Abstract
Melatonin or N-acetyl-5-methoxytryptamine, is a compound derived from tryptophan that is found in all organisms from single cells to vertebrates and the human. It is one of the most evolutionarily conserved and pleiotropic hormone still active in humans and has been implicated in vital skin functions such as hair growth, fur pigmentation as well as melanoma control. Being a main secretory product of the pineal gland, melatonin regulates seasonal biorhythms, reproductive mechanisms or mammary gland metabolism. Due to its wide range endocrine properties it is also recognized to modulate numerous additional functions ranging from scavenging free radicals, immunomodulation-mediated DNA repair, wound healing, involvement in gene expression connected with circadian clocks and modulation of secondary endocrine signaling including prolactin release. Recently, apart from above mentioned entities, it was shown that melatonin suppresses ultraviolet (UV)-induced damage in human skin and human derived cell lines (e.g., keratinocytes, fibroblasts). The magnitude of UV-induced damage is mediated apparently by various molecular mechanisms related to generation of reactive oxygen species (ROS), apoptosis and mitochondrial-mediated cell death which are all counteracted or modulated by melatonin. We provide here an update of the relevant protective effects and molecular mechanisms of action of melatonin in the skin.
Key words: melatonin, skin, ultraviolet radiation, antioxidant, oxidative stress, mitochondria, apoptosis
Introduction
Melatonin is a phylogenetically ancient methoxyindole, first identified as the main secretory product of the pineal gland.1 This highly conserved molecule is present in all organisms from unicells to vertebrates. Melatonin is well described as a neuroendocrine mediator with pleiotropic bioactivities such as hormonal, neurotransmitter, immunomodulator and biological modifier actions.2,3 Melatonin was found in the blood, where its concentration exhibits a circadian day-night-rhythm and seasonal rhythms. These effects are indirectly connected with modulation of endocrine systems such as oesterus activity or prolactin secretion.4 Moreover, melatonin was recently found to play an important role in immunological responses,5 inhibition of tumour growth6,7 or reproduction.8 Recent reports showed that melatonin exerts many direct, receptor-independent activities as a potent antioxidant,9,10 chemotoxicity reducing agent11,12 and as an anti-aging substance.13
The last decades of investigations concerning occurrence of melatonin in different body compartments revealed that significant high concentrations of melatonin were found in the bile fluid, bone marrow, cerebrospinal fluid, ovary, eye, lymphocytes or skin14 and is differentially distributed also in subcellular organelles.15,16 Its wide extracellular and intracellular distribution may explain the complexity and pluripotency of melatonin's role in modulating a diverse number of physiological processes through different mechanisms of action.17 Melatonin as a highly lipophilic compound penetrates easily through cellular membranes and therefore is able to efficiently protect readily every intracellular structure including enzymes, proteins, lipids, mitochondria and the nucleus against oxidative damage.18,19 Especially mitochondrial function has recently been found to be a target of protective melatonin action. Melatonin seems to be responsible for mitochondrial homeostasis or intracellular calcium balance, thus leading simultaneously to prevention of Alzheimer's disease, Parkinson's disease, aging, epilepsy, all of them related to mitochondrial dysfunctions.20
Some of the antioxidant ability of melatonin is possible because of genomic effects in regulating gene expression and activity of numerous antioxidant enzymes such as glutathione peroxidase (GPx), catalase (CAT), Mn-superoxide dismutase (Mn-SOD) and Cu/Zn-superoxide dismutase (Cu/Zn-SOD).21 Besides, melatonin does not act just as a potent antioxidant, but is also capable to activate other endogenous enzymes involved in antioxidative mechanisms against oxidative stress.22 For instance, isoforms of nitric oxide synthase, inducible (iNOS) and mitochondrial (mtNOS), are regulated by melatonin using its ability to bind to the calcium-calmodulin complex leading in this way to inhibition of nNOS.23 Additionally, according to Tan et al.24 structural metabolites of melatonin such as N-acetyl-5-methoxykynuramine (AMK) or N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), formed during its enzymatic metabolism in the brain, are also reported to be efficient scavengers of ROS and reactive nitrogen species (RNS). It is commonly known that there are many exogenous factors such as xenobiotics25 as well as various types of radiation such as ionisation and UV14 inducing ROS generation. Additionally, UV-induced formation of free radicals causes damage to important intra- and extracellular structures such as lipids, lipid membranes, nucleic acids and proteins.26 Apart from the above mentioned properties of melatonin, this compound is reported to exert UV protective effects via modulation of proinflammatory mediators.27
In this review, updated investigations and statements describing different mechanisms of action of melatonin against UV radiation are presented.
Cutaneous Synthesis of Melatonin
Most of investigations regarding the different aspects of melatonin confirm that, both, biosynthetic and biodegradative pathways of melatonin have been initially characterized in whole human and rodent skin and in the major cutaneous cell populations.28 The most important compound for intracutaneous synthesis of melatonin (Fig. 1) is the essential amino acid tryptophan (Trp) which is converted by tryptophan hydroxylase (TPH) to 5-OH-Trp and further to serotonin by activity of aromatic amino acid decarboxylase (AAD) which is available in almost every tissue.2,28 In fact, serotonin is essential in the melatonin biosynthesis pathway, nevertheless it has independent biological actions by itself and enters degradation independently of melatonin.28 Next, the acetylation of serotonin occurs forming N-acetylserotonin (NAS) catalyzed by either arylalkylamine N-acetyltransferase (AANAT) and/or arylamine N-acetyltransferase (NAT). Finally, NAS produced in the skin may be released into the circulation or stay in the cutaneous tissue and thereafter could be transformed into melatonin after active hydroxyindole-O-methyltransferase (HIOMT).28,29
Figure 1.
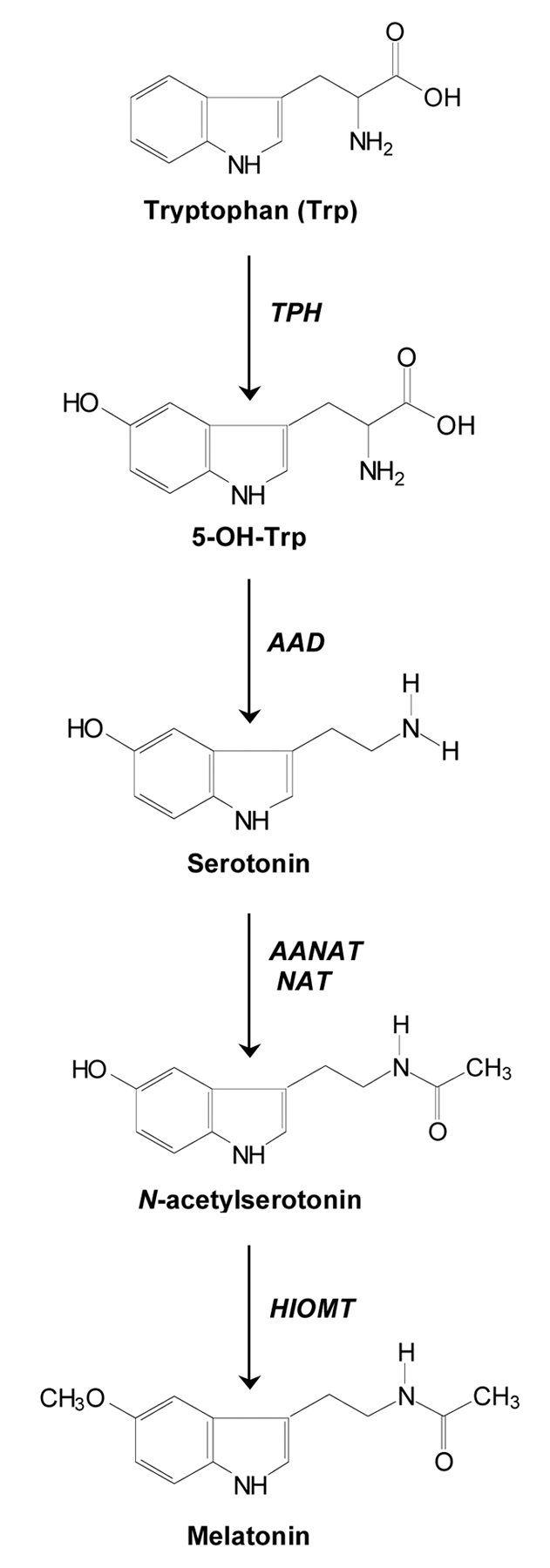
Biosynthesis pathway of melatonin in the skin. Melatonin is synthesized from tryptophan in a cascade of enzymatic reactions catalyzed by tryptophan hydroxylase (TPH), amino acid decarboxylase (AAD), arylalkylamine N-acetyltransferase (AANAT), arylamine N-acetyltransferase (NAT) and hydroxyindole-O-methyltransferase (HIOMT).
Melatonin as a Protectant against UV-Induced Oxidative Stress
Ultraviolet radiation (UVR) is the most important pathological environmental factor that directly affects the function of various cell systems in the skin, including keratinocytes, melanocytes and leukocytes as well as Langerhans cells. In addition to acute response resulting in sunburn cell formation, UVR induces generation of ROS while melatonin effectively suppresses this inflammatory reaction.18,27 There are two antioxidant actions of melatonin: a direct, due to its ability to act as a free radical scavenger, and an indirect due to gene expression and activity upregulation of the main antioxidant enzymes.
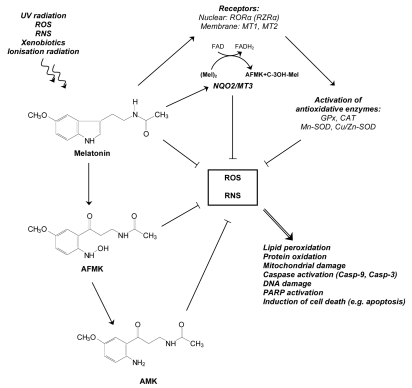
The direct antioxidant function uses physicochemical properties of melatonin. Thus, as an electron-rich molecule, melatonin is able to interact with various forms of free radicals such as H2O2, •OH, singlet oxygen (1O2), superoxide anion (O2•−), peroxynitrite anion (ONOO−) and peroxyl radical (LOO•).21 It should be noted that melatonin's antioxidant mechanism of action implies a free radical scavengers cascade in case of UV irradiation. Metabolites of melatonin degradation such as AFMK or AMK are known to be the main photoproducts and simultaneously potent antioxidants (Fig. 2).14,22 Cell-free system investigations carried out by our group using mixed UV-source (UVB: 60%, UVA: 30%) corresponding to natural solar irradiation, revealed four metabolites identified by HPLC and LC-MS: 2-OH-melatonin, 4-OH-melatonin, 6-OH-melatonin and AFMK.14 This evidence may indicate that melatonin metabolites, unlike classic antioxidants, do not induce prooxidant reactions. Even more, AFMK and AMK were found in mitochondrial studies to be much more potent antioxidants than melatonin itself.22
Figure 2.
Oxidative stress-induced melatonin response. Melatonin and its metabolites AFMK and/or AMK are found to significantly protect against oxidative damage by scavenging ROS (direct activity) or enhancing gene and activity expression of the main antioxidant enzymes (GPx, CAT, SOD) via nuclear (RORα), membrane (MT1, MT2) and cytosolic (NQO2/MT3) receptors (indirect activity).
The second mechanism of antioxidative action of melatonin is developed through indirect antioxidant function by activating antioxidant enzymes (Fig. 2).2 Here, melatonin was determined as an upregulating agent of GPx, CAT or SOD. It should be added that Pablos et al.30 observed different ratio of activity of these particular enzymes in various tissues. It was dependent on the accumulation rate of exogenous melatonin and its distribution in the tissue. Based on these observations, it is hypothesized that melatonin might upregulate GPx, CAT or SOD gene expression and activities also in oxidative stress induced by UV-irradiation in the skin. However, this hypothesis still needs to be investigated in future studies.
Intriguingly, not only enzyme activity, but also gene expression of antioxidative enzymes (Mn-SOD, Cu/Zn-SOD) is upregulated by melatonin.2 In fact, it has been shown that enzyme levels are increased, but it is more pronounced in case of chronically than acute melatonin administration.31 Currently, there are some proposals that melatonin-mediated expression of antioxidant enzymes is dependent of signal transduction pathways related to membrane, cytosolic and nuclear receptors32 but these hypothesis should be still experimentally demonstrated.
Protective Effects of Melatonin against Skin Photodamage
There is clear evidence that the protective effects of melatonin against photobiological disturbances are mediated by the strong antioxidative properties of this compound. It was shown that melatonin has a higher reduction potential (0.73 V) than vitamin C (0.23 V).21 Formation of highly toxic hydroxyl radicals occurred in presence of certain concentrations of vitamin C, while to date melatonin has not demonstrated such pro-oxidant properties. Regarding UV-induced ROS generation tightly connected with photodamage, it was shown that melatonin is a strong scavenger of free radicals compared to vitamin C or trolox, a vitamin E analogue.33 According to investigations carried out by Ryoo et al.34 in UV-exposed fibroblasts, only 56% of the cells survived upon UV exposure (140 mJ/cm2), while cells preincubated with 1 nmol melatonin revealed a cell survival rate of 92.5% which was paralleled by significant decrease of lipid peroxidation and cell death. Comparative experiments using UV-treated fibroblasts showed similar correlation in cell viability in presence of 100 nmol melatonin.35
Moreover, human keratinocytes, the main target cell population in epidermal photodamage, irradiated at increasing doses (10, 25, 50 and 100 mJ/cm2) were investigated for proliferation, colony formation and induction of apoptosis (TUNEL positivity), respectively. Here, melatonin at 10−3 and 10−4 M significantly protected keratinocytes against UV-mediated apoptosis.36 Melatonin was also determined as a crucial agent that downregulates expression of genes playing an important role in the execution of UV-induced skin photodamage: aldehyde dehydrogenase 3 type A1, interstitial collagenase (MMP-1), stromelysin 1 (MMP-3) or stromelysin 2 (MMP-10).3 Besides, melatonin was described as an effective anti-apoptotic compound that inhibits mitochondria-dependent (intrinsic) apoptosis through inhibition of caspase 9 and caspase 3, but does not inhibit the receptor-dependent (extrinsic) pathway of apoptosis mediated by caspase 8.37 It reduces dissipation of mitochondrial transmembrane potential, cleavage of caspases or activation of poly(ADP-ribose) polymerase (PARP), a key DNA-repair-mediating enzyme.37 All these events are caused by UV-induced mitochondrial ROS (mROS) generation which are effectively reduced by melatonin at the concentrations of 10−6, 10−4 and 10−3 M.37 Here, melatonin also significantly reduced detachment of UV-induced keratinocytes preventing appearance of apoptotic cells. These observations confirm direct and potent protective actions of melatonin in vitro related to molecular consequences of UV-induced apoptosis.
Conclusions
As seen throughout this review, melatonin exerts several mechanisms of action to develop its large number of functions. Apart from its role as a regulator of the circadian rhythm, another important aspect that has to be taken into account is the implication of melatonin in those situations where free radical production is enhanced. One of those is UV radiation, where melatonin has been demonstrated to be more effective than other common antioxidants, with the advantage that even lower doses are already sufficient. In fact, investigations using cell-free system and/or skin cells exposed to UVR revealed its close association with inhibition of formation of reactive oxygen species leading to skin aging or photocarcinogenesis. Regarding this property, melatonin can act as a direct free radical scavenger as well as indirect antioxidant through the regulation of antioxidant enzymes. So far, it was well documented that UV irradiation affects mitochondria-dependent downstream activation of executive caspases that mediate the intrinsic pathway of apoptosis in HaCaT keratinocytes. Apparently, besides antioxidative action, preincubation with melatonin revealed also anti-apoptotic activity, but at the same time DNA-protective effects which is essential for the survival of a non-malignant skin cell population. Just recently, prevention of mitochondrial disturbances by melatonin came “on stage” of the numerous protective effects of this amazingly pluripotent substance, and together with its metabolites, a new chapter of melatonin in dermato-endocrine research has just been opened.
Acknowledgements
The authors wish to acknowledge the funding agencies that have supported some of the original work cited in this review: German Academy of Natural Scientists Leopoldina, Halle and ‘Federal Ministry of Education and Research’ BMBF-LPD 9901/8-113 (T.W.F.), Foundation ‘Rene Touraine’ Short-Term International Fellowship, France (T.W.F.), Aaron B. Lerner scholarship from the Friedrich-Schiller-University, Jena, Germany (T.W.F.) and University of Tennessee Cancer Center Pilot Grant (T.W.F.). The senior author (T.W.F.) is also grateful to Prof. Andrzej Slominski, who has been the mentor and host of T.W.F. during his research stay at the Department of Pathology and Laboratory Medicine at the University of Tennessee Health Science Center, Memphis, Tennessee, that enabled him to set new milestones in characterizing the role of melatonin in the skin.
References
- 1.Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, a pineal factor that lightens melanocytes. J Am Chem Soc. 1958;80:2587. [Google Scholar]
- 2.Fischer TW, Slominski A, Zmijewski MA, Reiter RJ, Paus R. Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair. Exp Dermatol. 2008;17:713–730. doi: 10.1111/j.1600-0625.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- 3.Slominski A, Fischer TW, Zmijewski MA, Wortsman J, Semak I, Zbytek B, et al. On the role of melatonin in skin physiology and pathology. Endocrine. 2005;27:137–148. doi: 10.1385/ENDO:27:2:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rato AG, Pedrero JG, Martinez MA, del Rio B, Lazo PS, Ramos S. Melatonin blocks the activation of estrogen receptor for DNA binding. FASEB J. 1999;13:857–868. doi: 10.1096/fasebj.13.8.857. [DOI] [PubMed] [Google Scholar]
- 5.Guerrero JM, Reiter RJ. Melatonin-immune system relationships. Curr Top Med Chem. 2002;2:167–169. doi: 10.2174/1568026023394335. [DOI] [PubMed] [Google Scholar]
- 6.Fischer TW, Zmijewski MA, Zbytek B. Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int J Oncol. 2006;29:665–672. doi: 10.3892/ijo.29.3.665. [DOI] [PubMed] [Google Scholar]
- 7.Helton RA, Harrison WA, Kelley K, Kane MA. Melatonin interactions with cultured murine B16 melanoma cells. Melanoma Res. 1993;3:403–413. doi: 10.1097/00008390-199311000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Lerchl A, Schlatt S. Influence of photoperiod on pineal melatonin synthesis, fur color, body weight and reproductive function in the female Djungarian hamster, Phodopus sungorus. Neuroendocrinology. 1993;57:359–364. doi: 10.1159/000126380. [DOI] [PubMed] [Google Scholar]
- 9.Fischer TW, Elsner P. The antioxidative potential of melatonin in the skin. Curr Probl Dermatol. 2001;29:165–174. doi: 10.1159/000060665. [DOI] [PubMed] [Google Scholar]
- 10.Reiter RJ, Tan DX, Poeggeler B, Menendez-Pelaez A, Chen LD, Saarela S. Melatonin as a free radical scavenger: implications for aging and age-related diseases. Ann NY Acad Sci. 1994;719:1–12. doi: 10.1111/j.1749-6632.1994.tb56817.x. [DOI] [PubMed] [Google Scholar]
- 11.Atessahin A, Sahna E, Türk G, Ceribasi AO, Yilmaz S, Yüce A, Bulmus O. Chemoprotective effect of melatonin against cisplatin-induced testicular toxicity in rats. J Pineal Res. 2006;41:21–27. doi: 10.1111/j.1600-079X.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- 12.Reiter RJ, Tan DX, Sainz RM, Mayo JC, Lopez-Burillo S. Melatonin: reducing the toxicity and increasing the efficacy of drugs. J Pharm Pharmacol. 2002;54:1299–1321. doi: 10.1211/002235702760345374. [DOI] [PubMed] [Google Scholar]
- 13.Reiter RJ, Tan DX, Manchester LC, El Sawi MR. Melatonin reduces oxidant damage and promotes mitochondrial respiration: implications for aging. Ann NY Acad Sci. 2002;959:238–250. doi: 10.1111/j.1749-6632.2002.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 14.Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006;20:1564–1566. doi: 10.1096/fj.05-5227fje. [DOI] [PubMed] [Google Scholar]
- 15.Menendez-Pelaez A, Reiter RJ. Distribution of melatonin in mammalian tissues: the relative importance of nuclear versus cytosolic localization. J Pineal Res. 1993;15:59–69. doi: 10.1111/j.1600-079x.1993.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 16.Reiter RJ, Tan DX. What constitutes a physiological concentration of melatonin? J Pineal Res. 2003;34:79–80. doi: 10.1034/j.1600-079x.2003.2e114.x. [DOI] [PubMed] [Google Scholar]
- 17.León J, Acuña-Castroviejo D, Escames G, Tan DX, Reiter RJ. Melatonin mitigrates mitochondrial malfunction. J Pineal Res. 2005;38:1–9. doi: 10.1111/j.1600-079X.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 18.Fischer TW, Scholz G, Knöll B, Hipler UC, Elsner P. Melatonin suppresses reactive oxygen species induced by UV irradiation in leukocytes. J Pineal Res. 2004;37:107–112. doi: 10.1111/j.1600-079X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 19.Reiter RJ, Poeggeler B, Tan DX, Chen LD, Manchester LC, Guerrero JM. Antioxidant capacity of melatonin: a novel action not requiring a receptor. Neuroendocrinol Lett. 1993;15:103–116. [Google Scholar]
- 20.Acuña-Castroviejo D, Martín M, Macías M, Escames G, León J, Khaldy H, Reiter RJ. Melatonin, mitochondria and cellular bioenergetics. J Pineal Res. 2001;30:65–74. doi: 10.1034/j.1600-079x.2001.300201.x. [DOI] [PubMed] [Google Scholar]
- 21.Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, et al. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2:181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 22.Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 23.Escames G, León J, Macias M, Khaldy H, Acuña-Castroviejo D. Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxides synthase in rats. FASEB J. 2003;17:932–934. doi: 10.1096/fj.02-0692fje. [DOI] [PubMed] [Google Scholar]
- 24.Tan DX, Manchester LC, Burkhardt S, Sainz RM, Mayo JC, Kohen R, et al. N1-acetyl-N2-formyl-5methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 2001;15:2294–2296. doi: 10.1096/fj.01-0309fje. [DOI] [PubMed] [Google Scholar]
- 25.Kleszczynski K, Stepnowski P, Skadanowski AC. Mechanism of cytotoxic action of perfluorinated acids. II. Disruption of mitochondrial bioenergetics. Toxicol Appl Pharmacol. 2009;235:182–190. doi: 10.1016/j.taap.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Shindo Y, Witt E, Han D, Packer L. Dose-response effects of acute ultraviolet irradiation on antioxidants and molecular markers of oxidation in murine epidermis and dermis. J Invest Dermatol. 1994;102:470–475. doi: 10.1111/1523-1747.ep12373027. [DOI] [PubMed] [Google Scholar]
- 27.Fischer TW, Scholz G, Knöll B, Hipler UC, Elsner P. Melatonin reduces UV induced reactive oxygen species in dose-dependent manner in IL-3-stimulated leukocytes. J Pineal Res. 2001;31:39–45. doi: 10.1034/j.1600-079x.2001.310106.x. [DOI] [PubMed] [Google Scholar]
- 28.Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J, Szczesniewski A, et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002;16:896–898. doi: 10.1096/fj.01-0952fje. [DOI] [PubMed] [Google Scholar]
- 29.Conti A, Conconi S, Hertens E, Skwarlo-Sonta K, Markowska M, Maestroni JM. Evidence for melatonin synthesis in mouse and human bone marrow cells. J Pineal Res. 2000;28:193–202. doi: 10.1034/j.1600-079x.2000.280401.x. [DOI] [PubMed] [Google Scholar]
- 30.Pablos MI, Agapito MT, Gutierrez R, Recio JM, Reiter RJ, Barlow-Walden L, et al. Melatonin stimulates the activity of the detoxyfying enzyme glutathione peroxidase in several tissues of chicks. J Pineal Res. 1995;19:111–115. doi: 10.1111/j.1600-079x.1995.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 31.Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Rodriguez C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci. 2002;59:1706–1713. doi: 10.1007/PL00012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomás-Zapico C, Boga JA, Caballero B, Vega-Naredo I, Sierra V, Alvarez-García O, et al. Coexpression of MT1 and RORalpha1 melatonin receptors in the Syrian hamster Harderian gland. J Pineal Res. 2005;39:21–26. doi: 10.1111/j.1600-079X.2005.00210.x. [DOI] [PubMed] [Google Scholar]
- 33.Fischer TW, Scholz G, Knoll B, Hipler UC, Elsner P. Melatonin suppresses reactive oxygen species in UV-irradiated leukocytes more than vitamin C and trolox. Skin Pharmacol Appl Skin Physiol. 2002;15:367–373. doi: 10.1159/000064543. [DOI] [PubMed] [Google Scholar]
- 34.Ryoo YW, Suh SI, Mun KC, Kim BC, Lee KS. The effects of the melatonin on ultraviolet-B irradiated cultured dermal fibroblasts. J Dermatol Sci. 2001;27:162–169. doi: 10.1016/s0923-1811(01)00133-5. [DOI] [PubMed] [Google Scholar]
- 35.Lee KS, Lee WS, Suh SI, Kim SP, Lee SR, Ryoo YW, Kim BC. Melatonin reduces ultraviolet-B induced cell damages and polyamine levels in human skin fibroblasts in culture. Exp Mol Med. 2003;35:263–268. doi: 10.1038/emm.2003.35. [DOI] [PubMed] [Google Scholar]
- 36.Fischer TW, Zbytek B, Sayre RM, Apostolov EO, Basnakian AG, Sweatman TW, et al. Melatonin increases survival of HaCaT keratinocytes by suppressing UV-induced apoptosis. J Pineal Res. 2006;40:18–26. doi: 10.1111/j.1600-079X.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 37.Fischer TW, Zmijewski MA, Wortsman J, Slominski A. Melatonin maintains mitochondrial membrane potential and attenuates activation of initiator (casp-9) and effector caspases (casp-3/casp-7) and PARP in UVR-exposed HaCaT keratinocytes. J Pineal Res. 2008;44:397–407. doi: 10.1111/j.1600-079X.2007.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]