Abstract
Purpose
We evaluated rapid androgen cycling in combination with docetaxel for men with progressive non-castrate prostate cancers.
Methods
Non-castrate patients with ≤ 6 months of hormones were eligible. Cohort 1 (63 patients ) received 6 28-day cycles of docetaxel (75 mg/m2), leuprolide and 7 days of topical testosterone. Cohort 2 (39 patients) received 9 21-day cycles of docetaxel (70 mg/m2), leuprolide and 3 days of testosterone. The primary endpoint was the proportion of patients at 18 months who achieved non -castrate testosterone levels (>150 ng/dl) and an undetectable PSA (≤ 0.05, ≤0.5, or ≤2.0 ng/ml with prior prostatectomy, radiotherapy, or no definitive therapy, respectively). Cytochrome P450 3A4 (CYP3A4) activity and docetaxel pharmacokinetics were evaluated.
Results
A higher proportion of patients achieved the undetectable PSA outcome at 18 months in cohort 2 relative to cohort 1 (13% vs. 0%). The 16% incidence of febrile neutropenia was higher than observed in patients was castration-resistant disease, which may have been related to a 50% reduction in overall docetaxel clearance in the non-castrate group. There was no alteration in CYP3A4 activity (P=0.87) or docetaxel clearance (P=0.88) between cycles.
Conclusions
The undetectable PSA endpoint allows for a rapid screening of interventions for further study. Increasing the number of docetaxel cycles following a shorter period of testosterone repletion, and a longer duration of testosterone depletion, increased the proportion of men who achieved an undetectable PSA. The higher-than-expected incidence of febrile neutropenia may have been related to the reduced overall docetaxel clearance in patients with non-castrate vs castrate testosterone levels.
INTRODUCTION
Hormonal manipulations alone do not cure prostate cancer because cells that can resist and or survive the effects of androgen depletion are present when the disease is first manifest. Even when the sole manifestation of progression is a rising PSA and overall tumor burden is low, hormonal manipulations are not curative. Serial histologic studies of prostate cancer xenografts and human prostate cancers show that as early as one week following androgen depletion, the surviving cells are non-proliferating and there is little evidence of apoptosis.12 These arrested cells resume proliferating after testosterone is administered,1,3 or when testosterone levels are allowed to increase using an intermittent androgen depletion approach. Previously, in an effort to increase apoptotic rates, we explored a strategy of rapid androgen depletion and repletion in 28 day cycles in the clinic. While the results showed that repetitive apoptotic cycles could be induced, the overall response rate was not increased. 4
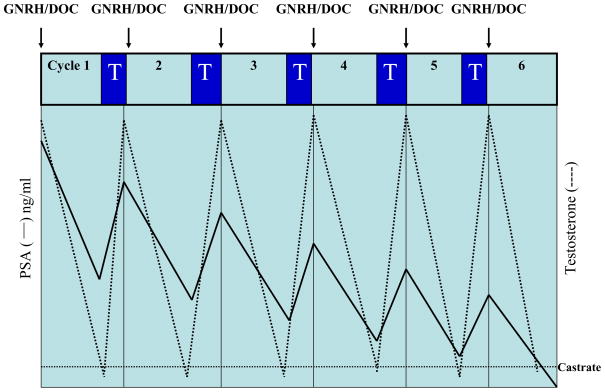
Chemotherapy in combination with androgen depletion has also been explored in patients with a rising PSA and/or clinical metastases5 as a way to enhance the efficacy of androgen depletion. Unfortunately, the results to date have been inconclusive, as no trial has been shown to improve outcomes or prolong life relative to androgen depletion alone.6–12 A drawback to this approach is that at a time of cell cycle arrest chemotherapy may be of limited benefit. The current trial is based on the hypothesis that prostate cancer cells will be more sensitive to docetaxel when they are proliferating during the androgen repletion phases of rapid androgen cycling (Figure 1).
Figure 1.
Idealized patient outcome on docetaxel (Doc) and rapid androgen cycling with leuprolide (GNRH) and testosterone repletion (T). The dotted line represents peak and trough testosterone levels in response to testosterone depletion and repletion. The solid line represents successive declines in peak and trough PSA values in response to each cycle of treatment.
Survival-based trials for patients with non-castrate prostate cancer are difficult to conduct due to issues of patient selection, the requirement for long-term follow-up, and too few meaningful clinical events on which to assess outcomes. Since designing and conducting large -scale phase III trials for patients with early disease is both difficult and expensive, it is important to minimize the chance of negative phase III trials by developing methods to improve the reliability of phase II studies. Toward this goal, we have proposed as an endpoint for phase II trials an undetectable PSA level with non -castrate levels of testosterone,13 a prerequisite to, but admittedly no guarantee of, cure. In this trial, we evaluated the outcomes and safety of docetaxel with repeated cycles of androgen depletion and short-term androgen repletion in patients with progressive non-castrate prostate cancers using a treatment-specific undetectable PSA endpoint (≤0.05, ≤0.5, or ≤2.0 ng/ml for patients with prior prostatectomy, radiotherapy, or no definitive therapy, respectively). We also evaluated the effects of rapid androgen cycling on cytochrome P450 3A4 (CYP3A4) and docetaxel pharmacokinetics.
PATIENTS AND METHODS
This was an IRB-approved study including Memorial Sloan-Kettering Cancer Center (MSKCC) and the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (JHU). All patients signed an IRB-approved informed consent.
Patient Eligibility
Eligible patients had prostate cancer histologically confirmed at their treating institution, non-castrate testosterone levels (>150 ng/dl), and progressive metastatic disease or rising PSA. Rising PSA was defined as a minimum baseline of 2 ng/ml with at least 3 determinations obtained more than 2 weeks apart demonstrating a 50% or greater increase in value. Additional eligibility requirements included: 1) ≤6 months of prior hormone therapy, 2) Karnofsky performance status of ≤70%, and 3) adequate organ function.
Study Design
In cohort 1, patients received six 4-week cycles consisting of monthly leuprolide (7.5 mg IM) and docetaxel (75 mg/m2) on day 1 of each cycle, with testosterone repletion (AndroGel 1%, 5 g topical daily) for 7 days prior to initiation of each subsequent cycle. In cohort 2, patients received nine 3-week cycles of 3-month depot leuprolide (22.5 mg IM) on day 1 of cycles 1, 5, and 9, docetaxel (70 mg/m2) on day 1, and testosterone repletion (AndroGel 1%, 5 g topical daily) for 3 days prior to initiation of each subsequent cycle. The total duration of leuprolide therapy was 24 weeks in cohort 1 and 36 weeks in cohort 2.
Patient Evaluation
Patients were examined and assessed for adverse events monthly while receiving treatment. Toxicity was assessed using the National Cancer Institute Common Toxicity Criteria (version 2.0). Patients were required to submit a drug diary for recording testosterone gel administration and toxicities during the cycling phase. Patients who had metastatic disease evident on baseline imaging (CT scan, MRI, or bone scan) had follow-up studies at 3-month intervals. Patients with no radiographic abnormalities were imaged every 6 months.
PSA and Testosterone Measurements
Blood for PSA and testosterone measurements was collected twice during each cycle: peak samples before docetaxel on day 1, and trough samples before androgen repletion (day 22 in cohort 1 and day 19 in cohort 2 ). PSA was determined by a two-site immunoenzymometric assay using the Nexia analytical system (Tosoh Medics, South San Francisco, CA). Total testosterone concentrations at MSKCC were determined by a solid-phase radioimmunoassay (Diagnostic Products Corp., Los Angeles, CA) with an analytical sensitivity of 10 ng/dL. For patients at JHU, a chemiluminescence immunoassay with an analytical sensitivity of 20 ng/dL was used.
CYP3A4 Activity
CYP3A4 activity was assessed using the erythromycin breath test as previously described.14 The test was administered to 12 cohort 1 patients before cycles 1 (without testosterone administration) and 2 (with testosterone administration), within 48 hours prior to docetaxel administration. A breath sample was obtained at 20 minutes after administration of 14C-labeled erythromycin, then shipped to Metabolic Solutions (Nashua, NH) for measurement of breath carbon dioxide. The data were reported as the flux of 14CO2. The parameter of interest was the percentage of 14 Cexhaled per minute at 20 minutes after administration of the radiolabeled erythromycin (C20min[% dose/min ]).
Pharmacokinetic Sampling and Analysis
Docetaxel pharmacokinetics were determined in 12 patients at the start of cycles 1 (without testosterone) and 2 (with testosterone administration) at the following time points: immediately before docetaxel treatment, 30 minutes into the infusion, at 59 minutes (just before the end of the docetaxel infusion), and postinfusion at 10 minutes, 30 minutes, 1, 3 and 7 hours, and the morning of days 2 (24 hours), 3 (48 hours), and 8, 15, and 22. Docetaxel concentrations in plasma were quantitated using a validated analytic assay consisting of high-performance liquid chromatography with mass spectrometric detection, as previously described.15 The lower limit of quantitation for docetaxel was 0.4 ng/mL.
Individual docetaxel pharmacokinetic parameters were estimated using model-dependent methods as implemented in Winnonlin version 5.1 (Pharsight Corp, Mountainview, CA). Concentration-time data were fit with a three-compartment model using PK Model 19 and the Gauss-Newton (Levenberg and Hartley) method for the fitting of non-linear regression functions. Calculated secondary pharmacokinetic parameters included systemic clearance, area under the concentration-time curve from time zero to infinity (AUCinf), half-life during the terminal phase of the disposition curve (t1/2,γ), and volume of distribution at steady-state (Vss). Maximum plasma concentration (Cmax) values were the observed values.
Statistical Analysis
The primary endpoint was the proportion of patients with a treatment-specific undetectable PSA and non-castrate testosterone (>150 ng/dl) at 18 months from the start of therapy. Treatment-specific undetectable PSA was defined as ≤ 0.05 ng/ml after prostatectomy, ≤ 0.5 ng/ml after radiation therapy, or ≤ 2.0 ng/ml for patients with metastatic disease without prior definitive therapy. We defined this event as a response and planned to declare the treatment active in either study cohort if the probability of a response in the population exceeded 0.15. A single-stage design that differentiated between population response probabilities of 0.15 and 0.35 was used. Progression was defined as 3 consecutive rises in PSA and/or evidence of disease progression on imaging.
For docetaxel pharmacokinetic studies, the sample size of 12 would detect an effect size of 1.25, assuming no period effects, with alpha = 0.01 and power = 95% using a pairwise 2-sided analysis. Sample size calculations were performed using NCSS 1001/Pass 2002 (Number Cruncher Statistical Systems, Kaysville, UT). For docetaxel pharmacokinetics and CYP3A activity, apaired t-test was used to compare parameters between cycles 1 and 2.
RESULTS
Patients
Between August 3, 2003 and January 6, 2006, 102 patients were enrolled on trial, 63 at MSKCC and 39 at JHU. The demographics, disease status, and prior treatment histories of the patients are detailed in Table 1.
Table 1.
Patient characteristics
| Cohort 1 | Cohort 2 | |
|---|---|---|
| n=63 | n=39 | |
|
Baseline Characteristics | ||
| Mean Age (range) | 62 (48–82) | 61 (40–77) |
| Median Gleason Score (range) | 7 (6–10) | 7 (6–9) |
| Median Baseline PSA Level, ng/ml (range) | 18 (0.67–122) | 13 (2–3692) |
| Median Baseline Testosterone, ng/dL (range) | 298 (169–579) | 306 (148–475) |
|
Disease State | ||
| Rising PSA | 25 (40%) | 18 (46%) |
| Non-castrate Metastatic | 38 (60%) | 21 (54%) |
|
Primary Therapy | ||
| Radical Prostatectomy Alone | 37 (59%) | 22 (56%) |
| Radiation Therapy Alone | 6 (10%) | 6 (15%) |
| Salvage Radiation Therapy | 16 (25%) | 9 (23%) |
| No Prior Treatment | 3 (5%) | 2 (5%) |
Treatment Outcome
All but 2 patients completed 6 months of chemotherapy and hormonal cycling without evidence of progression. Patients received 6 cycles of docetaxel and 24 weeks of GnRH exposure in cohort 1 versus 9 cycles of docetaxel and 36 weeks of GnRH agonist exposure in cohort 2.
Testosterone levels are detailed in Table 2. The median peak testosterone levels at baseline were in the normal physiologic range(≥ 150 ng/dl) for all but one patient, and in the normal physiologic range for all patients post-androgen repletion on both the 21-day and 28 -day schedules with 3 and 5 days of androgen repletion respectively. Trough values prior to testosterone repletion were all ≤50 ng/dL.
Table 2.
Testosterone levels (ng/dL) in evaluable patients
| Cohort 1 (6 Cycles) | Cohort 2 (9 Cycles) | |||
|---|---|---|---|---|
| Rising PSA | Metastatic | Rising PSA | Metastatic | |
| n=25 | n=38 | n=17 | n=21 | |
| Baseline, median (range) | 354 (210–575) | 317 (169–579) | 309 (188–466) | 316 (148–475) |
| Peaka, median (range) | 452 (47–898) | 462 (148–920) | 378 (65–723) | 277 (104–732) |
| Troughb, median (range) | 22 (10–41) | 19 (10–38) | 12 (10–36) | 10 (10–50) |
Peak: Day 1 (following testosterone repletion)
Trough: Cohort 1, Day 21 or Cohort 2, Day 19 (before testosterone repletion)
The PSA outcomes of evaluable patients at 6 and 18 months after the start of treatment are shown in Table 3. None of the patients in cohort 1 had an undetectable PSA at 18 months, while 5 patients (13%) in cohort 2 did (3 rising PSA and 2 with clinical metastases), which has been durable for a median of 23+ (range 18 +–26+) months. Imaging abnormalities have also resolved. Of these 5 patients, 3 had undergone a prostatectomy (1 with salvage radiation), and 2 had received primary radiation.
Table 3.
Evaluable patient outcomes and time to progression
| Cohort 1 | Cohort 2 | Total | |||
|---|---|---|---|---|---|
| Rising PSA | Metastatic | Rising PSA | Metastatic | ||
|
Treatment-specific undetectable PSA outcome | |||||
| No. evaluable | 25 | 37 | 17 | 21 | 100 |
| 6 monthsa, No. (%) | 9 (36%) | 14 (38%) | 13 (76%) | 12 (57%) | 48 (48%) |
| 18 months, No. (%) | 0 | 0 | 3 (18%) | 2 (10%) | 5b (5%) |
|
Time to progression | |||||
|---|---|---|---|---|---|
| Rising PSA | Metastatic | Rising PSA | Metastatic | Total | |
| No. evaluable | 25 | 37 | 14 | 19 | 95c |
| Median, months | 9 | 10 | 14 | 13 | 13 |
| Range, months | 8–15 | 8–14 | 11–19 | 6–21 | 8–21 |
6 28-day docetaxel cycles for cohort 1 and 9 21-day docetaxel cycles for cohort 2.
All 5 patients achieved an undetectable PSA by cycle 6.
5 patients in cohort 2 remain on study at a median of 23+ months (range 18+ to 26+)
Toxicity
As expected, Grade 1 and 2 toxicities in both cohorts included fatigue, hot flashes, and peripheral neuropathy(Table 4). Hyperglycemia was likely attributable to dexamethasone premedication. Grade 3 or 4 neutropenia (58% vs. 56%, respectively) and febrile neutropenia (14% vs. 18%)were similar between cohorts. Tthe duration of neutropenia was <7 days in the majority of patients and. no documented infections occurred. Eleven patients received pegylated filgastrim for febrile and/or recurrent episodes of grade 3 or 4 neutropenia.
Table 4.
Adverse events
| A. Cohort 1 (n=63) | |||||
|---|---|---|---|---|---|
| Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
| Fatigue | 54 (86%) | 3(5%) | 0 (0%) | 0 (0%) | 57 (91%) |
| Hot flashes | 37 (59%) | 4 (6%) | 0 (0%) | 0 (0%) | 41 (65%) |
| Neuropathy | 26 (41%) | 0 (0%) | 0 (0%) | 0 (0%) | 26 (41%) |
| Febrile Neutropenia | 0 (0%) | 0 (0%) | 4 (6%) | 5 (8%) | 9 (14%) |
| Neutropenia | 1 (2%) | 3 (5%) | 9 (14%) | 28 (44%) | 41 (65%) |
| Hyperglycemia | 15 (24%) | 25 (40%) | 14 (22%) | 0 (0%) | 54 (86%) |
| Mental Status Change | 0 (0%) | 0 (0%) | 1 (1.6%) | 0 (0%) | 1 (1.6%) |
| B. Cohort 2 (n=39) | |||||
|---|---|---|---|---|---|
| Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
| Fatigue | 33 (85%) | 5 (13%) | 0 (0%) | 0 (0%) | 38 (98%) |
| Hot flashes | 29 (74%) | 1 (3%) | 0 (0%) | 0 (0%) | 30 (77%) |
| Neuropathy | 23 (59%) | 2 (5%) | 0 (0%) | 0 (0%) | 25 (64%) |
| Febrile Neutropenia | 0 (0%) | 0 (0%) | 6 (15%) | 1 (3%) | 7 (18%) |
| Neutropenia | 0 (0%) | 5 (13%) | 6 (15%) | 16 (41%) | 27 (70%) |
| Hyperglycemia | 10 (26%) | 20 (51%) | 8 (21%) | 0 (0%) | 38 (98%) |
CYP3A Activity and Docetaxel Pharmacokinetics
CYP3A4 activity was not altered by androgen cycling. Mean erythromycin breath test C20min parameter values (% dose/min) were 0.044 (standard deviation [SD] 0.0082) before cycle 1 and 0.044 (SD 0.0091) before cycle 2 (P = 0.87). Likewise, androgen cycling did not alter docetaxel pharmacokinetic parameters. Docetaxel pharmacokinetic parameters for cycles 1 and 2 are summarized in Table 5. Docetaxel clearance was similar during cycles 1 and 2 with mean values of 23.9 L/h (SD 12.1) and 23.6 L/h (SD 7.43), respectively (P = 0.88). No differences were observed between cycles 1 and 2 for other docetaxel pharmacokinetic parameters listed in Table 5 (P >0.05).
Table 5.
CYP3A4 activity and docetaxel pharmacokinetics during cycles 1 and 2.
| Parameter | Cycle 1, mean (SD) | Cycle 2, mean (SD) | P-value |
|---|---|---|---|
| ERMBT C20min, %dose/min | 0.044 (0.0082) | 0.044 (0.0091) | 0.8734 |
| Cmax, μg/mL | 4.83 (1.60) | 4.86 (1.55) | 0.9060 |
| AUCinf, μg/mL*h | 7.82 (3.01) | 7.25 (2.12) | 0.5561 |
| CL, L/h | 23.9 (12.1) | 23.6 (7.43) | 0.8822 |
| t1/2,γ, h | 86.2 (37.5) | 65.8 (15.0) | 0.0554 |
| Vss, L | 871 (433) | 667 (189) | 0.1513 |
Abbreviations: ERMBT, erythromycin breath test; C20min, flux of 14 CO2 at 20 min after administration of the ERMBT; Cmax, maximum plasma concentration; AUCinf, area under the concentration-time curve from time zero to infinity; CL, clearance; t1/2,γ, gamma half-life; Vss, volume of distribution at steady-state, SD, standard deviation.
DISCUSSION
Improving the outcomes that can be achieved with androgen depletion alone has been a focus of research for over six decades.16,17 The efforts have accelerated with the demonstration that docetaxel can prolong the lives of patients with progressive castration-resistant disease, and several groups have reported combinations of androgen depletion and docetaxelin localized disease as neo-adjuvant or adjuvant therapy, and separately in patients with a rising PSA after local therapy with or without metastatic disease.9 11 This study is the first to evaluate docetaxel with rapid androgen cycling in non-castrate patients with prostate cancer. Overall, we observed a treatment -specific undetectable PSA in 48% of patients(48 of 100) at 6 months, which was not durable for any of the patients in cohort 1, but has been maintained for a median of 23+ months (range 18+ to 26+) in 13% (5 of 38) of patients treated in cohort 2. Although neither cohort showed sufficient activity to be pursued further, the results show how the treatment-specific undetectable PSA endpoint can be used to rapidly screen approaches in this patient group.
The trial builds upon our phase I study of rapid androgen cycling which showed that serially declining PSA peaks and troughs to undetectable levels could be achieved.4 The design extrapolates from studies in other tumor types showing that a chemotherapy regimen that is not curative in advanced disease can increase cure rates in patients with minimal tumor burdens. We hypothesized that administering chemotherapy to quiescent non-proliferating cells would be of limited value, a concept supported by recent data showing that androgen receptor activation significantly enhanced the response to docetaxel in androgen receptor (AR) proficient cells that were actively cycling. The mitogenic effects of androgen were distinct from an effect on PSA secretion, while the proapoptotic effects of docetaxel were reduced significantly by the coadministration of an AR antagonist.18 Separately, a microarray analysis of human radical prostatectomy specimens removed following neoadjuvant docetaxel showed a coordinate increase in the enzymes associated with androgen catabolism and a decrease in the levels of those associated with androgen synthesis. The net result was a decrease in androgen bioavailability, and reduced androgen signaling.19 Repetitive treatments with docetaxel reduced the anti-proliferative effects of androgen depletion even when testosterone levels were in the non-castrate range.
To identify strategies likely to succeed in the phase III setting, it is essential to establish a clinical regimen and define a relevant endpoint that allows the results to be placed in the context of other phase II trials in the same patient group. We have focused our phase II efforts on patients who have a PSA recurrence alone or PSA recurrence with clinical metastases after definitive local treatment, using the unambiguous endpoint of a treatment-specific undetectable PSA nadir and non -castrate levels of testosterone levels.13 This endpoint allows patients and physicians alike to clearly demarcate those who have had a complete response, a prerequisite for cure, versus those with residual disease evidenced by detectable PSA values. The endpoint was derived from our work showing that the likelihood of achieving an undetectable PSA varies as a function of disease extent and the presence or absence of detectable disease on scans.13 The endpoint has also been shown to provide prognostic significance in an interim analysis of patients treated with androgen depletion for a rising PSA following primary therapy20 and separately in a Southwest Oncology Group phase III trial of intermittent androgen depletion for patients with non-castrate clinical metastases.21 Both analyses showed that patients who did not achieve an undetectable PSA nadir following androgen depletion had an increased risk of prostate cancer-specific mortality.
The results of the current study show how the undetectable PSA endpoint can be utilized in the clinic. In cohort 1, 36% (9 of 25, 95% confidence interval 20–55%) of patients with a rising PSA, and 38% (14 of 37, 95% confidence interval 24–54%) of those with clinical metastases achieved an undetectable PSA at 6 months, but responses were not durable. Consequently, the docetaxel schedule was shortened to every 21-days, (consistent with the approved standard),22,23 the number of cycles was increased from 6 to 9, the period of androgen depletion increased from 24 to 36 weeks, and the duration of testosterone administration reduced from 7 days to 3. The rationale for the latter was based on studies in transgenic mouse models showing that after testosterone repletion the proliferation rates of quiescent prostate cancer cells peak at 3 days and decline to baseline after 7 to 20 days.3
In cohort 2, 18 of 28 patients achieved the endpoint after 6 docetaxel cycles, and an additional 7 patients achieved the endpoint after 9 docetaxel cycles. At 18 months, none of the patients in cohort 1, and 5 (13%) in cohort 2, had undetectable PSA levels. While it is possible that the additional 3 cycles of docetaxel and 36 vs 24 weeks of GnRH a gonist exposure contributed to the durability of outcomes in cohort 2, it is noteworthy that all of the patients with an undetectable PSA at 18 months had achieved it by 6 cycles of treatment. This suggests that the higher dose intensity of a 21 vs. 28 day cycle, and shorter duration of testosterone repletion, was most contributory to the improved outcomes.
The adverse events were similar to those observed with androgen depletion and chemotherapy administered separately.21 Noteworthy, however, was the incidence of febrile neutropenia in this population (14 – 18%) which was higher than that observed in patients with castration resistant disease (3%) or advanced non-small cell lung cancer (6%) using comparable docetaxel schedules (75 mg/m23 -weekly).22 24 This may be explained in part by the approximately 50% reduction in clearance and increase in AUC for docetaxel in the non-castrate population enrolled in the current trial, relative to a group with castration-resistant disease.25 The potential significance of these changes was shown in a population pharmacokinetic-pharmacodynamic model for docetaxel in which a 27% reduction of docetaxel clearance was associated with a 1.5-fold increase in the odds of developing febrile neutropenia.26
Two trials have explored docetaxel and androgen depletion for patients with a rising PSA and non-castrate testosterone levels. In one study, after six cycles of docetaxel followed by 12–20 months of androgen depletion, 5 of 39 (12%) patients achieved a serum PSA of 0.1 ng/mL following treatment although testosterone levels were not reported. 21 This response correlated with our results at 18 months in cohort 2. A similar study administering four cycles of estramustine and docetaxel followed by 15 months of androgen depletion, reported 20 of 56 (36%) patients achieving an undetectable PSA at one year after completion of therapy. It is of note that this patient cohort did not include metastatic or untreated primary disease which may have contributed to the relatively high response rate 11
To demonstrate that an intervention in the non-castrate state delays disease progression or prolongs life can be difficult because of patient heterogeneity and an often protracted natural history of disease. This study shows how an objective, unambiguous, and reproducible endpoint that can be assessed in a reasonable timeframe, can be used to screen different treatment approaches. Given the low durable response rate and increased toxicity, we would not recommend this regimen for further study. Future trials must continue to seek to improve upon standard hormonal regimens, using endpoints appropriate to both the disease state under study and individual patient risk. Towards that end, we plan to utilize the undetectable PSA endpoint in a randomized phase III study of androgen depletion with or without docetaxel for patients with a rapid PSA doubling time and high risk of cancer-specific mortality.
Acknowledgments
Grant Support: Prostate Cancer Foundation, P50-CA92629 SPORE in Prostate Cancer grant from the National Cancer Institute, Sanofi-Aventis Grant in Aid #16143 for Investigator Sponsored Trial #16143), DOD Consortium
Footnotes
The material in this work has previously been presented in abstract format at the American Society of Clinical Oncology Genitourinary and Annual Symposiums.
References
- 1.Agus DB, Cordon-Cardo C, Fox W, et al. Alterations of cell cycle regulators in prostate cancer: response to androgen withdrawal and development of androgen independence. J Natl Cancer Inst. 1999;91:1869–76. doi: 10.1093/jnci/91.21.1869. [DOI] [PubMed] [Google Scholar]
- 2.Ohlson N, Wikstrom P, Stattin P, et al. Cell proliferation and apoptosis in prostate tumors and adjacent non-malignant prostate tissue in patients at different time-points after castration treatment. Prostate. 2005;62:307–15. doi: 10.1002/pros.20139. [DOI] [PubMed] [Google Scholar]
- 3.Shaffer DR, Viale A, Ishiwata R, et al. Evidence for a p27 tumor suppressive function independent of its role regulating cell proliferation in the prostate. Proc Natl Acad Sci U S A. 2005;102:210–5. doi: 10.1073/pnas.0407362102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feltquate D, Nordquist L, Eicher C, et al. Rapid androgen cycling as treatment for patients with prostate cancer. Clin Cancer Res. 2006;12:7414–21. doi: 10.1158/1078-0432.CCR-06-1496. [DOI] [PubMed] [Google Scholar]
- 5.Scher HI, Heller G. Clinical states in prostate cancer: towards a dynamic model of disease progression. Urology. 2000;55:323–327. doi: 10.1016/s0090-4295(99)00471-9. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Halford S, Rigg A, et al. Adjuvant mitozantrone chemotherapy in advanced prostate cancer. BJU Int. 2000;86:675–80. doi: 10.1046/j.1464-410x.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- 7.DiPaola RS, Chenven ES, Shih WJ, et al. Mitoxantrone in patients with prostate specific antigen progression after local therapy for prostate carcinoma. Cancer. 2001;92:2065–71. doi: 10.1002/1097-0142(20011015)92:8<2065::aid-cncr1546>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Vaishampayan U, Fontana J, Du W, et al. Phase II trial of estramustine and etoposide in androgen-sensitive metastatic prostate carcinoma. Am J Clin Oncol. 2004;27:550–4. doi: 10.1097/01.coc.0000135922.12198.e4. [DOI] [PubMed] [Google Scholar]
- 9.Hussain A, Dawson N, Amin P, et al. Docetaxel followed by hormone therapy in men experiencing increasing prostate-specific antigen after primary local treatments for prostate cancer. J Clin Oncol. 2005;23:2789–96. doi: 10.1200/JCO.2005.07.152. [DOI] [PubMed] [Google Scholar]
- 10.Goodin S, Medina P, Capanna T, et al. Effect of docetaxel in patients with hormone -dependent prostate-specific antigen progression after local therapy for prostate cancer. J Clin Oncol. 2005;23:3352–7. doi: 10.1200/JCO.2005.11.111. [DOI] [PubMed] [Google Scholar]
- 11.Taplin ME, Xie W, Bubley GJ, et al. Docetaxel, estramustine, and 15-month androgen deprivation for men with prostate-specific antigen progression after definitive local therapy for prostate cancer. J Clin Oncol. 2006;24:5408–13. doi: 10.1200/JCO.2006.06.6589. [DOI] [PubMed] [Google Scholar]
- 12.Mackler NJ, Pienta KJ, Dunn RL, et al. Phase II evaluation of oral estramustine, oral etoposide, and intravenous paclitaxel in patients with hormone-sensitive prostate adenocarcinoma. Clin Genitourin Cancer. 2007;5:318–22. doi: 10.3816/CGC.2007.n.010. [DOI] [PubMed] [Google Scholar]
- 13.Beekman K, Morris M, Slovin S, et al. Androgen deprivation for minimal metastatic disease: threshold for achieving undetectable prostate-specific antigen. Urology. 2005;65:947–52. doi: 10.1016/j.urology.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Baker SD, van Schaik RH, Rivory LP, et al. Factors affecting cytochrome P-450 3A activity in cancer patients. Clin Cancer Res. 2004;10:8341–50. doi: 10.1158/1078-0432.CCR-04-1371. [DOI] [PubMed] [Google Scholar]
- 15.Baker SD, Zhao M, He P, et al. Simultaneous analysis of docetaxel and the formulation vehicle polysorbate 80 in human plasma by liquid chromatography/tandem mass spectrometry. Anal Biochem. 2004;324:276–84. doi: 10.1016/j.ab.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 16.Huggins C, Hodges CV. Studies on prostatic cancer I. the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:193–197. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 17.Huggins C, Stevens RE, Jr, Hodges CV. Studies on prostatic cancer. II. The effect of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–223. [Google Scholar]
- 18.Hess-Wilson JK, Daly HK, Zagorski WA, et al. Mitogenic action of the androgen receptor sensitizes prostate cancer cells to taxane-based cytotoxic insult. Cancer Res. 2006;66:11998–2008. doi: 10.1158/0008-5472.CAN-06-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Febbo PG, Richie JP, George DJ, et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2005;11:5233–40. doi: 10.1158/1078-0432.CCR-05-0299. [DOI] [PubMed] [Google Scholar]
- 20.Stewart AJ, Scher HI, Chen MH, et al. Prostate-specific antigen nadir and cancer-specific mortality following hormonal therapy for prostate-specific antigen failure. J Clin Oncol. 2005;23:6556–60. doi: 10.1200/JCO.2005.20.966. [DOI] [PubMed] [Google Scholar]
- 21.Hussain M, Tangen CM, Schellhammer PF, et al. Absolute PSA value after androgen deprivation (AD) is a strong independent predictor of survival in new metastatic (D2) prostate cancer (PCa): Data from the Southwest Oncology Group Trial 9346 (INT-0162). Proc Am Soc Clin Onc; 2006. p. 18S. (Abstract # 4517) [DOI] [PubMed] [Google Scholar]
- 22.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 23.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 24.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 25.Sinibaldi VJ, Elza-Brown K, Schmidt J, et al. Phase II evaluation of docetaxel plus exisulind in patients with androgen independent prostate carcinoma. Am J Clin Oncol. 2006;29:395–8. doi: 10.1097/01.coc.0000225411.95479.b4. [DOI] [PubMed] [Google Scholar]
- 26.Bruno R, Hille D, Riva A, et al. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16:187–96. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]