108:1–8. doi: 10.3109/00016488909107385. [DOI] [PubMed] [Google Scholar]
- 27.Palla A, Straumann D. Recovery of the high-acceleration vestibulo-ocular reflex after vestibular neuritis. J Assoc Res Otolaryngol. 2004;5:427–435. doi: 10.1007/s10162-004-4035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith PF, Curthoys IS. Mechanisms of Recovery following unilateral labyrinthectomy: a review. Brain Res Brain Res Rev. 1988;14:308–319. doi: 10.1016/0165-0173(89)90013-1. [DOI] [PubMed] [Google Scholar]
- 29.Straka H, Lambert FM, Pfanzelt S, Beraneck M. Vestibulo-ocular Signal Transformation in Frequency-Tuned Channels. Ann NY Acad Sci. 2009;1164:37–44. doi: 10.1111/j.1749-6632.2008.03740.x. [DOI] [PubMed] [Google Scholar]
- 30.Ushio M, Lasker DM, Minor LB. Unidirectional rotations in macaques produce asymmetric changes in gain of the horizontal VOR before and after unilateral labyrinthectomy. Soc Neurosci Abstr. 2009 doi: 10.1007/s00221-011-2622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zee DS, Fetter M, Proctor L. Recovery from unilateral labyrinthectomy in primate: Effects of visual inputs and considerations upon Ewalds second law. In: Hwang JC, Daunton NG, Wilson VJ, editors. Basic and Applied Aspects of Vestibular Function. pp. 125–131. [Google Scholar]