Abstract
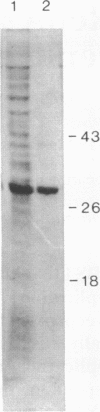
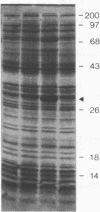
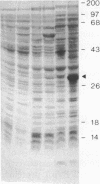
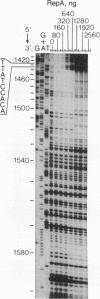
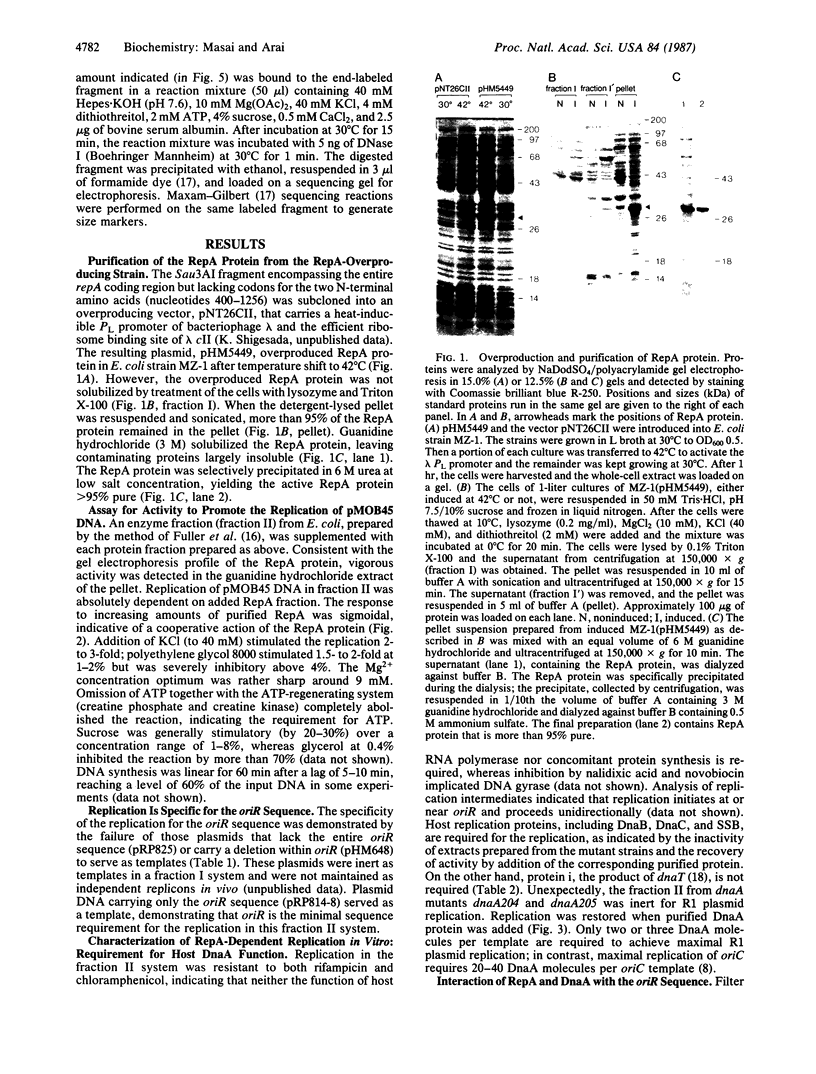
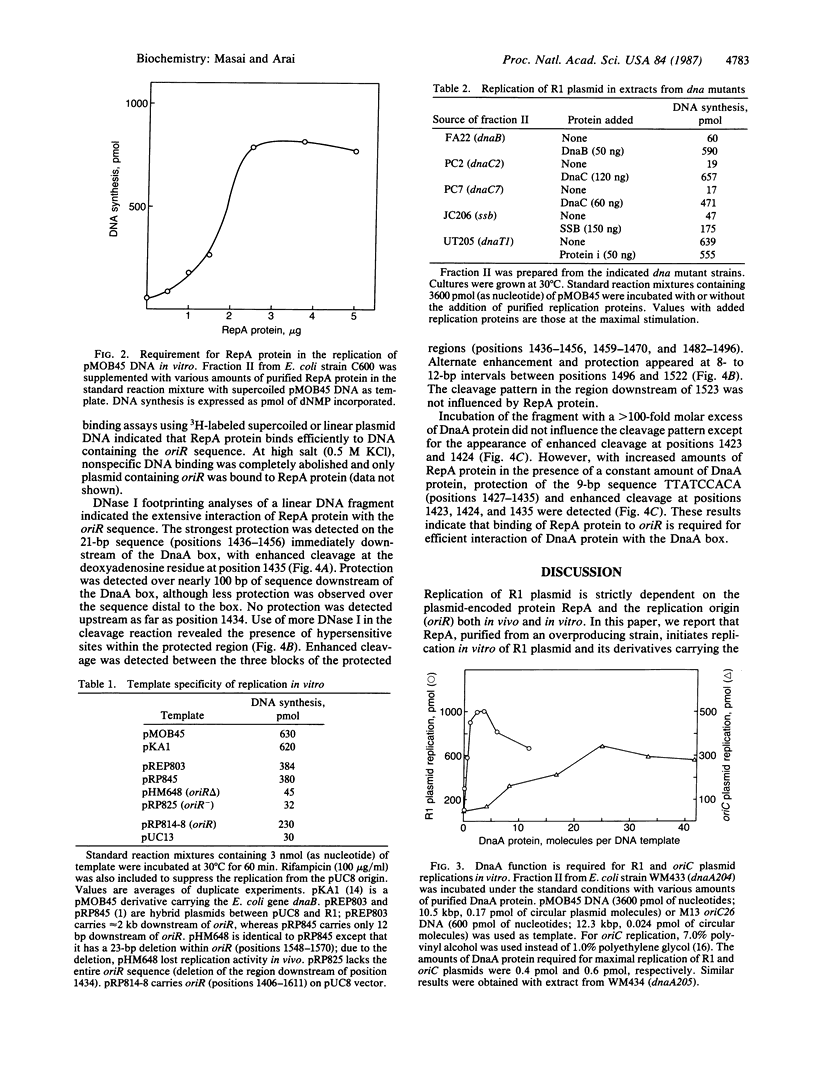
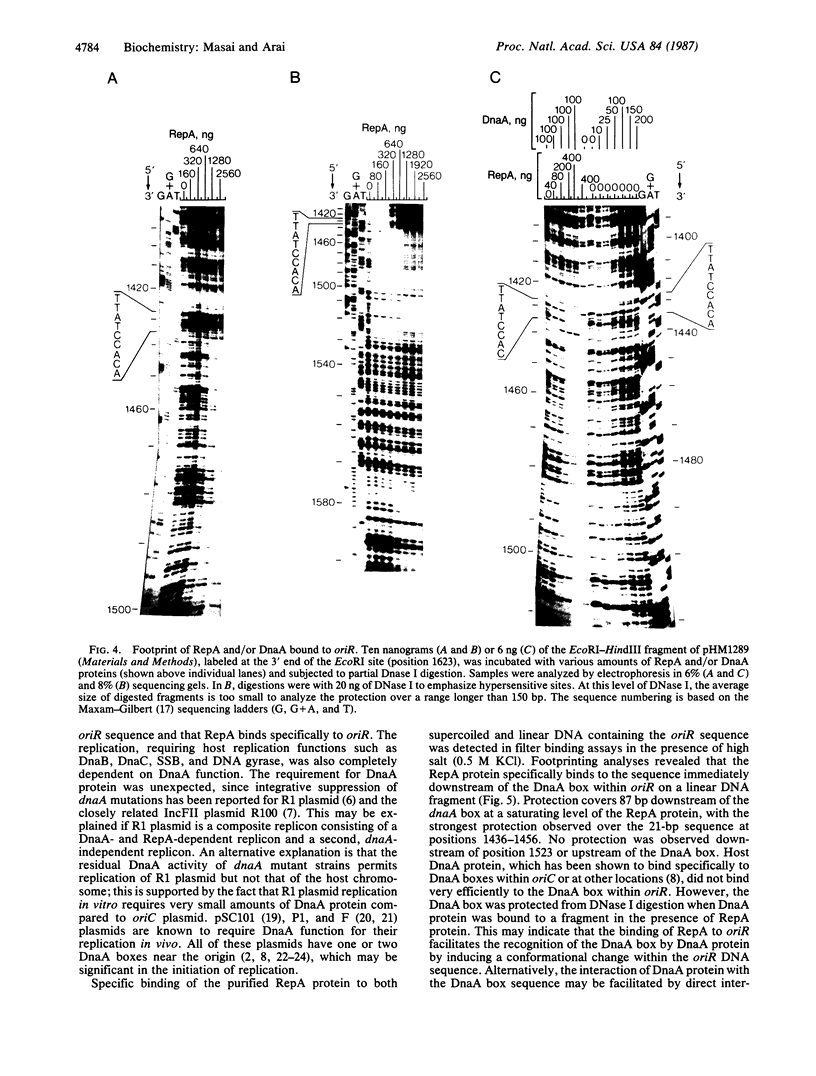
RepA, an initiation protein of R1 plasmid replication, was purified from an Escherichia coli strain overproducing the protein. The purified RepA protein specifically initiated replication in vitro of plasmid DNA bearing the replication origin of R1 plasmid (oriR). The replication, strictly dependent on added RepA protein, was independent of host RNA polymerase but required other host replication functions (DnaB and DnaC proteins, the single-stranded-DNA-binding protein SSB, and DNA gyrase). The replication was also completely dependent on the host DnaA function. In filter binding assays in high salt (0.5 M KCl) conditions, RepA specifically binds to both supercoiled and linear plasmid DNA containing the oriR sequence, whereas it binds to nonspecific DNA in low salt. DNase I-protection studies on a linearized DNA fragment revealed that DnaA protein specifically binds to a 9-base-pair DnaA-recognition sequence ("DnaA box") within oriR only when RepA is bound to the sequence immediately downstream of the DnaA box. These results indicate that initiation of R1 plasmid replication is triggered by interaction of RepA and DnaA proteins with the oriR sequence.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles A. L., Snyder K. M., Chattoraj D. K. P1 plasmid replication: replicon structure. J Mol Biol. 1984 Mar 5;173(3):307–324. doi: 10.1016/0022-2836(84)90123-2. [DOI] [PubMed] [Google Scholar]
- Arai K., Yasuda S., Kornberg A. Mechanism of dnaB protein action. I. Crystallization and properties of dnaB protein, an essential replication protein in Escherichia coli. J Biol Chem. 1981 May 25;256(10):5247–5252. [PubMed] [Google Scholar]
- Armstrong K. A., Acosta R., Ledner E., Machida Y., Pancotto M., McCormick M., Ohtsubo H., Ohtsubo E. A 37 X 10(3) molecular weight plasmid-encoded protein is required for replication and copy number control in the plasmid pSC101 and its temperature-sensitive derivative pHS1. J Mol Biol. 1984 May 25;175(3):331–348. doi: 10.1016/0022-2836(84)90352-8. [DOI] [PubMed] [Google Scholar]
- Bittner M., Vapnek D. Versatile cloning vectors derived from the runaway-replication plasmid pKN402. Gene. 1981 Dec;15(4):319–329. doi: 10.1016/0378-1119(81)90175-x. [DOI] [PubMed] [Google Scholar]
- Carl P. L. Escherichia coli mutants with temperature-sensitive synthesis of DNA. Mol Gen Genet. 1970;109(2):107–122. doi: 10.1007/BF00269647. [DOI] [PubMed] [Google Scholar]
- Diaz R., Nordström K., Staudenbauer W. L. Plasmid R1 DNA replication dependent on protein synthesis in cell-free extracts of E. coli. Nature. 1981 Jan 22;289(5795):326–328. doi: 10.1038/289326a0. [DOI] [PubMed] [Google Scholar]
- Diaz R., Ortega S. Initiation of plasmid R1 replication in vitro is independent of transcription by host RNA polymerase. Nucleic Acids Res. 1984 Jul 11;12(13):5175–5191. doi: 10.1093/nar/12.13.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Funnell B. E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984 Oct;38(3):889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassberg J., Meyer R. R., Kornberg A. Mutant single-strand binding protein of Escherichia coli: genetic and physiological characterization. J Bacteriol. 1979 Oct;140(1):14–19. doi: 10.1128/jb.140.1.14-19.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. B., Yarmolinsky M. B. Host participation in plasmid maintenance: dependence upon dnaA of replicons derived from P1 and F. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4423–4427. doi: 10.1073/pnas.83.12.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasunuma K., Sekiguchi M. Replication of plasmid pSC101 in Escherichia coli K12: requirement for dnaA function. Mol Gen Genet. 1977 Sep 9;154(3):225–230. doi: 10.1007/BF00571277. [DOI] [PubMed] [Google Scholar]
- Hatten M. E., Scandella C. J., Horwitz A. F., Burger M. M. Similarities in the membrane fluidity of 3T3 and SV101-3T3 cells and its relation to concanavalin A- and wheat germ agglutinin-induced agglutination. J Biol Chem. 1978 Mar 25;253(6):1972–1977. [PubMed] [Google Scholar]
- Kline B. C., Kogoma T., Tam J. E., Shields M. S. Requirement of the Escherichia coli dnaA gene product for plasmid F maintenance. J Bacteriol. 1986 Oct;168(1):440–443. doi: 10.1128/jb.168.1.440-443.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai H., Bond M. W., Arai K. Cloning of the Escherichia coli gene for primosomal protein i: the relationship to dnaT, essential for chromosomal DNA replication. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1256–1260. doi: 10.1073/pnas.83.5.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai H., Kaziro Y., Arai K. Definition of oriR, the minimum DNA segment essential for initiation of R1 plasmid replication in vitro. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6814–6818. doi: 10.1073/pnas.80.22.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Molin S., Nordström K. Control of plasmid R1 replication: functions involved in replication, copy number control, incompatibility, and switch-off of replication. J Bacteriol. 1980 Jan;141(1):111–120. doi: 10.1128/jb.141.1.111-120.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murotsu T., Matsubara K., Sugisaki H., Takanami M. Nine unique repeating sequences in a region essential for replication and incompatibility of the mini-F plasmid. Gene. 1981 Nov;15(2-3):257–271. doi: 10.1016/0378-1119(81)90135-9. [DOI] [PubMed] [Google Scholar]
- Ortega S., Lanka E., Diaz R. The involvement of host replication proteins and of specific origin sequences in the in vitro replication of miniplasmid R1 DNA. Nucleic Acids Res. 1986 Jun 25;14(12):4865–4879. doi: 10.1093/nar/14.12.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippe-Schindler R., Zahn G., Messer W. Control of the initiation of DNA replication in Escherichia coli. I. Negative control of initiation. Mol Gen Genet. 1979 Jan 10;168(2):185–195. doi: 10.1007/BF00431444. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M. Identification and mapping of the replication genes of an R factor, R100-1, integrated into the chromosome of Escherichia coli K-12. J Bacteriol. 1974 Jun;118(3):1123–1131. doi: 10.1128/jb.118.3.1123-1131.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]