Abstract
Antimicrobial peptides (AMPs) are effectors of cutaneous innate immunity and protect primarily against microbial infections. An array of AMPs can be found in and on the skin. Those include peptides that were first discovered for their antimicrobial properties but also proteins with antimicrobial activity first characterized for their activity as chemokines, enzymes, enzyme inhibitors and neuropeptides. Cathelicidins were among the first families of AMPs discovered in skin. They are now known to exert a dual role in innate immune defense: they have direct antimicrobial activity and will also initiate a host cellular response resulting in cytokine release, inflammation and angiogenesis. Altered cathelicidin expression and function was observed in several common inflammatory skin diseases such as atopic dermatitis, rosacea and psoriasis. Until recently the molecular mechanisms underlying cathelicidin regulation were not known. Lately, vitamin D3 was identified as the major regulator of cathelicidin expression and entered the spotlight as an immune modulator with impact on both, innate and adaptive immunity. Therapies targeting vitamin D3 signalling may provide novel approaches for the treatment of infectious and inflammatory skin diseases by affecting both innate and adaptive immune functions through AMP regulation.
Key words: 1a,25-dihydroxyvitamin D3; antimicrobial peptides; alarmins; cathelicidin; psoriasis; skin
Vitamin D3 and Immune Defense
Besides its crucial functions in calcium homeostasis there is increasing evidence that vitamin D3 serves as an immune regulator with impact on both innate and adaptive immunity.1,2 Several cell types of the adaptive immune system were found to be directly influenced by vitamin D3 signalling e.g. dendritic cells and T-cells.1,3 In a distinct immune regulatory role vitamin D3 affects innate antimicrobial defence mechanisms at epithelial barriers such as the airway epithelium or the skin.4,5 Either role might be of clinical importance as even today the prevalence of vitamin D3 deficiency is unexpectedly high and hypovitaminosis D is assumed to contribute to increased susceptibility to infection and a higher mortality risk.6 Sparse sun exposure and an unbalanced and insufficient diet contribute to a remarkable prevalence of vitamin D3 deficiency related diseases.7 Recommendations to prevent sun exposure especially in immunosuppressed patients further complicate this situation.8
Nevertheless, our bodies would be able to synthesize sufficient vitamin D3 from precursors under the influence of UVB irradiation.9 Synthesis of pre-vitamin D3 from 7-dehydrocholesterol occurs in skin and involves UVB irradiation that penetrates the epidermis. 7-dehydrocholesterol absorbs UV light most effectively at wavelengths between 270–290 nm and thus the production of vitamin D3 will occur at those wavelengths. The product of this fast photochemical process is calciol (previtamin D3) which is an inactive form of vitamin D3. To form an active hormone, hydroxylation of calciol by vitamin D 25-hydroxylase (CYP27A1) in the liver and by 25-hydroxyvitamin D3 1-a-hydroxylase (CYP27B1) in the kidney leads to active 1α,25-dihydroxyvitamin D3 (calcitriol).10 Keratinocytes express both relevant enzymes and can therefore activate vitamin D3 independent of renal and hepatic hydroxylation steps.11 This is especially important as vitamin D3 plays a crucial role in normal keratinocyte proliferation, differentiation and function.12,13
Vitamin D3: A Key Player in Adaptive Immunity
Almost all cells of the adaptive immune system express the vitamin D receptor (VDR), a nuclear receptor which renders them responsive to vitamin D3.1 Vitamin D3 suppresses adaptive immunity by inhibition of antigen presenting cells and by the generation of a TH2 micromilieu.14–16 Upon vitamin D3 treatment of T-cells TH2 cytokines such as IL-4, IL-5 and IL-10 were increased while IL-2 and IFNγ—typical TH1 cytokines needed for T-cell proliferation and activation—were decreased.15,17–18 Additionally, TH17 cell functions were inhibited while the number of regulatory T-cells (Tregs) increased after vitamin D3 treatment in a mouse model with experimental inflammatory bowel disease.19,20 TH17 cells are involved in psoriasis pathogenesis which might explain in part the beneficial effect of vitamin D analogues in this disease.21 As T-cells overexpress the chemokine receptor CXCR10 upon vitamin D3 treatment it enables these cells to home to the skin upon sensing the skin-specific chemokine CCL27 secreted by keratinocytes.22 In contrast, vitamin D3 decreased the expression of cutaneous lymphocyte-associated antigen (CLA) on T-cells.23 It is therefore likely that treatment with vitamin D3 analogues could attenuate T-cell mediated skin diseases through effects on this homing receptor but would enhance CXCR10 positive cell infiltrates.
Besides its effects on T-cells, proliferation and immunoglobulin production was shown to be inhibited by vitamin D3 in B-cells.24 Dendritic cells (DC) were identified as further targets of vitamin D3 and activation of the VDR in these cells inhibits cell differentiation, maturation and alters expression of co-stimulatory molecules and cytokines leading to an impaired antigen presenting capability of DCs.25–27
The Skin's Innate Defense Mechanisms
Our skin is equipped with an initial defense mechanism against invading microbial pathogens that does not require the specific recognition of the pathogen. Epidermal keratinocytes together with professional innate immune cells such as dendritic cells and macrophages which reside in the skin are ready to form an active barrier against infections.28 In addition, small cationic peptides classified as antimicrobial peptides (AMPs) contribute to the chemical shield on the surface of the skin and other epithelia. AMPs were first defined as endogenous antibiotics due to their capability to kill various pathogens, e.g. gram-positive and gram-negative bacteria, viruses and fungi. However, further functions of AMPs were identified recently: today it is well established that AMPs act as immune modulators with impact on innate and adaptive immune functions. They are therefore broadly considered as key players in the host defense system.29 Keratinocytes and other resident cells in the skin such as cells in eccrine glands, mast cells and sebocytes produce and secrete AMPs. Beyond that, invading immune cells (e.g. neutrophils, NK cells) contribute to the pool of AMPs in the skin.29–32
To date, several dozens of different peptides with antimicrobial function in the skin are known.33 Several AMPs were first known for other biological activities as their antimicrobial function was later identified. The leukocyte protease inhibitor, better known as α-melanocyte stimulating hormone is a prominent example.33
Two important and well studied AMP gene families in the skin are the defensins and cathelicidins.34,35 The first skin-derived antimicrobial peptide found in humans was β-defensin 2 (HBD2) which is most effective against gram-negative bacteria whereas HBD3 from the same AMP family has a broader spectrum of antimicrobial action.36–37 HBD2 is induced in skin inflammation and infection.38 Gläser et al. recently showed that HBD2 and 3 are inducible in vitro and in vivo by UVB irradiation whereas HBD1 is constitutively expressed.39,27
Another well-studied AMP in the skin is cathelicidin, often referred to its peptide forms hCAP18 or LL-37. The human genome contains only one cathelicidin gene (CAMP) which encodes for a pro-peptide, composed of an N-terminal cathelin domain and a C-terminal 37 amino acid sequence with antimicrobial activity. This part can be cleaved from the inactive precursor by serine proteases of the kallikrein family to form active LL-37.34,40–41 LL-37 forms an α-helix in aqueous solution which enables the peptide to disrupt both bacterial membranes and viral envelopes.33 Even antifungal activity of LL-37 in Candida infections was reported.42 Additionally, LL-37 interacts with mammalian cells in a ligand-receptor mediated or a receptor-independent manner resulting in a host response which is referred to as the “alarmin” function of LL-37.43 Furthermore, LL-37 influences ATP-receptor P2X7 and TLR signalling in immune cells, EGF receptor transactivation and intracellular Ca2+ mobilization.44–47 Also, chemokine and cytokine release was induced by LL-37.47 In synergy with the immune modulator IL-1β LL-37 enhances innate immune responses by multiple pathways.29,48 The dual role of cathelicidin, namely the antimicrobial and the alarmin function, suggests an important role for this peptide in cutaneous innate immune defense.
Regulation of Cathelicidin Expression by Vitamin D3
Cathelicidin expression is upregulated in infection and injury in the skin32,49 and cathelicidin promotes neovascularization, wound healing, but also chemokine expression and migration of immune cells (neutrophils, mast cells, monocytes and T-cells).50–53 Although LL-37 is induced in these conditions the regulation of cathelicidin gene expression was long unclear since mediators of infection did not influence gene expression.54
Several recent reports suggest a connection between vitamin D3 and AMP expression in keratinocytes. Wang et al. demonstrated that vitamin D3 has a pivotal role in antimicrobial immunity of the skin when they identified a vitamin D3 response element (VDRE) in the promoter region of the cathelicidin gene.55 Subsequently other groups confirmed that cathelicidin is a direct target of vitamin D3 in keratinocytes.56,57 Furthermore, conservation of cathelicidin gene regulation by the vitamin D3 pathway in humans and non-human primates during evolution has been demonstrated recently.58 To date several elements in the molecular mechanisms of vitamin D3 mediated cathelicidin expression have been characterized: Epigenetic changes such as histone acetylation and co-activator activity enable the control of cathelicidin gene expression by vitamin D3 in keratinocytes.59,60 Furthermore it was shown by our group that IL-17 and vitamin D3 pathways act in synergy to enhance the expression of cathelicidin in keratinocytes.61 In particular, vitamin D3 enables IL-17A to increase cathelicidin expression through activation of Act1 and MEK-ERK.
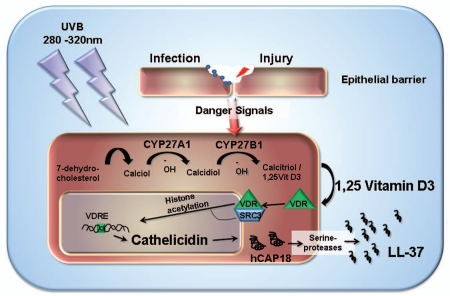
In cutaneous wounds or bacterial infections the expression of cathelicidin LL-37 is increased as is TLR2 and its co-receptor CD14. Additionally it was shown that vitamin D3 is responsible for the increased expression of TLR2 and CD14 in vitro and in vivo.5 However, a sudden change in the vitamin D3 concentration in the wound milieu seemed unlikely. As mentioned above, vitamin D3 is activated through hydroxylation by CYP27B1 in keratinocytes and monocytes in the skin.5 Also, the expression of CYP27B1 is under the control of danger signals that are active during skin infection and injury.5,62 Thus, the local increase of CYP27B1 in infected or injured tissue and the subsequent activation of vitamin D3 induce cathelicidin expression and function in cutaneous wounds (Fig. 1). Therefore, vitamin D3 predominantly suppresses adaptive immunity, whereas innate immune mechanisms such as cathelicidin expression in wounds and infections are activated.
Figure 1.
Model for UVB triggered vitamin D3 activation and cathelicidin response in the skin. Photochemical conversion of 7-dehydrocholesterol to calciol in the skin requires UVB irradiation. In keratinocytes two subsequent hydroxylation steps mediated by CYP27A1 and CYP27B1 result in active calcitriol/1a,25-dihydroxyvitamin D3 (1,25VitD3) that binds to and activates the vitamin D receptor (VDR) in an autocrine manner. Steroid receptor coactivator 3 (SRC3) complexes with the VDR and recruits histone acetyltransferases which open up chromatin and facilitate access to the cathelicidin gene. VDR binds to the vitamin D response element (VDRE) in the cathelicidin promoter region and activates transcription. Cathelicidin is synthesized as an inactive pro-peptide (hCAP18) which is cleaved upon release to active LL-37 by serine proteases.
Role of Cathelicidin in Skin Diseases
Under physiological conditions cathelicidin and other AMPs protect the skin from microbial infection. In healthy skin only very low amounts of cathelicidin LL-37 are detectable. Upon infection or wounding cathelicidin expression is strongly increased.5,32,49 Yet in some skin diseases the barrier function of the skin and control of inflammation is disturbed and cathelicidin levels are altered compared to healthy skin. In atopic dermatitis for example, viral or bacterial superinfections are common and this might be due to disturbed expression of AMPs.63 An altered cytokine milieu was suggested to be the reason for the diminished inducibility of cathelicidin expression and other AMPs such as HBD2 in the course of this disease.64 In particular, TH2 cytokines like IL-4 and IL-13 suppressed the induction of AMPs. However, this view has been challenged recently by other authors.65 Ballardini and co-workers found increased cathelicidin transcripts and protein in lesional skin from patients with atopic eczema compared to non-lesional skin or skin from healthy controls.65 Harder et al. reported increased functional HBD2 and other antimicrobial peptide expression in lesional skin in atopic eczema patients as compared to healthy individuals.66 Still, a third group reported downregulated cathelicidin expression in injured skin in atopic eczema following acute wounding in contrast to enhanced expression in wounds in healthy skin.67
Another disease with possible involvement of cathelicidin LL-37 is rosacea which is characterised by centrofacial skin inflammation and vascular reactivity. It was observed that patients suffering from this disease express abnormally high levels of LL-37 peptide whereas in healthy individuals smaller, processed forms predominate.68 These processed peptides mediate antimicrobial rather than immune-modulatory functions. Furthermore, in rosacea the processed forms of LL-37 peptide were different from the peptides found in healthy individuals due to an abnormal and increased activity of cutaneous protease activity.68 Thus, in rosacea an unknown molecular mechanism leading to increased LL-37 expression and abnormal protease activity results in skin inflammation.
Psoriasis is a third example of an inflammatory skin disease associated with abnormal AMP activity. Psoriasis is suggested to be an autoimmune disease with T-cell involvement and an overreacting innate immune response resulting in chronic cutaneous inflammation.28,69 Still, the auto-antigen triggering the immune response remains unknown. Recently, Lande et al. showed that plasmacytoid dentritic cells (pDCs) in psoriasis are activated by self-DNA complexed with LL-37.70 Cathelicidin LL-37 expression is increased in lesional skin63 and self-DNA-LL-37 complexes signalled through TLR9 resulting in IFN-γ release by pDCs. Consecutively, secreted IFN-γ activates a T-cell response that leads to cutaneous inflammation.70 The self-DNA needed for the initiation of this inflammatory cascade might come from dead and dying cells in the psoriatic plaque. Thus, in psoriasis overexpression of cathelicidin together with a second factor (self-DNA) which under healthy conditions is not present in the skin might lead to skin inflammation.71 Another recent study published by Ganguly et al. revealed that LL-37 not only forms complexes with self-DNA but also with self-RNA. Again the LL-37-nucleic acid complexes activated pDCs and additionally myeloid DCs (mDCs) via TLR7 and TLR8 signalling, respectively. Thus, the transformation of inert nucleic acids (self-DNA, self-RNA) into immunologically relevant immune activators by cathelicidin is thought to drive autoinflammatory response in psoriasis.72
Using vVitamin D3 to Target Cathelicidin in Inflammatory Skin Disease
As cathelicidin expression and function is altered in several common inflammatory skin disorders novel therapeutic approaches to restore normal cathelicidin expression and function might prove beneficial for the treatment of these skin diseases. Targeting the vitamin D3 pathway—so far the only known pathway that directly influences cathelicidin expression in keratinocytes—might be such a therapeutic strategy. In the following paragraph possible approaches will be discussed.
Several clinical studies show that skin inflammation in atopic dermatitis can be ameliorated by UVB irradiation.73 So far the positive effects of this therapy were attributed to the effect of UVB on T-cells. However, the beneficial effects could also be a result of the UVB induced activation of AMPs and/or the UVB induced vitamin D3 synthesis and subsequent cathelicidin expression in the skin.39,73,74 In addition, Malbris et al. showed recently that the vitamin D3 activated pathways leading to induced cathelicidin in keratinocytes in atopic skin are functional.67 Also, Hata et al. showed increased cathelicidin in skin biopsies in patients suffering from atopic eczema following a course of oral vitamin D supplementation.75 Thus, increasing vitamin D3 synthesis and elevating vitamin D3 levels in the serum could help to strengthen the innate defense barriers to prevent cutaneous infections which trigger skin inflammation in atopic dermatitis.
In rosacea, blockade of cathelicidin expression and LL-37 processing might ameliorate skin inflammation. A vitamin D receptor gene polymorphism was described in patients with severe rosacea indicating that vitamin D signalling is involved in disease pathogenesis.76 By targeting the vitamin D3 pathway cathelicidin overexpression could be blocked and the production of the disease aggravating cathelicidin fragments inhibited. Another possible therapeutic approach could be the inhibition of the increased proteolytic activity in the skin of rosacea patients by blocking serine protease activity.
In psoriasis, blocking or decreasing cathelicidin expression could disrupt the vicious circle of cutaneous inflammation driven by high levels of LL-37 complexed with self-DNA or self-RNA released from dead cells resulting in pDC activation.70,72 Vitamin D3 analogues have been used in the topical treatment of psoriasis for a long time although the molecular mechanisms behind their anti-psoriatic action are not completely understood. Vitamin D3 analogues bind to VDR which in turn should bind to the vitamin D responsive element in the promoter region of cathelicidin gene and thus increase LL-37 expression and skin inflammation.77 However, the opposite is true: Vitamin D analogues decrease inflammation in psoriatic plaques.78 Surprisingly, the expression of cathelicidin hCAP18 was upregulated by calcipotriol in keratinocytes in vitro and in vivo at the same time.77 So far this discrepancy could not be explained and further studies are needed to elucidate the role of cathelicidin in psoriasis pathogenesis.
Conclusion
In summary, influencing cathelicidin expression by targeting the vitamin D3 pathway might present a novel therapeutic approach for the treatment of skin diseases with disturbed hCAP18/LL-37 expression such as psoriasis, rosacea or atopic dermatitis. However, a deeper insight and understanding of the molecular mechanisms of AMP biology and their regulation by the vitamin D3 pathway is needed before this knowledge can be translated into therapeutic use.
Acknowledgements
J.S. has received grants from the Deutsche Forschungsgemeinschaft (Emmy Noether Programm; Scha 979/3-1; www.dfg.de) and the Fritz Thyssen Stiftung (www.fritz-thyssen-stiftung.de).
References
- 1.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Bikle DD. What is new in vitamin D: 2006–2007. Curr Opin Rheumatol. 2007;19:383–388. doi: 10.1097/BOR.0b013e32818e9d58. [DOI] [PubMed] [Google Scholar]
- 3.van Etten E, Stoffels K, Gysemans C, Mathieu C, Overbergh L. Regulation of vitamin D homeostasis: implications for the immune system. Nutr Rev. 2008;66:S125–S134. doi: 10.1111/j.1753-4887.2008.00096.x. [DOI] [PubMed] [Google Scholar]
- 4.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3) J Cyst Fibros. 2007;6:403–410. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allain TJ, Dhesi J. Hypovitaminosis D in older adults. Gerontology. 2003;49:273–278. doi: 10.1159/000071707. [DOI] [PubMed] [Google Scholar]
- 7.Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618–625. doi: 10.1111/j.1600-0625.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 8.Moan J, Porojnicu AC, Dahlback A, Setlow RB. Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proc Natl Acad Sci U S A. 2008;105:668–673. doi: 10.1073/pnas.0710615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bikle D, Teichert A, Hawker N, Xie Z, Oda Y. Sequential regulation of keratinocyte differentiation by 1,25(OH)2D3, VDR, and its coregulators. J Steroid Biochem Mol Biol. 2007;103:396–404. doi: 10.1016/j.jsbmb.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 10.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29:664–673. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Bikle DD, Pillai S, Gee E, Hincenbergs M. Regulation of 1,25-dihydroxyvitamin D production in human keratinocytes by interferon-gamma. Endocrinology. 1989;124:655–660. doi: 10.1210/endo-124-2-655. [DOI] [PubMed] [Google Scholar]
- 12.Bikle DD. Vitamin D regulated keratinocyte differentiation. J Cell Biochem. 2004;92:436–444. doi: 10.1002/jcb.20095. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann B. Role of the vitamin D3 pathway in healthy and diseased skin--facts, contradictions and hypotheses. Exp Dermatol. 2009;18:97–108. doi: 10.1111/j.1600-0625.2008.00810.x. [DOI] [PubMed] [Google Scholar]
- 14.Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–145. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 15.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 16.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol) J Clin Invest. 1984;74:1451–1455. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemire JM. Immunomodulatory actions of 1,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 1995;53:599–602. doi: 10.1016/0960-0760(95)00106-a. [DOI] [PubMed] [Google Scholar]
- 18.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr. 1995;125:1704S–1708S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 19.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 20.Gorman S, Kuritzky LA, Judge MA, Dixon KM, McGlade JP, Mason RS, et al. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 21.Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez-Farinas M, Fuentes-Duculan J, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 23.Yamanaka K, Dimitroff CJ, Fuhlbrigge RC, Kakeda M, Kurokawa I, Mizutani H, et al. Vitamins A and D are potent inhibitors of cutaneous lymphocyte-associated antigen expression. J Allergy Clin Immunol. 2008;121:148–157. doi: 10.1016/j.jaci.2007.08.014. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S, Sims GP, Chen XX, Gu YY, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 25.Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 26.D'Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao C, Wang I, Lehrer RI. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]
- 28.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009 doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schauber J, Gallo RL. Expanding the roles of antimicrobial peptides in skin: alarming and arming keratinocytes. J Invest Dermatol. 2007;127:510–512. doi: 10.1038/sj.jid.5700761. [DOI] [PubMed] [Google Scholar]
- 30.Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005;73:6771–6781. doi: 10.1128/IAI.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami M, Ohtake T, Dorschner RA, Schittek B, Garbe C, Gallo RL. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J Invest Dermatol. 2002;119:1090–1095. doi: 10.1046/j.1523-1747.2002.19507.x. [DOI] [PubMed] [Google Scholar]
- 32.Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 33.Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006;306:91–110. doi: 10.1007/3-540-29916-5_4. [DOI] [PubMed] [Google Scholar]
- 34.Zaiou M, Gallo RL. Cathelicidins, essential gene-encoded mammalian antibiotics. J Mol Med. 2002;80:549–561. doi: 10.1007/s00109-002-0350-6. [DOI] [PubMed] [Google Scholar]
- 35.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 36.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 37.Harder J, Siebert R, Zhang Y, Matthiesen P, Christophers E, Schlegelberger B, et al. Mapping of the gene encoding human beta-defensin-2 (DEFB2) to chromosome region 8p22-p23.1. Genomics. 1997;46:472–475. doi: 10.1006/geno.1997.5074. [DOI] [PubMed] [Google Scholar]
- 38.Schroder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999;31:645–651. doi: 10.1016/s1357-2725(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 39.Glaser R, Navid F, Schuller W, Jantschitsch C, Harder J, Schroder JM, et al. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. J Allergy Clin Immunol. 2009;123:1117–1123. doi: 10.1016/j.jaci.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 40.Braff MH, Hawkins MA, Di Nardo A, Lopez-Garcia B, Howell MD, Wong C, et al. Structure-function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J Immunol. 2005;174:4271–4278. doi: 10.4049/jimmunol.174.7.4271. [DOI] [PubMed] [Google Scholar]
- 41.Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. Faseb J. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Garcia B, Lee PH, Yamasaki K, Gallo RL. Antifungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005;125:108–115. doi: 10.1111/j.0022-202X.2005.23713.x. [DOI] [PubMed] [Google Scholar]
- 43.Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol. 2007;601:185–194. doi: 10.1007/978-0-387-72005-0_19. [DOI] [PubMed] [Google Scholar]
- 44.Tomasinsig L, Pizzirani C, Skerlavaj B, Pellegatti P, Gulinelli S, Tossi A, et al. The human cathelicidin LL-37 modulates the activities of the P2X7 receptor in a structure-dependent manner. J Biol Chem. 2008;283:30471–30481. doi: 10.1074/jbc.M802185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Nardo A, Braff MH, Taylor KR, Na C, Granstein RD, McInturff JE, et al. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J Immunol. 2007;178:1829–1834. doi: 10.4049/jimmunol.178.3.1829. [DOI] [PubMed] [Google Scholar]
- 46.Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–4668. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 47.Zheng Y, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils Antimicrobial peptides human beta-defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. Br J Dermatol. 2007;157:1124–1131. doi: 10.1111/j.1365-2133.2007.08196.x. Epub 2007 Oct 4. [DOI] [PubMed] [Google Scholar]
- 48.Yu J, Mookherjee N, Wee K, Bowdish DM, Pistolic J, Li Y, et al. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. J Immunol. 2007;179:7684–7691. doi: 10.4049/jimmunol.179.11.7684. [DOI] [PubMed] [Google Scholar]
- 49.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 50.Heilborn JD, Frohm Nilsson M, Kratz G, Weber G, Sorensen O, Borregaard N, et al. The cathelicidin antimicrobial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 51.Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 53.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–519. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang T-T, Nestel F, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting Edge: 1,25-Dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–292. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 56.Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124:1080–1082. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 57.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. Faseb J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 58.Gombart AF, Saito T, Koeffler HP. Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates. BMC Genomics. 2009;10:321. doi: 10.1186/1471-2164-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schauber J, Iffland K, Frisch S, Kudlich T, Schmausser B, Eck M, et al. Histone-deacetylase inhibitors induce the cathelicidin LL-37 in gastrointestinal cells. Mol Immunol. 2004;41:847–854. doi: 10.1016/j.molimm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Schauber J, Oda Y, Buchau AS, Yun QC, Steinmeyer A, Zugel U, et al. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-Dihydroxyvitamin D(3) J Invest Dermatol. 2008;128:816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- 61.Peric M, Koglin S, Kim SM, Morizane S, Besch R, Prinz JC, et al. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol. 2008;181:8504–8512. doi: 10.4049/jimmunol.181.12.8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 63.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 64.Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–348. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 65.Ballardini N, Johansson C, Lilja G, Lindh M, Linde Y, Scheynius A, et al. Enhanced expression of the antimicrobial peptide LL-37 in lesional skin of adults with atopic eczema. Br J Dermatol. 2009;161:40–47. doi: 10.1111/j.1365-2133.2009.09095.x. [DOI] [PubMed] [Google Scholar]
- 66.Harder J, Dressel S, Wittersheim M, Cordes J, Meyer-Hoffert U, Mrowietz U, et al. Enhanced Expression and Secretion of Antimicrobial Peptides in Atopic Dermatitis and after Superficial Skin Injury. J Invest Dermatol. doi: 10.1038/jid.2009.432. [DOI] [PubMed] [Google Scholar]
- 67.Mallbris L, Carlen L, Wei T, Heilborn J, Nilsson MF, Granath F, et al. Injury downregulates the expression of the human cathelicidin protein hCAP18/LL-37 in atopic dermatitis. Exp Dermatol. 2009 doi: 10.1111/j.1600-0625.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- 68.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 69.Bos JD, de Rie MA, Teunissen MB, Piskin G. Psoriasis: dysregulation of innate immunity. Br J Dermatol. 2005;152:1098–1107. doi: 10.1111/j.1365-2133.2005.06645.x. [DOI] [PubMed] [Google Scholar]
- 70.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 71.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 72.Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206:1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vahavihu K, Ala-Houhala M, Peric M, Karisola P, Kautiainen H, Hasan T, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010;163:321–328. doi: 10.1111/j.1365-2133.2010.09767.x. [DOI] [PubMed] [Google Scholar]
- 74.Peric M, Lehmann B, Vashina G, Dombrowski Y, Koglin S, Meurer M, et al. UV-B-triggered induction of vitamin D3 metabolism differentially affects antimicrobial peptide expression in keratinocytes. J Allergy ClinImmunol. 2010;125:746–749. doi: 10.1016/j.jaci.2009.12.933. [DOI] [PubMed] [Google Scholar]
- 75.Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008;122:829–831. doi: 10.1016/j.jaci.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jansen T, Krug S, Kind P, Plewig G, Messer G. BsmI polymorphism of the vitamin D receptor gene in patients with the fulminant course of rosacea conglobata (rosacea fulminans) J Dermatol. 2004;31:244–246. doi: 10.1111/j.1346-8138.2004.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 77.Peric M, Koglin S, Dombrowski Y, Gross K, Bradac E, Buchau A, et al. Vitamin D analogs differentially control antimicrobial peptide/”alarmin” expression in psoriasis. PLoS One. 2009;4:e6340. doi: 10.1371/journal.pone.0006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lebwohl M, Menter A, Koo J, Feldman SR. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004;50:416–430. doi: 10.1016/j.jaad.2002.12.002. [DOI] [PubMed] [Google Scholar]