Abstract
Background
Perioperative mortality is reported after abdominal aortic aneurysm (AAA) repair, but there is no agreed upon standard definition. Often, thirty-day mortality is reported because in-hospital mortality may be biased in favor of endovascular repair given the shorter length of stay. However, the duration of increased risk of death following aneurysm repair is unknown.
Study Design
We used propensity score modeling to create matched cohorts of US Medicare beneficiaries undergoing endovascular (n=22,830) and open (n=22,830) AAA repair from 2001–2004. We calculated perioperative mortality using several definitions including in-hospital, 30 day, and combined 30 day and in-hospital mortality. We determined the relative risk (RR) of death after open compared to endovascular repair as well as the absolute mortality difference. To define the duration of increased risk we calculated bi-weekly interval death rates for 12 months.
Results
In-hospital, 30 day, and combined 30 day and in-hospital mortality for open and endovascular repair were 4.6% vs. 1.1%, 4.8% vs. 1.6%, and 5.3% vs. 1.7% respectively. The absolute differences in mortality were similar at 3.5%, 3.2%, and 3.7%. The RR of death [95% confidence interval] was 4.2 [3.6–4.8], 3.1 [2.7–3.4], and 3.2 [2.8–3.5]. Bi-weekly interval death rates were highest during the first month after endovascular repair (0.6%), and during the first 2.5 months (0.5–2.1%) after open repair. After 2.5 months, rates were similar for both repairs (<0.5%) and stabilized after 3 months. The 90 day mortality for open and endovascular repair was 7.0% and 3.2% respectively.
Conclusions
In-hospital mortality comparisons overestimate the benefit of endovascular repair compared to 30 day or combined 30 day and in-hospital mortality. The total mortality impact of AAA repair is not realized until 3 months after repair and the duration of highest mortality risk extends longer for open repair.
Introduction
The “risk” of endovascular aneurysm repair (EVAR) or open surgical abdominal aortic aneurysm (AAA) repair may be defined in several ways. Typically perioperative mortality is the primary outcome reported. However, this may be defined as death during the initial hospitalization, within 30 days of surgery, or the union of the two – all deaths within 30 days plus any deaths beyond 30 days that occur prior to discharge from the hospital. Administrative datasets typically report only in-hospital mortality because many lack comprehensive follow-up data [1, 2]. Registry studies and some clinical trials typically only report 30 day mortality, whereas other clinical trials and single center studies report the combined 30 day and in-hospital mortality [3, 4]. This lack of uniformity hampers comparison of studies. Moreover, since length of stay is shorter after EVAR, measurement limited to in-hospital mortality may result in findings that are biased in favor of EVAR. As EVAR is being increasingly utilized to repair AAA, combined 30 day and in-hospital mortality may be a more appropriate measure of perioperative mortality.
Additionally, it is currently unknown to what extent any ongoing “risk” due to surgery persists beyond 30 days or hospital discharge and whether the duration of elevated risk is similar for EVAR and open repair. Such information would be useful to both patients and physicians in their decision making process. To address these issues and more accurately describe perioperative risk we determined mortality at a variety of time points after EVAR and open AAA repair in a matched comprehensive sample of US Medicare patients.
Methods
Patients
Medicare patients undergoing intact AAA repair during 2001–2004 were identified from Medicare Part A files. We required 2 years of Medicare enrollment prior to the AAA repair to allow for assignment of preoperative comorbidity based on prior inpatient and outpatient claims. We identified all patients age 67 or greater with a diagnosis of intact AAA (ICD-9 441.4) and a procedure code for open surgical AAA repair (38.44, 38.25) or EVAR (39.71). We excluded those with a diagnosis of AAA rupture (441.3), aortic dissection (441.0*), thoracic or thoracoabdominal aneurysm (441.1, 441.2, 441.6, 441.7), or unspecified aortic aneurysm (441.5, 441.9); and those with procedure codes for repair of the thoracic aorta (38.35, 38.45, 39.73), visceral/renal bypass (38.46, 39.24, 39.36; or CPT 35531, 35560, 35631), or thoracoabdominal or suprarenal AAA repair (33877, 35091, 35092). We required participation in both Medicare Part A and B and excluded those enrolled in health maintenance organizations because their claims data are not available. Procedures were classified as EVAR or open repair based on both physician and hospital claims. In those instances where physician and hospital claims differed (<5.5%), we assigned patients based on physician claims as we felt this would be more accurate.
Creating Matched Cohorts
We created matched cohorts of patients to control for the nonrandom assignment of patients to one of the two procedural groups. Logistic-regression models were used to predict the likelihood of EVAR (the propensity score). We used all available demographic and clinical characteristics for beneficiaries at baseline as explanatory variables. These data were obtained from claims during the 2-year period before the repair was performed, not including the index admission. We measured the rates of coexisting conditions using a version of the Elixhauser algorithm that was adapted to also include diagnoses made in the outpatient setting [5]. We matched each beneficiary who underwent EVAR to the beneficiary who underwent open repair with the closest estimated propensity score. To ensure close matches we required that the estimated logs-odds scores for endovascular repair of a patient who underwent endovascular repair and one who underwent open repair be within 0.60 SD of one another. This requirement ensures the removal of approximately 90% of the bias in estimates of effects due to differences in covariate distributions between the endovascular repair group and the open repair group [6, 7]. The algorithm and cohort have been described in detail previously [8–11].
Mortality
Date of death was determined using the Medicare denominator file and is complete through 2008. In-hospital mortality was defined as death during the initial hospitalization at the institution in which the AAA repair occurred (i.e. does not include death after transfer to a second acute care facility). Thirty-day mortality was defined as death within 30 days of AAA repair based on the date of surgery from Part A claims and date of death from the denominator file. The final definition of perioperative mortality included death either within 30 days of surgery or within the initial hospitalization (i.e. for hospitalizations that lasted > 30 days).
To determine whether the increased risk of death due to surgery persisted beyond the perioperative period, we also plotted mortality out to 1 year. We calculated and plotted bi-weekly interval death rates – the proportion of the surviving cohort that died within each 2 week interval. This allowed us to determine when the interval death rate decreased to a stable rate representing the end of the period of increased risk of death due to the surgical procedure.
We determined the absolute mortality difference between open repair and EVAR for the entire group as well as within age groups. We calculated the relative risk (RR) of death for open surgery compared to EVAR and associated 95% confidence interval (CI) for each of the definitions of perioperative mortality. Survival was compared using a log-rank test. The p-value was determined using a chi-square distribution with the appropriate degrees of freedom. P<0.05 was considered significant. We did this for the group overall and after stratifying by age. We modeled survival conditional on age and performed a pooled analysis as our matched pairs are sample induced clusters as opposed to true clusters. Mortality rates were compared over time and by age group. The institutional review board at Harvard Medical School determined that this study was exempt from review.
Results
We identified 61,598 patients undergoing repair of intact AAA from 2001–2004. Prior to propensity matching, patients who underwent endovascular repair were older and sicker than patients who underwent open repair. Table 1 includes the demographic and comorbidity characteristics of the pre- and post-propensity matched patients. After propensity score matching there were 45,660 patients (22,830 EVAR and 22,830 open).
Table 1.
Baseline Characteristics of Medicare Beneficiaries Undergoing Endovascular Repair or Open Repair of Abdominal Aortic Aneurysm (2001–2004), before and after Matching for Propensity Score
| Unmatched Cohort | Matched Cohort | |||||
|---|---|---|---|---|---|---|
| EVAR (n=29,542) | Open (n=32,056) | p Value | EVAR (n=22,830) | Open (n=22,830) | p Value | |
| Male sex | 83.2% | 74.6% | <0.001 | 80.3% | 80.6% | 0.38 |
| Age, y | ||||||
| 67–69 | 11.9% | 15.6% | <0.001 | 13.9% | 13.9% | 0.94 |
| 70–74 | 26.8% | 32.0% | <0.001 | 29.7% | 29.7% | 1.00 |
| 75–79 | 35.7% | 35.3% | 0.41 | 36.4% | 36.5% | 0.85 |
| 80–84 | 15.8% | 12.2% | <0.001 | 13.9% | 14.0% | 0.71 |
| ≥ 85 | 9.8% | 4.9% | <0.001 | 6.2% | 6.0% | 0.30 |
| White race | 95.8% | 95.6% | 0.16 | 95.8% | 95.9% | 0.67 |
| Comorbidities | ||||||
| Myocardial Infarction within past 6 mo | 1.9% | 1.8% | 0.73 | 1.9% | 1.8% | 0.48 |
| Myocardial Infarction within past 7–24 mo | 9.1% | 7.1% | <0.001 | 8.0% | 8.0% | 0.96 |
| Valvular heart disease | 12.2% | 9.6% | <0.001 | 10.9% | 10.5% | 0.17 |
| Congestive heart failure | 16.1% | 11.6% | <0.001 | 13.3% | 13.1% | 0.58 |
| Peripheral vascular disease | 21.2% | 21.4% | 0.53 | 21.3% | 20.8% | 0.22 |
| Cerebrovascular disease | 16.1% | 16.8% | 0.02 | 16.4% | 16.3% | 0.86 |
| Hypertension | 67.1% | 65.0% | <0.001 | 66.2% | 65.8% | 0.40 |
| Diabetes mellitus | 17.8% | 14.3% | <0.001 | 15.7% | 15.7% | 1.00 |
| Chronic obstructive pulmonary disease | 30.8% | 28.9% | <0.001 | 29.7% | 29.7% | 0.98 |
| Renal disease | 5.1% | 4.1% | <0.001 | 4.6% | 4.4% | 0.28 |
| End-stage renal disease | 0.6% | 0.3% | <0.001 | 0.3% | 0.3% | 1.00 |
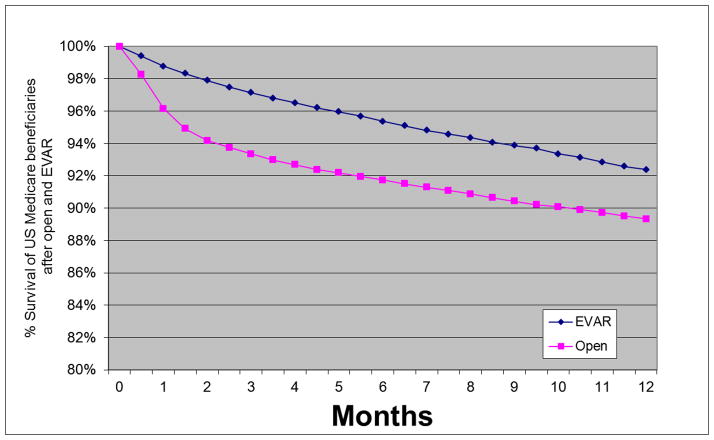
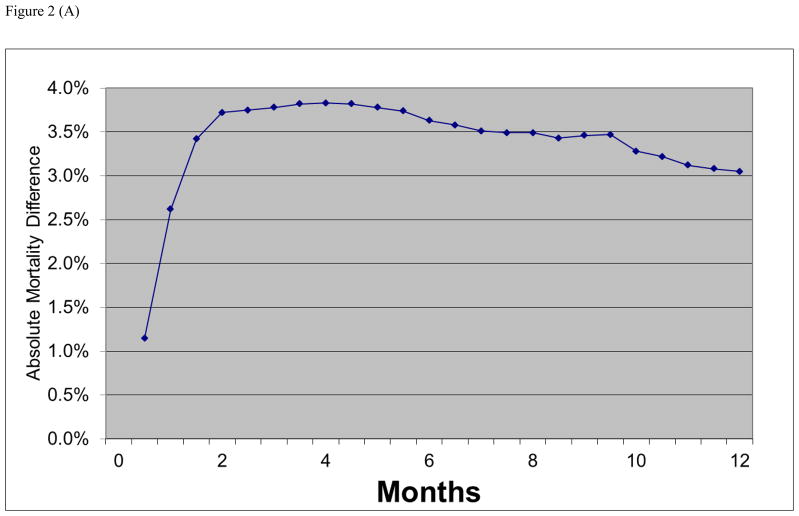
In-hospital mortality was 4.6% vs. 1.1% (p<0.001) for open repair and EVAR respectively (Table 1). Thirty day mortality was 4.8% vs. 1.6% (p<0.001), and combined in-hospital and 30 day mortality was 5.3% vs. 1.7% (p<0.001). EVAR mortality was significantly less than open repair mortality using all definitions. The absolute difference in mortality between open repair and EVAR remained relatively constant between the 3 definitions (3.5%, 3.2%, and 3.7% respectively). The absolute difference in mortality between open and EVAR was greatest between 2–4 months and then slowly declined over time. This is seen in the plot of early survival after EVAR and open AAA repair (Figure 1) as well as more directly in the plot of the absolute mortality difference between EVAR and open repair over time (Figure 2a).
Figure 1.
Survival through 1 year in US Medicare beneficiaries undergoing open and endovascular abdominal aortic aneurysm repair from 2001 to 2004.
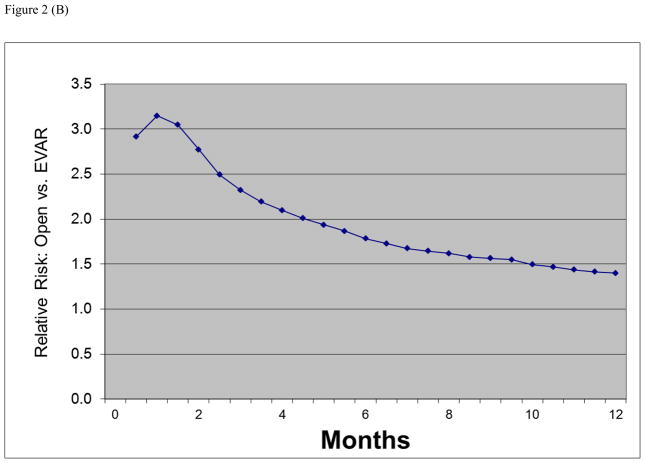
Figure 2.
(A) Absolute mortality differences during follow-up between open versus endovascular abdominal aortic aneurysm repair. (B) Relative risk of death after open versus endovascular abdominal aortic aneurysm repair in United States Medicare beneficiaries undergoing abdominal aortic aneurysm repair from 2001 to 2004.
The relative risk of mortality with open repair versus EVAR was higher using in-hospital mortality (RR 4.2, 95% CI 3.6–4.8) compared to 30 day mortality (RR 3.1, 95% CI 2.7–3.4) or combined 30 day and in-hospital mortality (RR 3.2, 95% CI 2.8–3.5). The relative risk then gradually declined over time as follow-up was extended, to a RR of 2.2 at 90 days and 1.8 at 6 months (Figure 2b).
The absolute difference in mortality between the two methods increased with age from approximately 2% for those age 67–69, to over 8% for those age > 85, but did not vary markedly by definition of perioperative mortality (Table 2). The RR of open repair vs. EVAR also was consistent across all age groups at each defined time point for each measure of mortality.
Table 2.
Perioperative Mortality after Open and Endovascular (EVAR) Abdominal Aortic Aneurysm Repair in US Medicare Beneficiaries Defined as In-Hospital, 30 day, Combined 30 day and In-Hospital, and 90 day Mortality
| EVAR (n=22,830) | Open (n=22,830) | p Value | Risk difference | Relative Risk | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
|
In-Hospital Mortality | ||||||
| All ages | 1.1% | 4.6% | <.001 | 3.5% | 4.2 | 3.6 to 4.8 |
| Age 67–69 | 0.4% | 2.3% | <.001 | 1.9% | 5.7 | 3.2 to 10.2 |
| Age 70–74 | 0.8% | 3.2% | <.001 | 2.4% | 4.0 | 3.0 to 5.4 |
| Age 75–79 | 1.3% | 4.7% | <.001 | 3.5% | 3.7 | 3.0 to 4.6 |
| Age 80–84 | 1.5% | 7.0% | <.001 | 5.5% | 4.8 | 3.5 to 6.6 |
| Age ≥85 | 2.5% | 10.8% | <.001 | 8.4% | 4.4 | 3.1 to 6.3 |
|
30 Day Mortality | ||||||
| All ages | 1.6% | 4.8% | <.001 | 3.2% | 3.1 | 2.7 to 3.4 |
| Age 67–69 | 0.8% | 2.6% | <.001 | 1.8% | 3.4 | 2.2 to 5.4 |
| Age 70–74 | 1.1% | 3.4% | <.001 | 2.4% | 3.2 | 2.5 to 4.1 |
| Age 75–79 | 1.7% | 4.6% | <.001 | 2.9% | 2.7 | 2.2 to 3.3 |
| Age 80–84 | 2.0% | 7.4% | <.001 | 5.4% | 3.7 | 2.8 to 4.8 |
| Age ≥85 | 3.9% | 11.5% | <.001 | 7.6% | 3.0 | 2.2 to 4.0 |
|
In-Hospital & 30 Day Mortality | ||||||
| All ages | 1.7% | 5.3% | <.001 | 3.7% | 3.2 | 2.8 to 3.5 |
| Age 67–69 | 0.8% | 2.8% | <.001 | 2.0% | 2.4 | 1.5 to 3.7 |
| Age 70–74 | 1.1% | 3.8% | <.001 | 2.6% | 3.3 | 2.6 to 4.3 |
| Age 75–79 | 1.8% | 5.3% | <.001 | 3.5% | 2.9 | 2.4 to 3.5 |
| Age 80–84 | 2.3% | 8.2% | <.001 | 5.9% | 3.6 | 2.8 to 4.6 |
| Age ≥85 | 4.2% | 12.7% | <.001 | 8.5% | 3.1 | 2.3 to 4.1 |
|
90 Day Mortality | ||||||
| All ages | 3.2% | 7.0% | <.001 | 3.8% | 2.2 | 2.0 to 2.4 |
| Age 67–74 | 2.2% | 4.4% | <.001 | 2.2% | 2.0 | 1.7 to 2.4 |
| Age 75–84 | 3.6% | 8.1% | <.001 | 4.6% | 2.3 | 2.0 to 2.6 |
| Age ≥85 | 7.6% | 16.8% | <.001 | 9.2% | 2.2 | 1.8 to 2.8 |
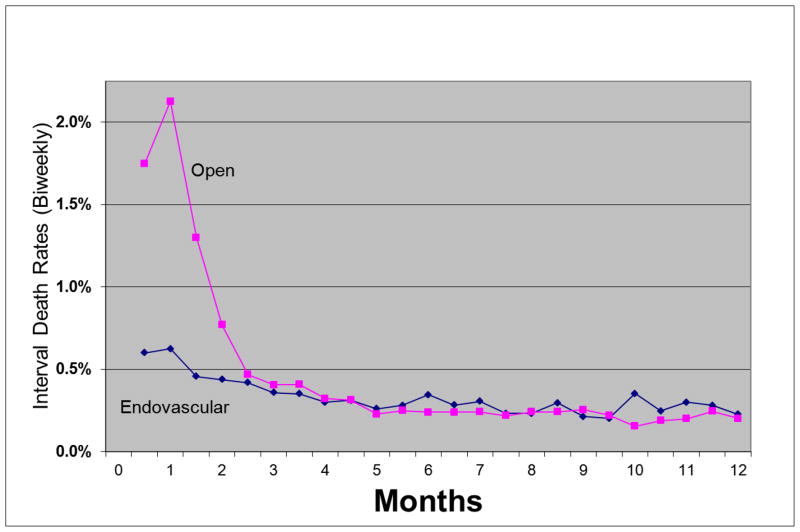
Bi-weekly interval death rates peaked at 1 month for both EVAR and open repair (Figure 3). After EVAR, the interval death rate dropped below 0.5% at 1.5 months whereas rates remained high (above 0.5%) after open repair for 2.5 months. After 2.5 months, interval death rates were similar for EVAR and open repair. The interval death rates continued to decline until approximately 3 months for EVAR and open repair after which they appeared to stabilize. The curve suggests that the highest risk of death due to AAA repair persists for 1.5 months after EVAR and 2.5 months after open repair and that the risk in the perioperative period would be underestimated using the conventional definitions used in most current reports. The total period of elevated risk extends beyond 30 days and persists for approximately 3 months after both EVAR and open AAA repair.
Figure 3.
Deaths occurring within bi-weekly intervals after open and endovascular abdominal aortic aneurysm repair in US Medicare beneficiaries undergoing abdominal aortic aneurysm repair from 2001 to 2004.
Discussion
In this study we compared commonly used definitions of perioperative mortality to see if the definition used could influence the findings of studies or impressions about the benefits of particular procedures. Our study has two notable findings that could influence the design of future comparative effectiveness studies of AAA repair, and if confirmed, of other surgical procedures. First, the perioperative mortality benefit of EVAR is confirmed using all common definitions of perioperative mortality and this finding is not sensitive to the definition of perioperative mortality used. The use of in-hospital mortality, however, overestimates the relative benefit of EVAR compared to the measures that include post-discharge follow-up. Second, our data suggest that none of the commonly used measures fully captures the excess surgical mortality associated with the perioperative period. The excess risk associated with open surgery does not appear to decline to the baseline trend until approximately 3 months. Thus, routine inclusion of three-month follow-up data will likely provide a more accurate picture of the results of interventions.
Comparative effectiveness studies of EVAR vs. open repair are commonly reported using one or more of the definitions we evaluated. Randomized trials have used 30 day as well as 30 day plus in-hospital mortality. The DREAM trial (n=345) found lower 30 day mortality with EVAR compared to open repair (1.2% vs. 4.6%) which did not reach statistical significance (p=0.10). The odds ratio (OR) was 3.9% (95% CI: 0.9–32.9) [12]. The EVAR 1 trial (n=1047) found significantly lower mortality with EVAR at both 30 days (1.7% vs. 4.7%, p=0.016, OR: 2.86 [95% CI: 1.21–5.88]) and for 30 day and in-hospital mortality (2.1% vs. 6.2%, p=0.0001, OR: 3.12 [95% CI: 1.61–7.14]) [13]. The recently published OVER trial (n=881) found significantly lower mortality with EVAR both at 30 days (0.2% vs. 2.3%, p=0.006, OR: 10.4 [95% CI: 1.32–81.4]) and within 30 days or in-hospital (0.5% vs. 3.0%, p=0.004, OR: 6.78 [95% CI: 1.52–30.2]) [14]. None of these studies, however, reported data at the three month mark, which might be a more accurate representation of the perioperative differences.
The use of different measures of perioperative mortality has implications for both clinicians and patients as they make decisions about AAA repair as well as researchers comparing the effectiveness of different treatments. When clinicians discuss the options available to patients, they need to counsel the patient that perioperative risk extends beyond discharge from the hospital and even beyond 30 days from the operative procedure. In addition, patients will be most interested in the absolute risk level that can be expected over time. Because most population based studies using administrative data have used in-hospital mortality they will tend to overestimate the perioperative benefit of EVAR according to our data [2, 15–17]. This same problem might also be present for other surgical procedures or interventions that do not require several days in the hospital for recovery. In contrast, registries such as the National Surgical Quality Improvement Program (NSQIP) and the SVS Lifeline registry have reported 30 day mortality [18, 19]. Thus, the results of these studies are not directly comparable. This is particularly important when comparing results of studies that used different definitions or when additional analyses such as meta-analyses are performed [20]. While the combined 30 day plus in-hospital mortality addresses these issues in part, even this definition does not capture the full period of “operative risk” associated with the surgical procedures.
Very few studies have reported 90 day mortality [21, 22]. To our knowledge, this is the first report to investigate the duration of elevated mortality risk after intact AAA repair. We estimate the period of high mortality risk after both open repair and EVAR and demonstrate the greater period of high mortality risk with both the plot of bi-weekly interval death rates as well as the plot of absolute mortality differences. While it may be preferable to use a longer time frame (e.g. 90 days) for perioperative mortality comparisons, one must also acknowledge that in these elderly patients there are competing risks of mortality from co-existing disease, highlighting the importance of using adequate control groups in study design. This is demonstrated by the ultimate (after 90 days) baseline bi-weekly death rate of 0.2 to 0.4% after both repair types.
Our analyses are subject to several limitations. First, as noted previously, this is not a randomized trial, rather a retrospective analysis using administrative data. It is therefore subject to coding errors. However, inclusion of Part B data from physician billing minimizes this substantially, and it is unlikely that the current analyses with respect to definitions of preoperative mortality would be biased in one way or the other. Additionally, the use of 2 years of prior claims helps distinguish pre-existing conditions from complications of surgery. The use of very large numbers of patients with propensity score matching overcomes much of potential selection bias. We do not have information regarding anatomy or prior laparotomy, both of which may influence the choice of repair method. However, despite this, our results are qualitatively and to a large extent quantitatively consistent with the randomized trials.
In summary, the absolute benefit of EVAR vs. open AAA repair in perioperative mortality is demonstrated with all common metrics. However, the relative benefit of EVAR is overestimated with in-hospital mortality compared to 30 day or combined 30 day plus in-hospital. The true period of perioperative risk persists for at least 3 months after EVAR and open repair.
Acknowledgments
This work was supported by the NIH T32 Harvard-Longwood Research Training in Vascular Surgery grant HL007734.
Abbreviations/Acronyms
- AAA
abdominal aortic aneurysm
- CI
confidence interval
- EVAR
endovascular aortic aneurysm repair
- NSQIP
National Surgical Quality Improvement Program
- OR
odds ratio
- RR
relative risk
- SVS
Society for Vascular Surgery
Footnotes
Disclosure Information: Dr Schermerhorn received a Gore Unrestricted Educational Grant as a consultant for Gore; a consulting fee from Endologix Data Safety and Monitoring Board, and a consulting fee from Medtronic. Dr Landon received a Gore Unrestricted Educational Grant as a consultant for Gore. All other authors have nothing to disclose.
The opinions expressed do not necessarily represent the views or policy positions of the Centers for Medicare and Medicaid Services.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson PL, Arons RR, Moskowitz AJ, et al. A statewide experience with endovascular abdominal aortic aneurysm repair: rapid diffusion with excellent early results. J Vasc Surg. 2004;39:10–19. doi: 10.1016/j.jvs.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Lee WA, Carter JW, Upchurch G, et al. Perioperative outcomes after open and endovascular repair of intact abdominal aortic aneurysms in the United States during 2001. J Vasc Surg. 2004;39:491–496. doi: 10.1016/j.jvs.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 4.Hua HT, Cambria RP, Chuang SK, et al. Early outcomes of endovascular versus open abdominal aortic aneurysm repair in the National Surgical Quality Improvement Program-Private Sector (NSQIP-PS) J Vasc Surg. 2005;41:382–389. doi: 10.1016/j.jvs.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin LM, Klabunde CN, Green P, et al. In search of the perfect comorbidity measure for use with administrative claims data: does it exist? Med Care. 2006;44(8):745–53. doi: 10.1097/01.mlr.0000223475.70440.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cochran W, Rubin D. Controlling bias in observational studies: a review. Sankhya-A. 1973;35:417–446. [Google Scholar]
- 7.Gu XS, Rosenbaum PR. Comparison of multivariate matching methods: structure, distances, and algorithms. J Comput Graph Stat. 1993;2:405–420. [Google Scholar]
- 8.Giles KA, Schermerhorn ML, O’Malley AJ, et al. Risk prediction for perioperative mortality of endovascular vs open repair of abdominal aortic aneurysms using the Medicare population. J Vasc Surg. 2009;50:256–262. doi: 10.1016/j.jvs.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schermerhorn ML, O’Malley AJ, Jhaveri A, et al. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358:464–474. doi: 10.1056/NEJMoa0707348. [DOI] [PubMed] [Google Scholar]
- 10.Landon BE, O’Malley AJ, Giles K, et al. Volume Outcomes Relationships and AAA Repair. Circulation. 2010;122:1290–1297. doi: 10.1161/CIRCULATIONAHA.110.949172. [DOI] [PubMed] [Google Scholar]
- 11.Giles KA, Landon BE, Cotterill P, et al. Thirty-day mortality and late survival with reinterventions and readmissions after open and endovascular aortic aneurysm repair in Medicare beneficiaries. J Vasc Surg. doi: 10.1016/j.jvs.2010.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prinssen M, Verhoeven EL, Buth J, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351:1607–1618. doi: 10.1056/NEJMoa042002. [DOI] [PubMed] [Google Scholar]
- 13.Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365:2179–2186. doi: 10.1016/S0140-6736(05)66627-5. [DOI] [PubMed] [Google Scholar]
- 14.Lederle FA, Freischlag JA, Kyriakides TC, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302:1535–1542. doi: 10.1001/jama.2009.1426. [DOI] [PubMed] [Google Scholar]
- 15.Giles KA, Hamdan AD, Pomposelli FB, et al. Population-based outcomes following endovascular and open repair of ruptured abdominal aortic aneurysms. J Endovasc Ther. 2009;16:554–564. doi: 10.1583/09-2743.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giles KA, Pomposelli F, Hamdan A, et al. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg. 2009;49:543–550. doi: 10.1016/j.jvs.2008.09.067. discussion 550–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orandi BJ, Dimick JB, Deeb GM, et al. A population-based analysis of endovascular versus open thoracic aortic aneurysm repair. J Vasc Surg. 2009;49:1112–1126. doi: 10.1016/j.jvs.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Bush RL, Johnson ML, Hedayati N, et al. Performance of endovascular aortic aneurysm repair in high-risk patients: results from the Veterans Affairs National Surgical Quality Improvement Program. J Vasc Surg. 2007;45:227–233. doi: 10.1016/j.jvs.2006.10.005. discussion 233–235. [DOI] [PubMed] [Google Scholar]
- 19.Giles KA, Hamdan AD, Pomposelli FB, et al. Body mass index: surgical site infections and mortality after lower extremity bypass from the National Surgical Quality Improvement Program 2005–2007. Ann Vasc Surg. 2009;24:48–56. doi: 10.1016/j.avsg.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lifeline Registry of EVAR Publications Committee. Lifeline registry of endovascular aneurysm repair: long-term primary outcome measures. J Vasc Surg. 2005;42:1–10. doi: 10.1016/j.jvs.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Laukontaus SJ, Pettila V, Kantonen I, et al. Utility of surgery for ruptured abdominal aortic aneurysm. Ann Vasc Surg. 2006;20:42–48. doi: 10.1007/s10016-005-9283-1. [DOI] [PubMed] [Google Scholar]
- 22.Mani K, Bjorck M, Lundkvist J, Wanhainen A. Improved long-term survival after abdominal aortic aneurysm repair. Circulation. 2009;120:201–211. doi: 10.1161/CIRCULATIONAHA.108.832774. [DOI] [PubMed] [Google Scholar]