Abstract
Since the early days of psychosomatic thinking, atopic disease was considered exemplary. In the 70s and 80s numerous reports stated increased anxiety, depression or ill stresscoping in atopics in correlation with enhanced disease activity. Employed patient groups however were small and diverse and controls rare. Therefore, the question remained, whether psychopathological findings in atopics were of pathogenetic relevance or an epiphenomenon of chronic inflammatory disease. Recently, the discussion has been revived and refocused by psychoneuroimmunological findings. We now know that atopic disease is characterized by an imbalance of the classical stress-axis response along the hypothalamus-pituitary-adrenal axis (HPA) and the sympathetic axis (SA). This imbalance can be found shoulder-to-shoulder with enhanced expression of newly emerging neuroendocrine stress mediators such as substance P (SP) and nerve growth factor that form up to a third stress axis (neurotrophin neuropeptide axis: NNA). Together they can alter the inflammatory as well as the neuroendocrine stress-response on several levels. In skin, the immediate inflammatory response to stress involves neuropeptide release and mast cell degranulation, in short neurogenic inflammation. Systemically, antigen-presentation and TH2 cytokine bias are promoted under the influence of cortisol and neuropeptides. Imbalanced stress-responsiveness may therefore be at the core of exacerbated allergic disease and deserves re-evaluation of therapeutic options such as neutralization of SP-signaling by antagonists against its receptor NK1, cortisol treatment as supplementation and relaxation techniques to balance the stress-response.
Key words: neurotrophins, Trk, p75NTR, skin, psoriasis, melanoma
Introduction
The relevance of stress generated by psychological strain such as anxiety, depression, traumatic life events or daily hassles but also by environmental and behavioral factors such as heat, cold, microbes, tobacco smoke, exercise etc., is a matter of hot debate when it comes to development and aggravation of chronic inflammatory diseases. Most clinicians and patients with for example atopic dermatitis will agree that there is some connection and that stress indeed plays a role in the course of the disease.1 A patient presenting with a demanding job, small children or sick family members to care for, will certainly hear the question: “you do have a lot of stress, don't you?”, and if nothing else provides effective treatment, psychotherapeutic or psychoeducational programs may be considered. However, symptoms of neuroendocrine arousal and the immunological results of stress-dependent alterations in neuroendocrine responses are hardly ever discussed or verified. Also stress is always looked at as deleterious and potential beneficial effects especially of dosed exposure are rarely considered.
Stress and Inflammatory Response: Closely Connected Response Mechanisms to Environmental Change
Like with other chronic diseases, it was not possible to determine a typical atopic personality profile.2,3 However, our growing understanding of neuronal networks and their lifelong plasticity provides new insights into the hardwiring of neuro-immune interaction and its epigenetic modification beyond genetics and morphogenesis.4,5 Allergy and psychological aspects are as closely connected to each other as skin and brain, which both derive from the ectoderm. You could say, every neuronal factor ever found to play a role in the brain is also found in functional skin cells (keratinocytes, fibroblasts, etc.,) as well as skin resident (mast cells, Langerhans cells) or skin homing (T-effector cells, antigen presenting cells, macrophages, granulocytes etc.,) immune cells.6–12 The inflammatory response thereby is the most primitive defense mechanism of the organism and its rudiments developed even before the nervous system. The stress response has developed from the immune response and remained the closely associated and highly conserved oldest response mechanism to environmental changes. Stress has therefore a high potential to provoke adaptive changes in neuroendocrine-immune circuitry and respective interventions may well be able to improve even genetically determined disease.13
A Third Stress Axis Complements Neuro-Immune Adaptation to Inflammatory Challenge
The best known stress pathways commonly are activation of the hypothalamus pituitary adrenal axis (HPA) and the sympathetic axis (SA).14,15 Acute stress triggers high release of their key mediators cortisol and adrenalin/noradrenalin within minutes. The immune system responds by increased pro-inflammatory cytokine levels such as interferonγ (T helper cell type 1 [TH1] cytokine) and by mounting a fast but tissue damaging cellular immune response.16,17 By contrast, chronic stress exposure reduces the capacity to mount an acute stress response and increases basal cortisol levels.15 Now the immune response shifts from cellular to humoral and cytokines such as interleukin 4 and 5 (TH2) are most prominent.18 This enables the immune system to terminate acute inflammation but also facilitates development of autoimmune and atopic disease.19,20–24 Interestingly, epigenetic modification of the HPA stress axis renders the individual even more susceptible to mount a misbalanced chronic stress response.4,25,26
This simplifying model of the stress response and its immunological effects ignores the presence of a third stress axis that is always co-activated. Along this third stress axis neuropeptides and neurotrophins are released centrally and peripherally (NNA).11,27–33 Activation of the NNA on the level of the hypothalamus can suppress activation of the HPA axis, a phenomenon described in stressed atopics.34 In the periphery, neuropeptides released by stress cause massive mast cell degranulation. This activation was first shown by neuroimmunologists such as Bienstock in the 90ies and has been confirmed many times.11,27–29,31,33,35
Ever since this discovery review articles stated the pro-inflammatory potential of neurogenic inflammation in the development and aggravation of chronic inflammatory diseases such as atopic dermatitis. Also, skin is growingly recognized as a neuro-immuno-endocrine organ. This organ is not only the target of neuroendocrine mediators but also its source.36 But the relevance and impact of the cutaneous neuro-immune interaction remained unclear.
Stress during Challenge is Detrimental to Peripheral Inflammation: Evidence from a Mouse Model
In a mouse model for atopic dermatitis-like allergic dermatitis (AlD) and noise stress, as an example of environmental stress that acts through perception of a threat rather than physical harm, we were able to show for the first time, that stress indeed enhances neuronal plasticity and subsequently neurogenic inflammation in a peripheral inflammatory disease. This activation accounted for a worsening of disease parameters such as epidermal hyperplasia, vascular activation or infiltration by eosinophils by approximately 50% and depended on SP and partially on NGF.11,37 We concluded, that stress around the time of an inflammatory challenge enhances the inflammatory response with deleterious effects on chronic inflammatory diseases.11,38
Other have shown that this enhanced neuro-immune interaction also increases the susceptibility of mast cells to respond to non-neuronal mast cell activators such as IgE.39,40 Intriguingly, this situation may be further enhanced by the chronic-stress-cortisol-release-pattern which results in enhanced SP production by keratinocytes.41 Other neuropeptides that may contribute to the described immune imbalance include vasoactive intestinal peptide (VIP). Increased levels of this neuropeptide correlate for example with increased IL-4 levels in children of divorced parents.42
Interestingly, neurotrophins such as the nerve growth factor NGF are also detectable in increased levels in animals disturbed by stress on systemic as well as local level.43–45 Being a neurotrophin, NGF supports enhanced neuro-immune communication by promoting neuronal plasticity but also by directly affecting keratinocyte proliferation and immune cell function in inflamed skin.46–51 However, activation of this axis does not directly contribute to neurogenic inflammation but rather enhances the skin's level of resistance to any threat imposed upon it.43
The Stress Response is not a One Way Street
Summarizing the above, stress appears to be mainly detrimental to chronic inflammatory disease. However, it appears that stress can be trained to handle. Almost 30 years ago for example it was demonstrated that histamin release can be conditioned and mast cell dependent reactions can be modulated by psychoemotional intervention (i.e., hypnosis), which suppresses allergic inflammation.52–55 Ader was later able to show that it is possible to condition immune suppression via modification of the neuroendocrine stress response.56 This demonstrates that the stress response is not a one way street that once taken only leads to worsened disease. It can be used to “harden” the organism against repeated challenges. In a recent study, we found that certain stress paradigms enhance neuro-immune interaction with antigen presenting dendritic cells.57 As a result, T-regulatory cells are produced and suppress cutaneous inflammation in the AlD model.
Neuroimmune Interaction in Lymphoid Organs: High Potential for Systemic Immune Modulation
Some works also suggest that the stress-dependent activation of the noradrenergic innervation of lymphoid organs and subsequently increased production of TH2 cytokines may also play a role in TH2-mediated diseases.18,58,59 The spleen holds a special position among the lymphoid organ. Primarily noradrenergic innervated with a far smaller portion of peptidergic nerve fibers, the spleen responds to physical and psychological stimulants with a TH2 bias.60 In addition, Sloan and colleagues showed that social stress in primates lead to increased innervation of lymph nodes in parallel with increased NGF tissue levels and subsequent higher vulnerability to viral infections.58,61,62
This splenic TH2 bias is referred to as an anti-inflammatory pathway and protects the body from excessive tissue-destructive, inflammatory response e.g., to an invasion of bacteria, but apparently has also pathogenetic relevance. The amount of cytokines produced within the spleen can act peripherally as well as centrally.59,63 Like the skin, the spleen has a stress-dependent activated innervation, contacts between nerve fibers and immune cells are an interface between periphery and the CNS.64–68 It therefore offers itself as an intriguing site to examine the impact of lymphoid neuro-immune interaction and the role of the NNA within it on the onset or prolongation of diseases with allergic and/or inflammatory background.
Future Instructive Directions for the Therapy of Chronic Inflammatory Disease Need to Include Neuroendocrine Circuitry
Targeting the NNA in the treatment of chronic inflammatory disease is a promising target for effective therapeutic intervention. In doing so, the pointed pharmacological intervention to terminate neuro-immune activation in ongoing inflammation by neutralizing SP and/or NGF signaling can complement the classical cortisol therapy. At the same time, any relaxation technique that reduces chronic HPA, SA as well as NNA activation, ranging from laughter to conflict resolving psychotherapy, should prove effective. In analogy to sublingual immunotherapy, which follows the concept of tolerance induction through repeated exposure to minimal allergen challenges, we suggest adopting a popular slogan in establishing therapeutic strategies for the management of allergic disease: “a little stressor a day keeps the doctor away.”69,70 Providing evidence based research and guidelines for future employment of respective therapeutic concepts are important goals of neuroendocrine research in dermatology and it ought to be a primary target of clinical investigation and modern prevention strategies to define therapeutic stress-strategies that break the stress circuit and reestablish a balanced stress response.
In summary, to what degree stress affects inflammatory disease outcome or what pathways are involved is a black box to most patients and doctors. To improve our understanding of the mediators involved has therefore a great potential to optimize handling of chronic inflammatory diseases such as atopic disease and the frustrating treatment of the chronically diseased.71,72 It should therefore be implemented in standardized patient educational programs and additional pharmacological intervention is to be explored.
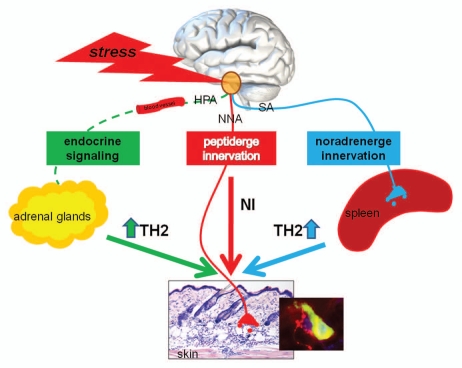
Figure 1.
Schematic presentation of neuroendocrine stress effects on skin health. Stress dependent activation of the endocrine pathway (HPA) and the sympathetic nervous system (SA) lead to a systemically TH2 bias, playing a role in the onset and/or extending of the allergic component in atopic diseases. The activation of peptidergic innervations directly effects healthy skin homeostasis inducing TH1 cytokines, mast cells degranulation and eosinophilia leading to a neurogenic inflammation (NI). Understanding of the linkage of these three stress axes—how they communicate with each other and how they influence each other opens the research to future targets of possible disease interventions.
Acknowledgements
This study was supported by grants from the Universitätsmedizin Charité Berlin, Germany and the German Research Foundation (DFG Pe 890/1-3, 4-1) to Eva Peters.
Abbreviations
- AD
atopic dermatitis and-like allergic dermatitis
- SP
substance P
- NK1
neurokinin-1
- SPR
SP receptor
- HPA
hypothalamic-pituitary-adrenocortical axis
- TH
T-helper cell
- IL-4 and -5
interleukin (IL)-4 and IL-5
- TNFγ
tumor necrosis factor alpha
- IFNγ
interferon gamma
- AL(OH)3
aluminium hydroxide
References
- 1.Gieler U, Ring J, Wahn V. Neurodermitisschulung. Ein neues Behandlungsprogramm zur sekundären Prävention. Dt Ärztebl. 2001;98:3202–3209. (Ger). [Google Scholar]
- 2.Richter R. Allergie. In: Meyer AE, Freyberger H, Kerekjarto Mv, et al., editors. Jores Praktische Psychosomatik—Einführung in die Psychosomatische und Psychotherapeutische Medizin. Bern: Huber; 1996. pp. 423–434. (Ger). [Google Scholar]
- 3.Richter R, Ahrens S. Asthma. In: Ahrens S, Schneider W, editors. Lehrbuch der Psychotherapie und Psychosomatik. Stuttgart, New York: Schattauer; 2002. pp. 410–419. (Ger). [Google Scholar]
- 4.Branchi I, Francia N, Alleva E. Epigenetic control of neurobehavioural plasticity: the role of neurotrophins. Behav Pharmacol. 2004;15:353–362. doi: 10.1097/00008877-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese F, Molteni R, Racagni G, Riva MA. Neuronal plasticity: a link between stress and mood disorders. Psychoneuroendocrinology. 2009;34:208–216. doi: 10.1016/j.psyneuen.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Luger TA, Lotti T. Neuropeptides: role in inflammatory skin diseases. J Eur Acad Dermatol Venereol. 1998;10:207–211. [PubMed] [Google Scholar]
- 7.Niemeier V, Kupfer J, Al-Abesie S, Schill WB, Gieler U. [From neuropeptides and cytokines to psychotherapy. Skin diseases between psychoneuroimmunology research and psychosomatic treatment] Forsch Komplementarmed. 1999;2:14–18. doi: 10.1159/000057141. (Ger). [DOI] [PubMed] [Google Scholar]
- 8.Stander S, Steinhoff M. Pathophysiology of pruritus in atopic dermatitis: an overview. Exp Dermatol. 2002;11:12–24. doi: 10.1034/j.1600-0625.2002.110102.x. [DOI] [PubMed] [Google Scholar]
- 9.Peters EM, Hansen MG, Overall RW, Nakamura M, Pertile P, Klapp BF, et al. Control of human hair growth by neurotrophins: brain-derived neurotrophic factor inhibits hair shaft elongation, induces catagen and stimulates follicular transforming growth factor beta2 expression. J Invest Dermatol. 2005;124:675–685. doi: 10.1111/j.0022-202X.2005.23648.x. [DOI] [PubMed] [Google Scholar]
- 10.Peters EM, Hendrix S, Golz G, Klapp BF, Arck PC, Paus R. Nerve growth factor and its precursor differentially regulate hair cycle progression in mice. J Histochem Cytochem. 2006;54:275–288. doi: 10.1369/jhc.4A6585.2005. [DOI] [PubMed] [Google Scholar]
- 11.Pavlovic S, Daniltchenko M, Tobin DJ, Hagen E, Hunt SP, Klapp BF, et al. Further exploring the brain-skin connection: stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. J Invest Dermatol. 2008;128:434–446. doi: 10.1038/sj.jid.5701079. [DOI] [PubMed] [Google Scholar]
- 12.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiological reviews. 2006;86:1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 13.Langkafel M, Lorenzen J, Richter-Görge H, Senf W. Entwicklungstheorie. In: Senf WBM, editor. Praxis der Psychotherapie. Stuttgart: Thieme; 2000. (Ger). [Google Scholar]
- 14.Marshall GD. Neuroendocrine mechanisms of immune dysregulation: applications to allergy and asthma. Ann Allergy Asthma Immunol. 2004;93:11–17. doi: 10.1016/s1081-1206(10)61482-2. [DOI] [PubMed] [Google Scholar]
- 15.Straub RH. Lehrbuch der klinischen Pathophysiologie komplexer chronischer Erkrankungen Vandenhoe & Ruprecht. 2006. Physiologische Grundlagen. (Ger). [Google Scholar]
- 16.Buske-Kirschbaum A, Ebrecht M, Kern S, Hellhammer DH. Endocrine stress responses in TH1-mediated chronic inflammatory skin disease (psoriasis vulgaris)—do they parallel stress-induced endocrine changes in TH2-mediated inflammatory dermatoses (atopic dermatitis)? Psychoneuroendocrinology. 2006;31:439–446. doi: 10.1016/j.psyneuen.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Dhabhar FS. Acute stress enhances while chronic stress suppresses skin immunity. The role of stress hormones and leukocyte trafficking. Ann NY Acad Sci. 2000;917:876–893. doi: 10.1111/j.1749-6632.2000.tb05454.x. [DOI] [PubMed] [Google Scholar]
- 18.Marshall GD, Jr, Agarwal SK. Stress, immune regulation and immunity: applications for asthma. Allergy Asthma Proc. 2000;21:241–246. doi: 10.2500/108854100778248917. [DOI] [PubMed] [Google Scholar]
- 19.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines and autoimmunity. Ann NY Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 20.Buske-Kirschbaum A, Gierens A, Hollig H, Hellhammer DH. Stress-induced immunomodulation is altered in patients with atopic dermatitis. Journal of neuroimmunology. 2002;129:161–167. doi: 10.1016/s0165-5728(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 21.Buske-Kirschbaum A, von Auer K, Krieger S, Weis S, Rauh W, Hellhammer D. Blunted cortisol responses to psychosocial stress in asthmatic children: a general feature of atopic disease? Psychosom Med. 2003;65:806–810. doi: 10.1097/01.psy.0000095916.25975.4f. [DOI] [PubMed] [Google Scholar]
- 22.Helmbold P, Gaisbauer G, Kupfer J, Haustein UF. Longitudinal case analysis in atopic dermatitis. Acta Derm Venereol. 2000;80:348–352. doi: 10.1080/000155500459286. [DOI] [PubMed] [Google Scholar]
- 23.Schmid-Ott G, Jaeger B, Meyer S, Stephan E, Kapp A, Werfel T. Different expression of cytokine and membrane molecules by circulating lymphocytes on acute mental stress in patients with atopic dermatitis in comparison with healthy controls. The J Allergy Clin Immunol. 2001;108:455–462. doi: 10.1067/mai.2001.117800. [DOI] [PubMed] [Google Scholar]
- 24.Buske-Kirschbaum A. Cortisol responses to stress in allergic children: interaction with the immune response. Neuroimmunomodulation. 2009;16:325–332. doi: 10.1159/000216190. [DOI] [PubMed] [Google Scholar]
- 25.Angelucci L. The hypothalamus-pituitary—adrenocortical axis: epigenetic determinants changes with aging, involvement of NGF. Neurochem Intl. 1994;25:53–59. doi: 10.1016/0197-0186(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 26.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 27.Djuric VJ, Bienenstock J. Learned sensitivity. Ann Allergy. 1993;71:5–14. [PubMed] [Google Scholar]
- 28.Joachim RA, Handjiski B, Blois SM, Hagen E, Paus R, Arck PC. Stress-induced neurogenic inflammation in murine skin skews dendritic cells towards maturation and migration: key role of intercellular adhesion molecule-1/leukocyte function-associated antigen interactions. Am J Pathol. 2008;173:1379–1388. doi: 10.2353/ajpath.2008.080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters EM, Kuhlmei A, Tobin DJ, Müller-Röver S, Klapp BF, Arck PC. Stress exposure modulates peptidergic innervation and degranulates mast cells in murine skin. Brain Behav Immun. 2005;19:252–262. doi: 10.1016/j.bbi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Wright RJ. Stress and atopic disorders. J Allergy Clin Immunol. 2005;116:1301–1306. doi: 10.1016/j.jaci.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 31.Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Curr Opin Allergy Clin Immunol. 2005;5:23–29. doi: 10.1097/00130832-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Bienenstock J, MacQueen G, Sestini P, Marshall JS, Stead RH, Perdue MH. Mast cell/nerve interactions in vitro and in vivo. Am Rev Respir Dir. 1991;143:55–58. doi: 10.1164/ajrccm/143.3_Pt_2.S55. [DOI] [PubMed] [Google Scholar]
- 33.Amano H, Negishi I, Akiyama H, Ishikawa O. Psychological stress can trigger atopic dermatitis in NC/Nga mice: an inhibitory effect of corticotropin-releasing factor. Neuropsychopharmacology. 2008;33:566–573. doi: 10.1038/sj.npp.1301435. [DOI] [PubMed] [Google Scholar]
- 34.Nussdorfer GG, Malendowicz LK. Role of tachykinins in the regulation of the hypothalamo-pituitary-adrenal axis. Peptides. 1998;19:949–968. doi: 10.1016/s0196-9781(98)00017-5. [DOI] [PubMed] [Google Scholar]
- 35.Bienenstock J. From IgA to neuro-immunomodulation:a travelogue through immunology. Neth J Med. 1991;39:183–187. [PubMed] [Google Scholar]
- 36.Fischer TW, Peters EMJ. 8th Annual Meeting of the German Dermatoendocrinology Working Group (ADE) of Arbeitsgemeinschaft Dermatologische Forschung (ADF) Exp Dermatol. 2009;18:1082–1084. doi: 10.1111/j.1600-0625.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- 37.Peters EM, Handjiski B, Kuhlmei A, Hagan E, Bielas H, Braun A, et al. Neurogenic inflammation in stress-induced termination of murine hair growth is promoted by nerve growth factor. Am J Pathol. 2004;165:259–271. doi: 10.1016/S0002-9440(10)63294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters EM, Liezmann C, Spatz K, Daniltchenko M, Joachim R, Gimenez-Rivera A. Nerve growth factor partially recovers inflamed skin from stress-induced worsening in allergic inflammation. J Invest Dermatol. 2010 doi: 10.1038/jid.2010.317. In press. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki R, Furuno T, McKay DM, Wolvers D, Teshima R, Nakanishi M, et al. Direct neurite-mast cell communication in vitro occurs via the neuropeptide substance P. J Immunol. 1999;163:2410–2415. [PubMed] [Google Scholar]
- 40.Siebenhaar F, Magerl M, Peters EM, Hendrix S, Metz M, Maurer M. Mast cell-driven skin inflammation is impaired in the absence of sensory nerves. J Allergy Clin Immunol. 2008;121:955–961. doi: 10.1016/j.jaci.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Katayama I, Bae SJ, Hamasaki Y, Igawa K, Miyazaki Y, Yokozeki H, et al. Stress response, tachykinin and cutaneous inflammation. J Investig Dermatol Symp Proc. 2001;6:81–86. doi: 10.1046/j.0022-202x.2001.00015.x. [DOI] [PubMed] [Google Scholar]
- 42.Herberth G, Weber A, Roder S, Elvers HD, Kramer U, Schin RP, et al. Relation between stressful life events, neuropeptides and cytokines: results from the LISA birth cohort study. Pediatr Allergy Immunol. 2008;19:722–729. doi: 10.1111/j.1399-3038.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 43.Peters EM, Liezmann C, Spatz K, et al. Nerve Growth Factor Partially Recovers Inflamed Skin from Stress-Induced Worsening in Allergic Inflammation. J Invest Dermatol. doi: 10.1038/jid.2010.317. [DOI] [PubMed] [Google Scholar]
- 44.Zhu SW, Yee BK, Nyffeler M, Winblad B, Feldon J, Mohammed AH. Influence of differential housing on emotional behaviour and neurotrophin levels in mice. Behav Brain Res. 2006;169:10–20. doi: 10.1016/j.bbr.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 45.Alleva E, Aloe L, Bigi S. An updated role for nerve growth factor in neurobehavioural regulation of adult vertebrates. Rev Neurosci. 1993;4:41–62. doi: 10.1515/revneuro.1993.4.1.41. [DOI] [PubMed] [Google Scholar]
- 46.Botchkarev VA, Botchkareva NV, Welker P, Metz M, Lewin GR, Subramaniam A, et al. A new role for neurotrophins: involvement of brain-derived neurotrophic factor and neurotrophin-4 in hair cycle control. FASEB J. 1999;13:395–410. doi: 10.1096/fasebj.13.2.395. [DOI] [PubMed] [Google Scholar]
- 47.Braun A, Appel E, Baruch R, Herz U, Botchkarev V, Paus R, et al. Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eu J Immunol. 1998;28:3240–3251. doi: 10.1002/(SICI)1521-4141(199810)28:10<3240::AID-IMMU3240>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 48.Peters EM, Raap U, Welker P, Tanaka A, Matsuda H, Pavlovic-Masnicosa S, et al. Neurotrophins act as neuroendocrine regulators of skin homeostasis in health and disease. Horm Metab Res. 2007;39:110–124. doi: 10.1055/s-2007-961812. [DOI] [PubMed] [Google Scholar]
- 49.Aloe L, Bracci-Laudiero L, Bonini S, Manni L. The expanding role of nerve growth factor: from neurotrophic activity to immunologic diseases. Allergy. 1997;52:883–894. doi: 10.1111/j.1398-9995.1997.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 50.Brodie C. Differential effects of Th1 and Th2 derived cytokines on NGF synthesis by mouse astrocytes. FEBS Lett. 1996;394:117–120. doi: 10.1016/0014-5793(96)00911-8. [DOI] [PubMed] [Google Scholar]
- 51.Kupfer J, Gieler U, Braun A, et al. Stress and atopic eczema. Int Arch Allergy Immunol. 2001:124. [Google Scholar]
- 52.Zachariae R, Bjerring P. The effect of hypnotically induced analgesia on flare reaction of the cutaneous histamine prick test. Arch Dermatol Res. 1990;282:539–543. doi: 10.1007/BF00371950. [DOI] [PubMed] [Google Scholar]
- 53.Russell M, Dark KA, Cummins RW, Ellman G, Callaway E, Peeke HV. Learned histamine release. Science. 1984;225:733–734. doi: 10.1126/science.6205449. [DOI] [PubMed] [Google Scholar]
- 54.Kossak HC. Ein Lehrbuch. Psychologie Verlags Union. 1997. Hypnose. (Ger). [Google Scholar]
- 55.Langewitz W, Izakovic J, Wyler J, Schindler C, Kiss A, Bircher AJ. Effect of self-hypnosis on hay fever symptoms—a randomised controlled intervention study. Psychother Psychosom. 2005;74:165–172. doi: 10.1159/000084001. [DOI] [PubMed] [Google Scholar]
- 56.Ader R. Classical conditioning in the treatment of psoriasis. Cutis. 2000;66:370–372. [PubMed] [Google Scholar]
- 57.Pavlovic S, Liezmann C, Blois SM, Joachim R, Kruse J, Romani N, et al. Substance P is a key mediator of stress-induced protection from allergic sensitization via modified antigen presentation. J Immunol. 2011;186:848–855. doi: 10.4049/jimmunol.0903878. [DOI] [PubMed] [Google Scholar]
- 58.Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. J Neurosci. 2007;27:8857–8865. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 60.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sloan EK, Capitanio JP, Cole SW. Stress-induced remodeling of lymphoid innervation. Brain Behav Immun. 2008;22:15–21. doi: 10.1016/j.bbi.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sloan EK, Capitanio JP, Tarara RP, Cole SW. Social temperament and lymph node innervation. Brain Behav Immun. 2008;22:717–726. doi: 10.1016/j.bbi.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meltzer JC, MacNeil BJ, Sanders V, Pylypas S, Jansen AH, Greenberg AH, et al. Stress-induced suppression of in vivo splenic cytokine production in the rat by neural and hormonal mechanisms. Brain Behav Immun. 2004;18:262–273. doi: 10.1016/j.bbi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Felten DL. Direct innervation of lymphoid organs: substrate for neurotransmitter signaling of cells of the immune system. Neuropsychobiology. 1993;28:110–112. doi: 10.1159/000119011. [DOI] [PubMed] [Google Scholar]
- 65.Straub RH. Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol Sci. 2004;25:640–646. doi: 10.1016/j.tips.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 66.Stevens-Felten SY, Bellinger DL. Noradrenergic and peptidergic innervation of lymphoid organs. Chem Immunol. 1997;69:99–131. doi: 10.1159/000058655. [DOI] [PubMed] [Google Scholar]
- 67.Besedovsky HO, del Rey A. The cytokine-HPA axis feed-back circuit. Zeitschrift fur Rheumatologie. 2000;59:26–30. doi: 10.1007/s003930070014. (Ger). [DOI] [PubMed] [Google Scholar]
- 68.Besedovsky H, Sorkin E, Felix D, Haas H. Hypothalamic changes during the immune response. European journal of immunology. 1977;7:323–325. doi: 10.1002/eji.1830070516. [DOI] [PubMed] [Google Scholar]
- 69.Allam JP, Novak N, Fuchs C, Asen S, Bergé S, Appel T, et al. Characterization of dendritic cells from human oral mucosa: a new Langerhans' cell type with high constitutive FcepsilonRI expression. J Allergy Clin Immunol. 2003;112:141–148. doi: 10.1067/mai.2003.1607. [DOI] [PubMed] [Google Scholar]
- 70.Broide DH. Immunomodulation of allergic disease. Annu Rev Med. 2009;60:279–291. doi: 10.1146/annurev.med.60.041807.123524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niemeier V, Nippesen M, Kupfer J, Schill WB, Gieler U. Psychological factors associated with hand dermatoses: which subgroup needs additional psychological care? Br J Dermatol. 2002;146:1031–1037. doi: 10.1046/j.1365-2133.2002.04716.x. [DOI] [PubMed] [Google Scholar]
- 72.Gieler U, Niemeier V, Brosig B. Psychoneuroimmunology and Evaluation of Therapeutic Approaches. In: Bieber T, Leung DYM, editors. Atopic Dermatitis. New York, Basel: Marcel Dekker, Inc.; 2002. pp. 43–65. [Google Scholar]