Abstract
The complete amino acid sequence of human plasma apolipoprotein C-II (apoC-II) has been synthesized chemically by the solid-phase method using phenylacetamidomethyl-resin. All amino acids were coupled to the peptide-resin in the presence of 1-hydroxybenzotriazole; tert-butyloxycarbonyl-protected amino acids with the appropriate side-chain-protecting groups that are stable to the reaction conditions used in the solid-phase methodology were used. After cleavage and deprotection, the crude apoC-II was purified by ion-exchange chromatography and then by reverse-phase high-performance liquid chromatography. The purified apolipoprotein was found to elute as a single peak under various chromatographic conditions, and the overall yield of the final purified protein was 20.7%. Synthetic apoC-II was characterized by several complementary analytical techniques including amino acid composition, Edman phenylisothiocyanate degradation, polyacrylamide gel electrophoresis, and high-performance liquid chromatography. The final product was found to be homogeneous and to activate normal human post-heparin lipase to the same extent as native apoC-II. The synthetic protein is also equally immunoreactive as native apoC-II.
Full text
PDF

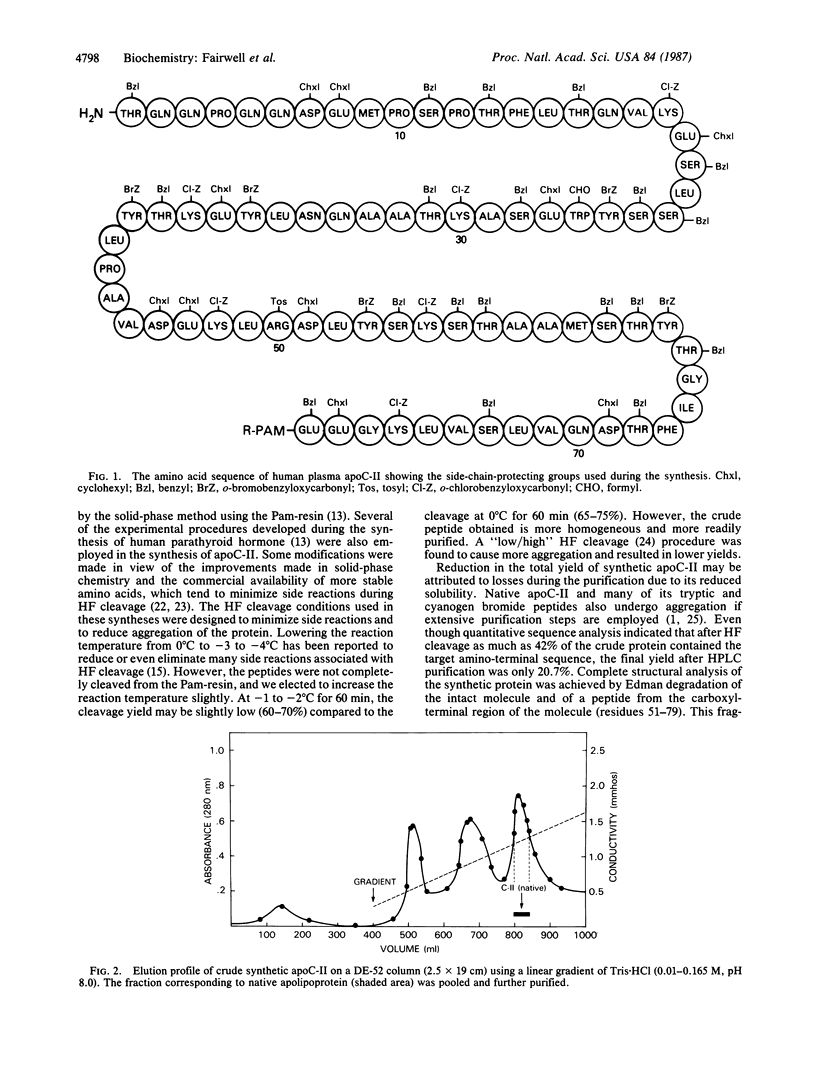
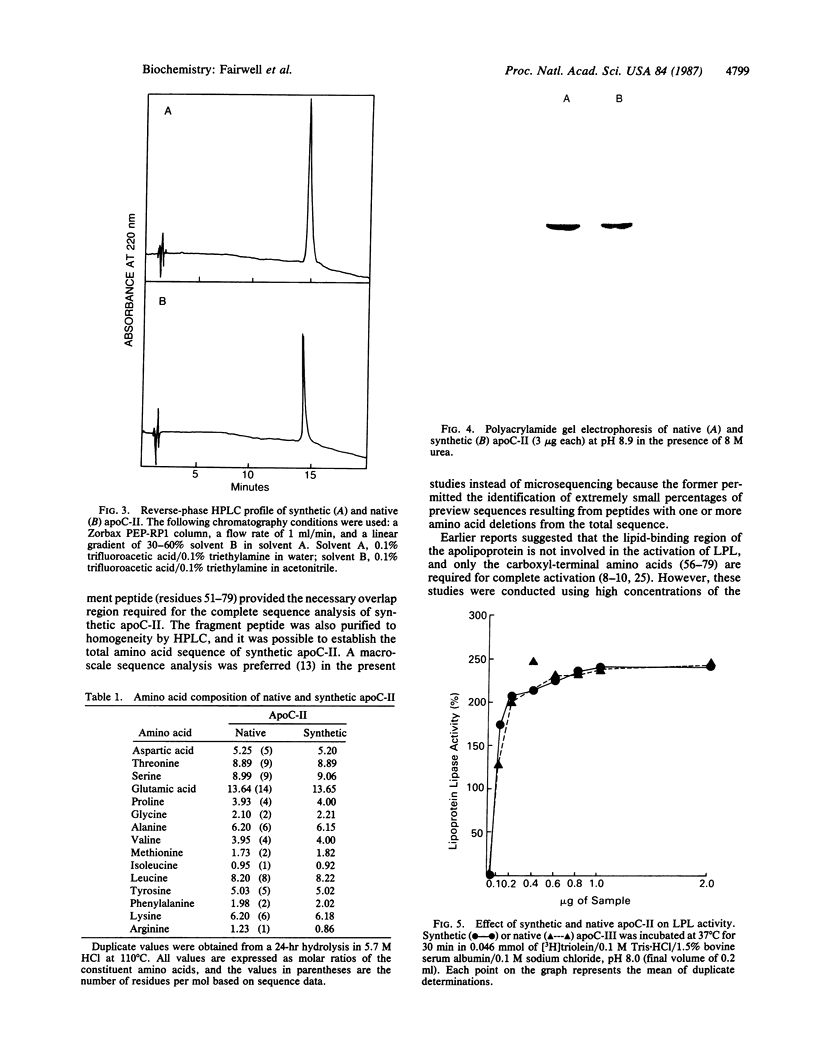

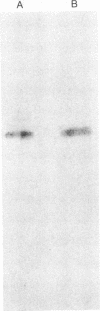
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breckenridge W. C., Little J. A., Steiner G., Chow A., Poapst M. Hypertriglyceridemia associated with deficiency of apolipoprotein C-II. N Engl J Med. 1978 Jun 8;298(23):1265–1273. doi: 10.1056/NEJM197806082982301. [DOI] [PubMed] [Google Scholar]
- Brewer H. B., Jr, Shulman R., Herbert P., Ronan R., Wehrly K. The complete amino acid sequence of alanine apolipoprotein (apoC-3), and apolipoprotein from human plasma very low density lipoproteins. J Biol Chem. 1974 Aug 10;249(15):4975–4984. [PubMed] [Google Scholar]
- Catapano A. L., Kinnunen P. K., Breckenridge W. C., Gotto A. M., Jr, Jackson R. L., Little J. A., Smith L. C., Sparrow J. T. Lipolysis of ApoC-II deficient very low density lipoproteins: enhancement of lipoprotein lipase action by synthetic fragments of apoC-II. Biochem Biophys Res Commun. 1979 Aug 13;89(3):951–957. doi: 10.1016/0006-291x(79)91870-9. [DOI] [PubMed] [Google Scholar]
- Dimarchi R. D., Tam J. P., Merrifield R. B. Solid phase synthesis of the protected 43-55 tridecapeptide of the heavy chain of myeloma immunoglobin M603, employing cyclohexyl ester protection for glutamic acid. Int J Pept Protein Res. 1982 Mar;19(3):270–279. doi: 10.1111/j.1399-3011.1982.tb03038.x. [DOI] [PubMed] [Google Scholar]
- Fairwell T. Chemical ionization mass spectral analysis of phenylthiohydantoin derivatives. Methods Enzymol. 1983;91:502–511. doi: 10.1016/s0076-6879(83)91047-9. [DOI] [PubMed] [Google Scholar]
- Fairwell T., Hospattankar A. V., Ronan R., Brewer H. B., Jr, Chang J. K., Shimizu M., Zitzner L., Arnaud C. D. Total solid-phase synthesis, purification, and characterization of human parathyroid hormone-(1-84). Biochemistry. 1983 May 24;22(11):2691–2697. doi: 10.1021/bi00280a016. [DOI] [PubMed] [Google Scholar]
- Hospattankar A. V., Fairwell T., Ronan R., Brewer H. B., Jr Amino acid sequence of human plasma apolipoprotein C-II from normal and hyperlipoproteinemic subjects. J Biol Chem. 1984 Jan 10;259(1):318–322. [PubMed] [Google Scholar]
- Jackson R. L., Balasubramaniam A., Murphy R. F., Demel R. A. Interaction of synthetic peptides of apolipoprotein C-II and lipoprotein lipase at monomolecular lipid films. Biochim Biophys Acta. 1986 Feb 12;875(2):203–210. doi: 10.1016/0005-2760(86)90169-4. [DOI] [PubMed] [Google Scholar]
- Jahn C. E., Osborne J. C., Jr, Schaefer E. J., Brewer H. B., Jr In vitro activation of the enzymic activity of hepatic lipase by apoA-II. FEBS Lett. 1981 Aug 31;131(2):366–368. doi: 10.1016/0014-5793(81)80405-x. [DOI] [PubMed] [Google Scholar]
- Kaiser E., Colescott R. L., Bossinger C. D., Cook P. I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970 Apr;34(2):595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- Kinnunen P. K., Jackson R. L., Smith L. C., Gotto A. M., Jr, Sparrow J. T. Activation of lipoprotein lipase by native and synthetic fragments of human plasma apolipoprotein C-II. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4848–4851. doi: 10.1073/pnas.74.11.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa J. C., Levy R. I., Herbert P., Lux S. E., Fredrickson D. S. A specific apoprotein activator for lipoprotein lipase. Biochem Biophys Res Commun. 1970 Oct 9;41(1):57–62. doi: 10.1016/0006-291x(70)90468-7. [DOI] [PubMed] [Google Scholar]
- Matsueda G. R. Deprotection of Nin-formyl tryptophan using 1,2-ethanedithiol in liquid hydrogen fluoride. Deformylation upon HF treatment of Merrifield peptidyl-resins. Int J Pept Protein Res. 1982 Jul;20(1):26–34. [PubMed] [Google Scholar]
- Merrifield R. B. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:221–296. doi: 10.1002/9780470122778.ch6. [DOI] [PubMed] [Google Scholar]
- Miller N. E., Rao S. N., Alaupovic P., Noble N., Slack J., Brunzell J. D., Lewis B. Familial apolipoprotein CII deficiency: plasma lipoproteins and apolipoproteins in heterozygous and homozygous subjects and the effects of plasma infusion. Eur J Clin Invest. 1981 Feb;11(1):69–76. doi: 10.1111/j.1365-2362.1981.tb01768.x. [DOI] [PubMed] [Google Scholar]
- Morrisett J. D., Jackson R. L., Gotto A. M., Jr Lipid-protein interactions in the plasma lipoproteins. Biochim Biophys Acta. 1977 Aug 9;472(2):93–133. doi: 10.1016/0304-4157(77)90015-6. [DOI] [PubMed] [Google Scholar]
- Smith L. C., Pownall H. J., Gotto A. M., Jr The plasma lipoproteins: structure and metabolism. Annu Rev Biochem. 1978;47:751–757. doi: 10.1146/annurev.bi.47.070178.003535. [DOI] [PubMed] [Google Scholar]
- Smith L. C., Pownall H. J., Gotto A. M., Jr The plasma lipoproteins: structure and metabolism. Annu Rev Biochem. 1978;47:751–757. doi: 10.1146/annurev.bi.47.070178.003535. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Debuch H. The lipids of the Golgi apparatus subfractions from rat liver. Biochim Biophys Acta. 1979 May 25;573(2):301–307. doi: 10.1016/0005-2760(79)90063-8. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]