This physician survey looks at the effect of the 21-gene recurrence score assay results on adjuvant treatment recommendations for patients with lymph node–positive, estrogen receptor–positive breast cancer.
Abstract
Purpose:
To survey the effect of the 21-gene recurrence score (RS) assay results on adjuvant treatment recommendations for patients with lymph node–positive (N+), estrogen receptor–positive (ER+) breast cancer.
Methods:
Medical oncologists who ordered the 21-gene RS assay were invited to complete a survey regarding their most recent patient with N+/ER+ breast cancer. We obtained responses from 160 (16%) of the 1,017 medical oncologists.
Results:
Most of the respondents were in community (71%) versus academic (25%) settings and had practiced for a median of 11 years. T1, T2, or T3 disease was reported in 62%, 35%, and 3% of patients, respectively. One, two, three, or ≥ 4 nodes were reported in 69%, 18%, 6%, and 3% of patients, respectively. Eighty-six percent of the oncologists made treatment recommendations before obtaining the RS; 51% changed their recommendations after receiving the RS. In 33%, treatment intensity decreased from chemotherapy plus hormonal therapy to hormonal therapy alone. In 9%, treatment intensity increased from hormonal therapy alone to chemotherapy plus hormonal therapy. In 8%, treatment recommendations changed in a way that did not fit the definition of either increased or decreased intensity.
Conclusion:
In this survey of physician practice, the RS result was used to guide adjuvant treatment decision making in N+/ER+ breast cancer more often in patients with tumors less than 5 cm in size and one to three positive lymph nodes than in patients with larger tumors and four or more positive nodes and yielded an overall reduction in recommendations for chemotherapy.
Introduction
Large clinical trials, including the National Surgical Adjuvant Breast and Bowel Project B-14 and B-20 studies, have demonstrated the benefit of hormonal therapy with tamoxifen and adjuvant chemotherapy in women with node-negative, estrogen receptor–positive (N−/ER+) breast cancer. The likelihood of distant recurrence in this population when treated with tamoxifen alone after surgery is approximately 15% at 10 years.1 Adding chemotherapy to reduce the risk of recurrence requires treatment of all patients. The 21-gene recurrence score (RS) assay provides a reliable method of defining the risk of locoregional and distant recurrence for individual patients with N−/ER+ breast cancer.1–3 The assay has enabled oncologists to identify specific patients with N−/ER+ breast cancer who are unlikely to benefit from chemotherapy.2 The assay has allowed oncologists treating N−/ER+ patients to move from a population-based estimate of recurrence to one that is individualized on the basis of the genetic features of a particular tumor, which has led to an overall reduction in treatment intensity for patients with a low RS.4–8
For patients with node-positive, estrogen receptor–positive (N+/ER+) breast cancer, the National Comprehensive Cancer Network (NCCN)9 and ASCO10 guidelines recommend adjuvant chemotherapy in addition to hormonal therapy, but many of these women would also remain disease free even without receiving chemotherapy. In 2007, scientific data were presented (and later published) showing that the 21-gene RS assay is prognostic and predictive for postmenopausal N+/ER+ patients11–13 and provides information distinct from and complementary to classic clinicopathologic features.12 The information led some medical oncologists in the United States to begin using the 21-gene RS assay with select patients. Through mid-2009, more than 1,000 assays were performed in the United States subsequent to the scientific presentations, so we conducted a practice pattern survey to better understand how oncologists actually used the 21-gene RS assay results for patients with N+/ER+ breast cancer. The study objectives were to determine whether the assay results affected adjuvant treatment recommendations in a group of medical oncologists who participated in a Web-based survey and to ascertain which factors were important in the decision to order the assay.
Methods
We conducted cognitive interviews with four practicing medical oncologists who used the 21-gene RS assay. Interviewees were from both single-specialty and academic medical groups, had been in practice for 22 to 30 years, and had extensive experience treating patients with breast cancer. We conducted the interviews by using a semistructured format, asking questions about the physicians' decision making regarding breast cancer treatment. Information from the interviews informed the development of a survey with sections that covered physician demographics, general use and views of the 21-gene RS assay, and their most recent use of the assay for a patient with N+/ER+ breast cancer. The survey was reviewed for clarity and content by the investigators and other health care professionals, including three practicing medical oncologists. It was pretested by two other medical oncologists. Once finalized, the survey was uploaded, tested, and activated for use on a Web-based platform that was password protected, used 128-bit secure sockets layer encryption, and had redundant firewalls. The study protocol was reviewed and approved by an independent institutional review board.
From a database maintained by Genomic Health (Redwood City, CA), we created a file of 1,017 medical oncologists who had ordered the 21-gene RS assay for patients with N+/ER+ breast cancer. This file represented all physicians who had ordered the assay for patients with N+/ER+ breast cancer at the time the study began. No patient information, identifiable or otherwise, was included in this file. The survey was conducted from April 2009 through June 2009. A prespecified limit of 150 responses was set as adequate for analysis in this descriptive study. Two of the authors (C.O. and M.B.) sent an initial e-mail or cover letter with an electronic link or Web address for the survey to all of the identified medical oncologists. Physicians were offered $150 to complete the survey. Second and third contacts were made via e-mail or postal service for all nonrespondents. To access the survey, respondents had to confirm that they were medical oncologists and had ordered the assay for at least one patient with N+/ER+ disease. We defined N+ as > 1 axillary lymph node with hematoxylin-eosin or immunohistochemical staining that was positive for nodal metastasis of > 2.0 mm; our definition excluded pN1mic tumors and lymph nodes with isolated tumor cells. The survey asked about provider-, practice-, and patient-related factors associated with the most recent order of the 21-gene RS assay for a patient with N+/ER+ disease. Respondents were instructed to refer to that patient's medical record to answer the patient-related questions.
To describe respondents' adoption of new diagnostic tests, we adapted category descriptions from a published model of the diffusion of innovations14,15: “first anywhere” (usually one of the first oncologists anywhere to order new diagnostic tests), “first locally” (usually one of the first oncologists in their area to order new diagnostic tests), “sooner than most locally” (usually adopts new diagnostic tests sooner than most oncologists in their area), “later than most locally” (usually waits until most of the others in their area are ordering new diagnostic tests before doing so), and “last locally” (usually one of the last in their area to order new diagnostic tests). The survey asked respondents about their views on the strength of the evidence for ordering the 21-gene RS assay for pre- and postmenopausal patients with N+/ER+ disease. The survey also asked whether inclusion as a recommended test in ASCO or NCCN guidelines would affect their ordering of the 21-gene RS assay for N+/ER+ patients.
For the respondents' most recent patient, the survey asked what treatment recommendations were made before and after receiving the 21-gene RS assay results. Treatment recommendations were categorized as hormonal therapy alone or chemotherapy plus hormonal therapy. We defined a decrease in treatment intensity as a change in treatment recommendation from chemotherapy plus hormonal therapy to hormonal therapy alone. An increase in treatment intensity was defined as the addition of chemotherapy to hormonal therapy. Treatment changes that did not fit those definitions (eg, changes in chemotherapy components only or changes from hormonal therapy alone to chemotherapy alone) were categorized as “other.” We performed a descriptive analysis summarizing frequency and percentage distributions of the survey responses and classified patients according to their RS group: low (RS < 18), intermediate (RS 18-30), and high (RS ≥ 31). Because this was an exploratory study, all analyses were descriptive in nature, and no formal statistical tests were conducted. The survey was closed shortly after reaching the prespecified limit of 150 respondents.
Results
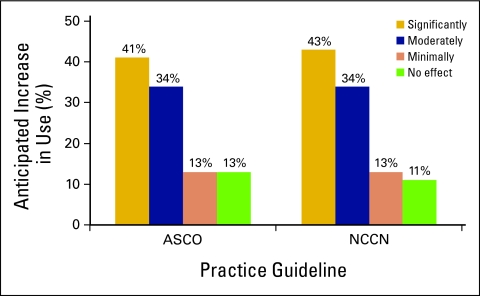
Of 1,017 physicians invited to participate, 232 (22%) accessed the Web-based survey within 6 weeks. Fourteen were either not medical oncologists or had not ordered the 21-gene RS assay for any patient with N+/ER+ disease. Fifty-eight did not complete the survey, leaving 160 (16%) completed surveys. Most of the oncologists classified their practices as community-based (71%), followed by academic (25%) and other (4%) settings. They were equally dispersed throughout the four major geographic regions of the United States and had a median of 11 years (range, 1-45 years) of practice experience. On the basis of their selection of the one statement that they believed best fit their rate of new technology adoption, our participating oncologists were categorized as 12% first anywhere, 24% first locally, 53% sooner than most locally, 11% later than most locally, and 1% last locally (Table 1). Of the 160 respondents, 112 (70%) reported being mostly or completely satisfied with the data supporting the use of the 21-gene RS assay in postmenopausal patients with N+/ER+ disease, and 75 (47%) reported the same level of satisfaction with the data regarding its use in premenopausal patients with N+/ER+ disease. One hundred twenty respondents (75%) predicted a moderate or significant increase their use of the assay in N+/ER+ patients if it were included in ASCO guidelines, and 123 (77%) predicted an increase in assay use if it were included in NCCN guidelines (Appendix Fig A1, online only).
Table 1.
Characteristics of Medical Oncologists Who Ordered the 21-Gene RS Assay for Patients With N+/ER+ Breast Cancer
| Characteristic | No. (N = 160) | % |
|---|---|---|
| Practice setting | ||
| Academic medical center | 40 | 25.0 |
| Community (multi-specialty, single-specialty, or solo practice) | 114 | 71.3 |
| Other | 6 | 3.8 |
| No. of years in practice* | ||
| Mean | 14.5 | |
| SD | 10.3 | |
| Median | 11 | |
| Range | 1-45 | |
| Geographic region | 41 | 25.6 |
| East | ||
| Midwest | 37 | 23.1 |
| South | 44 | 27.5 |
| West | 38 | 23.8 |
| Categorization by rate of new technology adoption | 19 | 11.9 |
| First anywhere | ||
| First locally | 38 | 23.8 |
| Sooner than most locally | 84 | 52.5 |
| Later than most locally | 18 | 11.3 |
| Last locally | 1 | 0.6 |
Abbreviations: RS, recurrence score; N+/ER+, node-positive/estrogen receptor–positive; SD, standard deviation.
Three missing values.
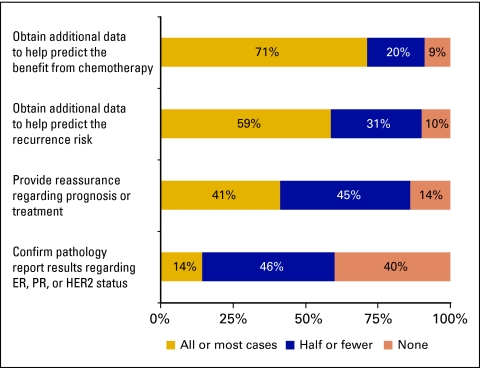
In the 3 months before completing the survey, oncologists ordered the assay for a mean of 1.9 N+/ER+ patients (median, 1; range, 0-20) and 9.5 N−/ER+ patients (median, 6; range, 0 to 75). Most respondents (91%) did not have an upper age limit for ordering the assay. Eighty-three percent reported that they did not have a lower age limit; 9% reported limiting its use to postmenopausal women only. Reasons for ordering the assay given by most or all respondents were as follows: to obtain additional data to help predict the benefit from chemotherapy (71%); to obtain additional data to help predict the patient's recurrence risk (59%); to provide reassurance regarding prognosis or treatment (41%); and to confirm pathology report results regarding ER, progesterone receptor, or human epidermal growth factor receptor 2 status (14%; Appendix Fig A2, online only). In most cases (67%), the patient contributed to the decision to order the 21-gene RS assay. The patient's reluctance to receive chemotherapy was most commonly cited as the most important factor in the oncologist's decision to order the assay (47%), followed by presence of comorbid conditions that increased risks of toxicity associated with adjuvant chemotherapy (19%), absence of medical conditions that significantly impaired quality of life or limited life expectancy (10%), advanced age of the patient (9%), a recommendation by a colleague or expert (2%), and other factors (14%).
The median age of respondents' last patient with N+/ER+ disease for whom the 21-gene assay was ordered was 61 years (range, 34-82 years), and 79% of patients were postmenopausal. T1, T2, or T3 disease was reported in 62%, 35%, and 3% of patients, respectively (unknown in 1 patient). One, two, three, or four or more positive nodes were reported in 69%, 18%, 6%, and 3% of patients, respectively (unknown in eight patients; Table 2). The 21-gene RS assay classified 87 (54%) patients as having a low RS, 60 (38%) as having an intermediate RS, and 13 (8%) as having a high RS. Oncologists' postassay risk assessments (low, intermediate, high) agreed with the assay results for 89% of patients.
Table 2.
Characteristics of Patients With N+/ER+ Breast Cancer
| Characteristic | No. (N = 160) | % |
|---|---|---|
| Age, years | ||
| Mean | 60.2 | |
| SD | 11.2 | |
| Median | 61 | |
| Range | 34-82 | |
| Menopausal status | ||
| Postmenopausal | 126 | 78.8 |
| Premenopausal | 30 | 18.8 |
| Unknown | 4 | 2.5 |
| Tumor classification | ||
| T1 (≤ 2 cm) | 99 | 61.9 |
| T2 (2-5 cm) | 56 | 35.0 |
| T3 (> 5 cm) | 4 | 2.5 |
| Unknown | 1 | 0.6 |
| No. of positive axillary lymph nodes* | ||
| 1 | 110 | 68.8 |
| 2 | 28 | 17.5 |
| 3 | 10 | 6.3 |
| ≥ 4 | 4 | 2.5 |
| Unknown | 8 | 5.0 |
| Comorbidities | ||
| Any listed comorbidity | 34 | 21.3 |
| Diabetes mellitus | 26 | 16.3 |
| Uncontrolled hypertension | 8 | 5.0 |
| History of stroke or other cerebrovascular disease | 0 | 0.0 |
| Congestive heart failure or other chronic heart disease | 0 | 0.0 |
| Pulmonary fibrosis or other chronic lung disease | 5 | 3.1 |
| Chronic renal insufficiency | 5 | 3.1 |
| Peripheral neuropathy | 0 | 0.0 |
| Cytopenias | 0 | 0.0 |
Abbreviations: N+/ER+, node-positive/estrogen receptor–positive; SD, standard deviation.
Excludes micrometastases and isolated tumor cells.
For 138 patients, oncologists indicated that they made treatment recommendations before ordering the assay. Among N+/ER+ patients who had a preassay treatment recommendation, 72 (52%) were found to have a low RS, 53 (38%) an intermediate RS, and 13 (9%) a high RS (Table 3). After obtaining the RS, oncologists indicated that they changed their treatment recommendation in 70 patients (51%). These changes were seen for patients with RSs in every risk category: 43 (60%) with a low RS, 20 (38%) with an intermediate RS, and seven (54%) with a high RS. The recommended treatment decreased in intensity from chemotherapy plus hormonal therapy to hormonal therapy alone in 46 patients (33%). Among the 72 patients with a low RS, chemotherapy was eliminated in 35 (49%); among the 53 patients with an intermediate RS, chemotherapy was eliminated in 11 (21%); and among the 13 patients with a high RS, chemotherapy was not eliminated for any patient. Overall, the recommended treatment intensity increased from hormonal therapy alone to chemotherapy plus hormonal therapy in 13 patients (9%): four (6%) with a low RS, six (11%) with an intermediate RS, and three (23%) with a high RS. In 11 patients (8%), treatment changes did not fit our definitions of either increased or decreased intensity (eg, changes in chemotherapy components only or changes from hormonal therapy alone to chemotherapy alone; Table 3).
Table 3.
Effect of the 21-Gene RS Assay on Treatment Recommendations for Patients With N+/ER+ Breast Cancer
| Effect on Recommendation | RS |
|||||||
|---|---|---|---|---|---|---|---|---|
| Low (< 18, n = 72) |
Intermediate (18-30, n = 53) |
High (≥ 31 n = 13) |
All (n = 138)* |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Any change | 43 | 60 | 20 | 38 | 7 | 54 | 70 | 51 |
| Decreased intensity† | 35 | 49 | 11 | 21 | 0 | 0 | 46 | 33 |
| Increased intensity‡ | 4 | 5 | 6 | 12 | 3 | 23 | 13 | 9 |
| Other§ | 4 | 5 | 3 | 6 | 4 | 31 | 11 | 8 |
| No change | 29 | 40 | 33 | 62 | 6 | 46 | 68 | 49 |
Abbreviations: RS, recurrence score; N+/ER+, node-positive/estrogen receptor–positive.
Excludes 22 patients with no treatment recommendations before assay.
Decreased intensity = chemotherapy plus hormonal therapy → hormonal therapy alone.
Increased intensity = hormonal therapy alone → chemotherapy plus hormonal therapy.
Eleven patients were recommended treatment changes that did not fit our definitions of either increased or decreased intensity (eg, changes in chemotherapy components only or changes from hormonal therapy alone to chemotherapy alone).
Discussion
In this survey of medical oncologists who ordered the 21-gene RS assay for patients with N+/ER+ breast cancer, recommendations for systemic adjuvant therapy were frequently altered by the RS result. This suggests that this small sample of oncologists use the test to determine the most appropriate adjuvant treatment for patients with N+/ER+ disease, particularly for those with tumors less than 5 cm in size and with one to three positive lymph nodes. Furthermore, most of the treatment changes involved the elimination of chemotherapy.
The ability of the 21-gene RS assay to predict both recurrence and the benefits of therapy has been demonstrated in different patient populations. In N−/ER+ disease, studies support the clinical utility of the assay in predicting the risk of recurrence and determining which individuals will derive the greatest benefit from adding chemotherapy to hormonal therapy.1,2,16 More recently, the assay has been reported to have similar prognostic and predictive ability in postmenopausal patients with N+/ER+ disease.12,13 Other studies suggest that the RS assay may predict increased rates of response to neoadjuvant chemotherapy17,18 and hormonal therapy19 for patients with larger tumors before breast-conserving surgery. The RS has been shown to provide independent, complementary information beyond that provided by standard clinicopathologic characteristics.11,20,21
In this study, respondents altered treatment recommendations for 51% of patients after reviewing the RS results. Studies in the N−/ER+ patient population have shown lower rates of treatment changes, with treatment recommendations being altered for 21% to 44% of patients. A single-center, retrospective study of 68 patients showed that for 21%, the oncologist's recommendation for adjuvant treatment changed after receiving the RS, and 82% of these treatment changes resulted in chemotherapy being eliminated.4 Another retrospective study of 85 patients at two academic centers found the RS altered the recommended treatment for 44%, eliminating chemotherapy for 89% of these patients.22 A retrospective study at a tertiary breast cancer referral center for the US Department of Defense found that the RS changed the treatment that an expert panel of breast oncologists would have recommended for 24% of patients, with 71% of the changes being the elimination of chemotherapy.23 A prospective study of 89 consecutive patients found treatment recommendations were altered as a result of the RS for 32% of patients, with 71% of those changes being the elimination of chemotherapy.26 The higher rate of change seen in our study may be explained by treatment guidelines for N+/ER+ disease, which recommend chemotherapy for all patients, making more patients in our survey eligible for the elimination of chemotherapy.9 Economic analyses have suggested that use of the 21-gene RS assay in the adjuvant treatment decision-making process for N−/ER+ patients saves money.24,25 By reducing chemotherapy use in N+/ER+ disease, the 21-gene RS assay may be cost effective, if not cost saving, although formal economic analyses in this patient population are needed to confirm this hypothesis.
In interpreting these results, a number of limitations must be considered. We used a voluntary, Web-based survey of medical oncologists who had ordered the 21-gene RS assay for at least one N+/ER+ patient. For this descriptive study, we intentionally closed the survey after reaching a prespecified number of at least 150 responses, which occurred after 1.5 months. Oncologists who responded to the survey invitation may have had a systematically different impression of the clinical utility of the assay than nonrespondents. Respondents were compensated for completing the survey. In prior surveys, nearly all oncologists stated that they would order the 21-gene RS assay again after having ordered it for their study patients, suggesting a high overall level of satisfaction.26 If there are systematic differences between respondents and nonrespondents, these results may not be representative of the population of all users of this assay. Furthermore, descriptions of the most recent N+/ER+ patient may have been affected by recall error. To minimize such errors, respondents were prompted to have their patient's chart open in front of them as they completed the survey. We did not attempt to confirm respondents' use of medical records or to assess the accuracy and completeness of the medical record itself. Data were collected on one patient per physician, and care for one patient may not be representative of all care provided by a particular physician. There is some evidence that the respondents and their patients are similar to the population of interest. Most of the participating oncologists practiced in community settings, had a wide range in years of experience and geographic locations, and were not in the “first anywhere” or “first locally” categories of new technology adoption. The geographic distribution of respondents was similar to the distribution of nonrespondents, and, overall, the physician factors in our final sample were similar in distribution to those in other large surveys of US oncologists.27,28 In addition, the proportion of N+/ER+ patients classified as having a low RS was similar to that seen in a study of N+/ER+ breast cancer.11
Results of this survey suggest that respondents order the 21-gene RS assay for patients with N+/ER+ breast cancer for reasons similar to those for patients with N−/ER+ disease,1,2,4,16,22 and that the RS results in both settings are interpreted in much the same manner. For more than half of the patients represented in this survey, the information obtained from the 21-gene RS assay changed the oncologist's initial treatment recommendations. This reduced the number of patients for whom adjuvant chemotherapy was prescribed, particularly among those with a low RS, a group that made up the majority of the sample. For this study, we used patients' RSs to categorize them into low-, intermediate-, and high-risk groups, but clinicians are likely to use RS results as a continuous variable, incorporating the RS into individualized treatment decisions based on the integration of patient preference, pathology results, and other clinical information. To our knowledge, our study is the first to investigate the impact of RS on treatment decisions in patients with N+/ER+ breast cancer. Additional data on the impact of the 21-gene RS assay on adjuvant treatment recommendations and outcomes will be gathered as medical oncologists continue to order the assay for this population.
Acknowledgment
Supported by Genomic Health. Presented in abstract form as a poster presentation at the 32nd Annual San Antonio Breast Cancer Symposium, San Antonio, TX, December 9-13, 2009.
Appendix
Figure A1.
Anticipated extent of increased use of the 21-gene recurrence score assay in lymph node–positive, estrogen receptor–positive breast cancer if the assay were included as a recommended test in clinical practice guidelines. NCCN, National Comprehensive Cancer Network.
Figure A2.
Reasons for ordering the 21-gene recurrence score assay in patients with lymph node–positive, estrogen receptor–positive breast cancer. ER, estrogen receptor, PR, progesterone receptor, HER2, human epidermal growth factor receptor 2.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Calvin Chao, Genomic Health (C); Stanley Skrzypczak, Genomic Health (C); Roberto Bugarini, Genomic Health (C) Consultant or Advisory Role: Michael Broder, Genomic Health (C) Stock Ownership: Calvin Chao, Genomic Health; Stanley Skrzypczak, Genomic Health Honoraria: None Research Funding: Caron Ory, Genomic Health; Michael Broder, Genomic Health Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: Ruth Oratz, Benjamin Kim, Calvin Chao, Stanley Skrzypczak, Caron Ory, Roberto Bugarini, Michael Broder
Financial support: Calvin Chao, Stanley Skrzypczak
Administrative support: Calvin Chao, Caron Ory
Collection and assembly of data: Caron Ory, Michael Broder
Data analysis and interpretation: Ruth Oratz, Benjamin Kim, Calvin Chao, Stanley Skrzypczak, Caron Ory, Roberto Bugarini, Michael Broder
Manuscript writing: Ruth Oratz, Benjamin Kim, Calvin Chao, Stanley Skrzypczak, Caron Ory, Roberto Bugarini, Michael Broder
Final approval of manuscript: Ruth Oratz, Benjamin Kim, Calvin Chao, Stanley Skrzypczak, Caron Ory, Roberto Bugarini, Michael Broder
References
- 1.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 2.Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: Results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28:1677–1683. doi: 10.1200/JCO.2009.23.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher B, Jeong JH, Bryant J, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: Long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet. 2004;364:858–868. doi: 10.1016/S0140-6736(04)16981-X. [DOI] [PubMed] [Google Scholar]
- 4.Oratz R, Paul D, Cohn AL, et al. Impact of a commercial reference laboratory test recurrence score on decision making in early-stage breast cancer. J Oncol Pract. 2007;3:182–186. doi: 10.1200/JOP.0742001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang H, Brufsky AM, Lembersky BB, et al. A retrospective analysis of the impact of Oncotype DX low recurrence score results on treatment decisions in a single academic breast cancer center. Presented at the 30th annual San Antonio Breast Cancer Symposium; December 13-16, 2007; San Antonio, TX. abstr 2061. [Google Scholar]
- 6.Mumby PB, Lo SS, Norton J, et al. Prospective multi-center study of the impact of the 21-gene recurrence score assay on patient satisfaction, anxiety and decisional conflict for adjuvant breast cancer treatment selection. Presented at the 30th annual San Antonio Breast Cancer Symposium; December 13-16, 2007; San Antonio, TX. abstr 2092. [Google Scholar]
- 7.Erb C, Fox KR, Patel M, et al. Evaluation of practice patterns in the treatment of node-negative, hormone-receptor positive breast cancer patients with the use of the Oncotype DX assay at the University of Pennsylvania. Presented at the 30th annual San Antonio Breast Cancer Symposium; December 13-16, 2007; San Antonio, TX. abstr 3082. [Google Scholar]
- 8.Ben-Baruch N, Hammerman A, Klang S, et al. A prospective study of the impact of the 21-gene recurrence score assay on treatment decisions in N-, ER+ early stage breast cancer patients. J Clin Oncol. 2007;25(suppl):18S. abstr 11008. [Google Scholar]
- 9.National Comprehensive Cancer Network. The NCCN breast cancer clinical practice guidelines (v. 2.2008) http://www.nccn.org.
- 10.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein LJ, Gray R, Badve S, et al. Prognostic utility of the 21-gene assay in hormone receptor–positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol. 2008;25:4063–4071. doi: 10.1200/JCO.2007.14.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A transATAC study. J Clin Oncol. 2010;28:1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 14.Rogers EM. Diffusion of Innovations. ed 4. New York, NY: Free Press; 1995. pp. 280–282. [Google Scholar]
- 15.Berwick DM. Disseminating innovations in health care. JAMA. 2003;289:1969–1975. doi: 10.1001/jama.289.15.1969. [DOI] [PubMed] [Google Scholar]
- 16.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2005;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 17.Gianni L, Zambetti M, Clark K, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally-advanced breast cancer. J Clin Oncol. 2005;23:7265–7277. doi: 10.1200/JCO.2005.02.0818. [DOI] [PubMed] [Google Scholar]
- 18.Chang JC, Makris A, Gutierrez MC, et al. Gene expression profiles in formalin-fixed, paraffin-embedded core biopsy tissue predict docetaxel chemosensitivity. Breast Cancer Res Treat. 2008;108:233–240. doi: 10.1007/s10549-007-9590-z. [DOI] [PubMed] [Google Scholar]
- 19.Akashi-Tanaka S, Shimizu C, Ando M, et al. 21-Gene expression profile assay on core needle biopsies predicts responses to neoadjuvant endocrine therapy in breast cancer patients. Breast. 2009;18:171–174. doi: 10.1016/j.breast.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Paik S, Tang G, Fumagalli D. An ideal prognostic test for estrogen receptor–positive breast cancer? J Clin Oncol. 2008;25:4058–4059. doi: 10.1200/JCO.2008.16.7528. [DOI] [PubMed] [Google Scholar]
- 21.Wolf I, Ben-Baruch N, Shapira-Frommer R, et al. Association between standard clinical and pathologic characteristics and the 21-gene recurrence score in breast cancer patients. Cancer. 2008;112:731–736. doi: 10.1002/cncr.23225. [DOI] [PubMed] [Google Scholar]
- 22.Asad J, Jacobson AF, Estabrook A, et al. Does Oncotype DX recurrence score affect the management of patients with early-stage breast cancer? Am J Surg: 2008;196:527–529. doi: 10.1016/j.amjsurg.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Henry LR, Stojadinovic A, Swain SM, et al. The influence of a gene expression profile on breast cancer decisions. J Surg Oncol. 2009;99:319–323. doi: 10.1002/jso.21244. [DOI] [PubMed] [Google Scholar]
- 24.Hornberger J, Cosler LE, Lyman GH. Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care. 2005;11:313–324. [PubMed] [Google Scholar]
- 25.Lyman GH, Cosler LE, Kuderer NM, et al. Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: An economic analysis based on prognostic and predictive validation studies. Cancer. 2007;109:1011–1018. doi: 10.1002/cncr.22506. [DOI] [PubMed] [Google Scholar]
- 26.Lo SS, Mumby PB, Norton J, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol. 2010;28:1671–1676. doi: 10.1200/JCO.2008.20.2119. [DOI] [PubMed] [Google Scholar]
- 27.Helft PR, Hlubocky F, Daugherty CK. American oncologists' views of Internet use by cancer patients: A mail survey of American Society of Clinical Oncology members. J Clin Oncol. 2003;21:942–947. doi: 10.1200/JCO.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Fenton L, Rigney M, Herbst RS. Clinical trial awareness, attitudes, and participation among patients with cancer and oncologists. Commun Oncol. 2009;6:207–213. [Google Scholar]