Copay for chemotherapy drugs is a financial hardship for many patients with cancer. The authors have developed a support program to work closely with copay assistance foundations to secure financial assistance for appropriate treatment.
Abstract
Purpose:
With the advent of newer cancer therapies (eg, biologic and cytotoxic), treatment is becoming increasingly expensive for patients with cancer. Patients enrolled in Medicare and commercial insurance plans often have large copay requirements with each treatment cycle. Often, these patients undergo significant financial hardship, and some patients decline treatment. We have developed a support program that works closely with all copay assistance foundations to secure financial assistance to facilitate appropriate treatment.
Methods:
In September, 2008 we initiated a coordinated program with various copay assistance foundations, including Healthwell, Cancer Care, Patient Access, Chronic Disease Fund, Beckstrand Cancer, Lilly Cares and the Leukemia and Lymphoma Society. Patients requesting assistance with chemotherapy copay were enrolled in this program. Information about income level, chemotherapy regimens, and associated copay was given to these foundations, who then determined the amount of monetary assistance.
Results:
Since the initiation of this program, of 201 patients who began receiving chemotherapy, 25 (12.4%) requested assistance with this program for either intravenous or oral treatments. The current results of time delays for foundation decision, success rates and administrative costs to secure funding will be presented at the time of the poster presentation.
Conclusion:
Copay for chemotherapy drugs is a financial hardship for a significant number of patients. Coordinated resources must be provided and reimbursed to facilitate appropriate and sustainable cancer care. This program is a successful model for other centers to adopt.
Introduction
With new anticancer treatment programs becoming increasingly expensive, there has been an increased need to address the costs of care. ASCO has developed a guidance statement1 and policy positions to address this issue. These high costs have made many patients discontinue therapy.2 It has been estimated that, in the United States, more than one million cancer patients are foregoing cancer care because of copayment costs.3 According to the American Cancer Society, 20% of cancer patients spend their entire life savings to get cancer care, and an increasing number of cancer deaths are a direct result of inadequate insurance.4
Pharmaceutical manufacturers have developed assistance programs to help patients access to their medications, including cancer-related or parenterally administered medications. Both pharmacists5–9 and physician practices have used these assistance programs. Optimal use of these assistance programs requires appropriate administrative support. The costs involved to enable indigent patients to obtain oral nonchemotherapy medications have been analyzed and found to be substantial.10 Other groups including the Cancer Financial Assistance Coalition (CFAC), a coalition of financial assistance organizations is trying to help cancer patients with their financial challenges.
Eighty-five percent of cancer care in the United States is given in community oncology settings. Funding cut backs have lead to staff reductions in many of these cancer centers at a time when patients are requiring more administrative assistance in navigating the increasingly complex reimbursement system. To help defray the costs of cancer care, pharmaceutical manufacturers have established foundations that can pay for otherwise unaffordable coshare or copayment costs of patients. However, the costs of coordinating patient application for such foundation support are unknown.
In response to this situation, Wilshire Oncology Medical Group (LaVerne, CA) has developed a patient support program to coordinate application to copay assistance support foundations for patients scheduled to receive intravenous cancer treatments. We performed the present study to determine the efficiency, costs, and cost effectiveness of this program.
Methods
Wilshire Oncology Medical Group is a community cancer program consisting of nine medical oncologists and two radiation oncologists, with intravenous chemotherapy and biotherapy administered in six infusion centers. There is a centralized administration office for billing and support staff. Physicians agreed to fund and operate a central coordination program for patients with cancer who apply to a patient support foundation.
Wilshire Oncology Medical Group assigned one half of a FTE (full-time equivalent) to administer the financial assistance from its centralized office. All patients scheduled for intravenous anticancer therapy received individual financial counseling by clinical office staff before initiating intravenous therapy. Managers in the six infusion centers determined the need for assistance on the basis of patient insurance and face-to-face financial counseling. For those patients with an established need for financial assistance, the centralized office completed applications (single or multiple) for each patient.
We then evaluated the time for applications, decision (approvals or denials), amount of funds collected from foundations, and factors associated with either approval or denial of foundation applications. Copay foundation assistance received through Wilshire Oncology Medical Group for oral chemotherapies and radiation oncology was not included in this analysis.
Results
This study was performed from September 1, 2008, through March 20, 2009. Nine medical oncologists and two radiation oncologists participated in this study together with the supportive staff. Duration of the study was 199 days. During that time, 500 patients received intravenous anticancer therapy. Five hundred fifty-five individual treatment plans were needed for those 500 patients. An increased number of plans relative to patients were required because some patients experienced disease progression during the first regimen and had applications submitted for a second or third regimen during the study.
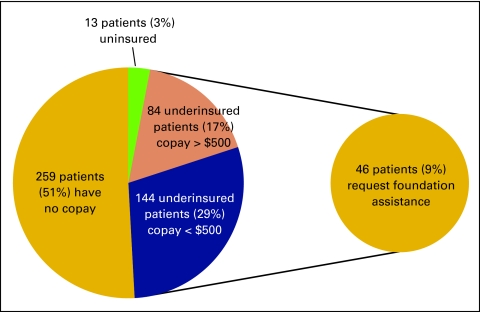
During the study period, 487 patients had insurance coverage, and 13 patients had no coverage. Of the uninsured patients, seven (54%) received free drugs from the manufacturer. For six patients (46%), manufacturers denied drug replacement for the cancer center because of availability of generic drugs for those individuals.
Wilshire Oncology Medical Group was unable to locate resources for these 13 patients for payments for infusions, physician evaluations and management services, imaging services, laboratory studies, and radiation oncology. These 13 patients accounted for 3% of the total patients given intravenous therapy.
Of the remaining 97% of patients, 259 patients (51%) had insurance that paid for all treatment, with no copay or share of costs to the patient (Fig 1). For 84 underinsured patients (17%), the copay amounts were more than $500. In 144 underinsured patients (29%), copay requirement was less than $500. Of the 228 patients who were underinsured, 46 (9% of all patients) requested assistance of Wilshire Oncology Medical Group to identify a patient support foundation to help with copayments.
Figure 1.
Comparison of copay responsibilities for uninsured and underinsured patients.
From these 46 patients, 161 individual applications were submitted (average of 3.5 per patient). Twenty-three applications were submitted per month. The foundations approached for potential support were HealthWell Foundation, Patient Advocacy Foundation Co-Pay Relief Program, Cancer Care Co-Payment Assistance Foundation, Patient Access Network Foundation, Chronic Disease Fund, Beckstrand Cancer Foundation, National Brain Tumor Society, The Leukemia and Lymphoma Society, and National Organization for Rare Disorders.
The average time to determination was 20 days. Of the 161 applications submitted, 76 (47%) were denied, and 85 (53%) were accepted for funding. Overall, for all 46 underinsured patients for whom foundation support was sought, 42 (91%) patients received funds. Four patients (9%) were unable to find foundation support.
The financial aspects of foundation approvals are shown in Table 1. An average of more than $6,000 was received per patient. The average amount required for patients to pay their out-of-pocket costs for cancer therapy ranged from $1,600 to $2,500 after receipt of foundation support. The amount received per physician was more than $28,000, or more than $4,000 per physician per month.
Table 1.
Financial Results of Foundation Application
| Item | Amount |
|---|---|
| Foundation grants approved, % | 85 |
| Total funds approved, $ | 252,747 |
| Average funds approved per patient, $ | 6,018 |
| Funds approved per month, $ | 36,107 |
| Funds approved per physician, $ | 28,083 |
| Funds approved per physician per month, $ | 4,012 |
For those patients who were denied any foundation support, the reasons given were that patient income was too high, the foundation was out of funds (but foundation support was invited when funds were made available again), or that treatment elements were not met. These treatment elements included medication that was not a covered benefit of the foundation, oral chemotherapy not covered by a foundation emphasizing intravenous therapy, or that the patient's diagnosis was not covered by the foundation.
The outcome of the four underinsured patients denied funding by all foundation programs was as follows: One patient received initial treatment that was paid for by the patient's children. One patient declined all therapy. One patient was referred to the County Health Facility. One patient received and paid for the coshare of an alternative, less expensive treatment program.
We next analyzed the administrative costs for foundation support (Table 2). The cost per month was $1,733 the cost per patient applying for support was $264, and the cost per application was $75. These administrative costs represented 5% of total funds collected on behalf of the patients.
Table 2.
Administrative Costs for Foundation Support Coordination
| Item | Amount |
|---|---|
| Salary of patient support coordinator, $ | 16.00/hour (+ benefits) |
| Cost per month, $ | 1,733 |
| Cost for 7 months, $ | 12,131 |
| Cost per year, $ | 20,796 |
| Cost per patient, $ | 264 |
| Cost per application, $ | 75 |
Discussion
Copay for intravenous anticancer therapy treatments was found to be a financial hardship for a significant number of our patients. This difficulty had previously resulted in many patients declining therapy or shortening their course of therapy. In many patients, copays were simply not paid by the patient or the family, resulting in a burden on the oncology practice for uncollected funds.
The development of this foundation assistance program within Wilshire Oncology Medical Group was found to be very successful. Although costs of patient care had previously been a substantial burden on our oncology practice (regardless of practice site or tumor diagnosis), we found that reimbursement obtained as a result of the foundation support coordinator was very cost effective, and we recommend that such support be sought from foundations or pharmaceutical manufacturers.
Because this model was successful, it is appropriate for adoption and implementation by other practices. Because copay assistance is necessary for optimal care of a significant number of patients, pharmaceutical manufacturers, health care insurers (including Medicare), and ASCO should recognize this service in the development of reimbursement codes to ensure that practices can hire the specialized personnel needed to assist patients. In that regard, it is recommended that State Oncology Societies and/or ASCO develop programs that facilitate foundation financial support offices within practices. For example, a universal application form could be developed for use by all foundations to streamline the process of application.
It is also recommended that grants from foundations be tailored to specific patient needs. It is likely that three to five times more patients could be helped with the same level of grant funding. We found that larger-than-needed awards tied up funds for 120 to 150 days. Although these unused grant funds were subsequently reallocated to patients who had qualified 3 to 5 months earlier, those patients had to make alternative therapy choices or forego therapy while unused grant funds were unavailable for distribution.
The limitations of our study are a small sample size limited to a single institution, single geographic area (with related limited social/insurance mix), and a limited number of copay foundations used. We conclude that coordinated resources should be developed in every treatment center to facilitate appropriate, timely, and sustainable cancer care for underinsured patients. Our model can be used for this purpose.
Acknowledgment
Presented in part as a poster at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
Conception and design: Swapnil P. Rajurkar, Cary A. Presant, Linda D. Bosserman, Wendy J. McNatt
Financial support: Linda D. Bosserman
Administrative support: Swapnil P. Rajurkar, Cary A. Presant, Linda D. Bosserman, Wendy J. McNatt
Collection and assembly of data: Swapnil P. Rajurkar, Cary A. Presant, Linda D. Bosserman, Wendy J. McNatt
Data analysis and interpretation: Swapnil P. Rajurkar, Cary A. Presant, Linda D. Bosserman
Manuscript writing: Swapnil P. Rajurkar, Cary A. Presant, Linda D. Bosserman
Final approval of manuscript: Swapnil P. Rajurkar, Cary A. Presant, Linda D. Bosserman, Wendy J. McNatt
References
- 1.Meropol NJ, Schrag D, Smith TJ, et al. American Society of Clinical Oncology guidance statement: The cost of cancer care. J Clin Oncol. 2009;27:3868–3874. doi: 10.1200/JCO.2009.23.1183. [DOI] [PubMed] [Google Scholar]
- 2.Partridge AH, Wang PS, Winer EP, et al. Non-adherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 3. Science Daily News, February 2, 2009.
- 4.Seffrin J. More cancer patients can't afford care. CBS Evening News. 2009 Feb 5; [Google Scholar]
- 5.Chishoelm MA, Tackett KL, Kendrick BD, et al. Assistance programs available for medications commonly used in transplant patients. Clin Transpl. 2000;14:269–281. doi: 10.1034/j.1399-0012.2000.140401.x. [DOI] [PubMed] [Google Scholar]
- 6.Chisholm MA, DiPiro JT. Pharmaceutical manufacturer assistance programs. Arch Intern Med. 2002;162:780–784. doi: 10.1001/archinte.162.7.780. [DOI] [PubMed] [Google Scholar]
- 7.Chisholm MA, Reinhardt BO, Vollenweider LJ, et al. Medication assistance programs for uninsured and indigent patients. Am J Health Syst Pharm. 2001;57:1131–1136. [PubMed] [Google Scholar]
- 8.Sarrafizadeh M, Waite NM, Hobson EH, Migden H. Pharmacist-facilitated enrollment in medication assistance programs in a private ambulatory care clinic. Am J Health Syst Pharm. 2004;61:1816–20. doi: 10.1093/ajhp/61.17.1816. [DOI] [PubMed] [Google Scholar]
- 9.Viale PH, Mister S. Utilization of medication-assistance programs for medically uninsured patients: One public teaching hospital's experience. Clin J Oncol Nurse. 2001;5:247–52. [PubMed] [Google Scholar]
- 10.Clay P, Vaught E, Glaros A, et al. Costs to physician offices of providing medications to medically indigent patients via pharmaceutical manufacturer prescription assistance programs. J Manag Care Pharm. 2007;13:506–514. doi: 10.18553/jmcp.2007.13.6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]