Abstract
The title compound, C10H11BrN2O3, exhibits a small twist between the amide residue and benzene ring [the C—N—C—C torsion angle = 12.7 (4)°]. The crystal structure is stabilized by weak N—H⋯O, C—H⋯Br and C—H⋯O interactions. These lead to supramolecular layers in the bc plane.
Related literature
For initiators in ATRP (polymerization by atom transfer radical) processes, see: Matyjaszewski & Xia (2001 ▶); Pietrasik & Tsarevsky (2010 ▶). For graph-set notation of hydrogen-bond patterns, see: Etter (1990 ▶).
Experimental
Crystal data
C10H11BrN2O3
M r = 287.11
Monoclinic,
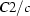
a = 24.1245 (12) Å
b = 5.8507 (3) Å
c = 15.4723 (8) Å
β = 91.837 (5)°
V = 2182.72 (19) Å3
Z = 8
Mo Kα radiation
μ = 3.76 mm−1
T = 123 K
0.60 × 0.05 × 0.05 mm
Data collection
Oxford Diffraction Xcalibur E diffractometer
Absorption correction: analytical (CrysAlis PRO; Oxford Diffraction, 2009 ▶) T min = 0.444, T max = 1.000
5100 measured reflections
2633 independent reflections
2197 reflections with I > 2σ(I)
R int = 0.032
Refinement
R[F 2 > 2σ(F 2)] = 0.032
wR(F 2) = 0.068
S = 1.06
2633 reflections
151 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.55 e Å−3
Δρmin = −0.60 e Å−3
Data collection: CrysAlis CCD (Oxford Diffraction, 2009 ▶); cell refinement: CrysAlis CCD; data reduction: CrysAlis CCD; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶) and Mercury (Macrae et al., 2006 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811005320/tk2720sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811005320/tk2720Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C6—H6⋯O1 | 0.95 | 2.27 | 2.862 (3) | 120 |
| C7—H7⋯O1i | 0.95 | 2.45 | 3.139 (3) | 129 |
| N1—H1n⋯O3ii | 0.84 (3) | 2.66 (3) | 3.316 (3) | 136 (2) |
| C10—H10⋯Br1iii | 0.95 | 2.91 | 3.812 (2) | 160 |
Symmetry codes: (i) 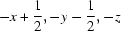 ; (ii)
; (ii) 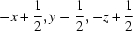 ; (iii)
; (iii) 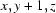 .
.
Acknowledgments
RMF is grateful to the Spanish Research Council (CSIC) for the use of a free-of-charge licence to the Cambridge Structural Database. RMF and FZ also thank the Universidad del Valle, Colombia, and the Instituto de Química de São Carlos, USP, Brasil for partial financial support. RLAH thanks CNPq for partial financial support.
supplementary crystallographic information
Comment
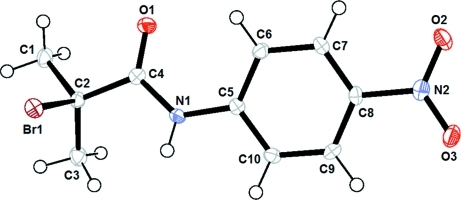
The title compound, (I), forms a part of a synthetic programme that the Polymer Research Group of Universidad del Valle is pursuing in order to obtain compounds that act as initiators of reactions in polymerization processes. Compound (I), Fig. 1, belongs to a series of polymeric ATRP (polymerization by atom transfer radical) initiators (Pietrasik & Tsarevsky, 2010); most initiators for ATRP processes are alkyl halides (Matyjaszewski & Xia, 2001). The C4—N1—C5—C6 torsion angle is 12.7 (4)°, indicating a small twist between the benzene ring and the amide. An intramolecular C—H···O interaction is observed (see Table 1).
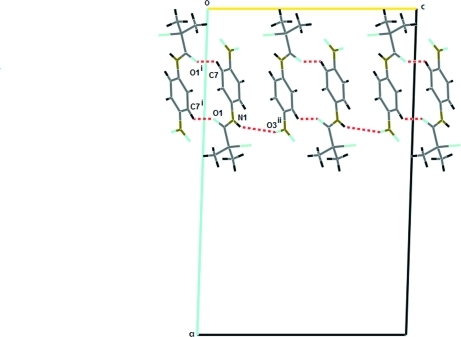
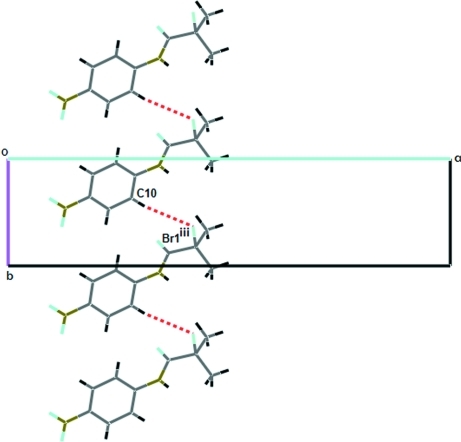
In the crystal structure, weak C—H···O and N—H···O intermolecular interactions are detected (Table 1). In a first substructure (Fig. 2), molecules are linked by C7—H···O1i contacts (i: -x+1/2,-y-1/2,-z) which leads to the formation of dimers with graphs-set notation, R22(14) (Etter, 1990). In turn, N—H···O interactions link the dimers running along the c axis. The N1 atom acts as hydrogen bond donor to O3ii (ii: -x+1/2,+y-1/2,-z+1/2). In a second substructure, C10—H···Br (Table 1) interactions provide further links between the dimers. Overall, the crystal structure comprises layers in the bc plane (Fig. 3).
Experimental
The initial reagents were purchased from Aldrich Chemical Co. and were used as received. In a 100 mL round bottom flask 4-nitroaniline (3.258 mmol, 0.450 g), triethylamine (0.653 mmol, 0.066 g) were mixed, then a solution of 2-bromo isobuturyl bromide (0.704 g) in anhydrous THF (5 mL) was added drop wise, under an argon stream. The reaction was carried out in a dry bag overnight under magnetic stirring. The solid was filtered off and dichloromethane (20 mL) added to the organic phase which was washed with brine (40 mL) followed by water (10 mL). The solution was concentrated at low pressure affording colourless crystals and recrystalized from a solution of hexane and ethyl acetate (80:20). M. pt. 356 (1) K.
Refinement
The H-atoms were placed geometrically [C—H = 0.95 Å for aromatic & C—H = 0.98 Å for methyl], refined in the riding model approximation with Uiso(H) = 1.2–1.5Ueq(C). The N-bound H atom was refined freely.
Figures
Fig. 1.
An ORTEP-3 (Farrugia, 1997) plot of (I) with the atomic labelling scheme. Displacement ellipsoids are drawn at the 50% probability level. H atoms are shown as spheres of arbitrary radius.
Fig. 2.
Part of the crystal structure of (I), showing the formation of R22(10) and R44(18) rings, running along [110]. Symmetry code: (i) -x+1,-y+1,-z; (ii) -x+2,-y+2,-z.
Fig. 3.
Part of the crystal structure of (I), showing the formation of an infinite zig-zag chain of dimers along [001] direction. Symmetry code: (iii) x,-y+1/2+1,+z-1/2.
Crystal data
| C10H11BrN2O3 | F(000) = 1152 |
| Mr = 287.11 | Dx = 1.747 Mg m−3 |
| Monoclinic, C2/c | Melting point: 385(1) K |
| Hall symbol: -C 2yc | Mo Kα radiation, λ = 0.71073 Å |
| a = 24.1245 (12) Å | Cell parameters from 2722 reflections |
| b = 5.8507 (3) Å | θ = 3.2–29.3° |
| c = 15.4723 (8) Å | µ = 3.76 mm−1 |
| β = 91.837 (5)° | T = 123 K |
| V = 2182.72 (19) Å3 | Fragment cut from needle, colourless |
| Z = 8 | 0.60 × 0.05 × 0.05 mm |
Data collection
| Oxford Diffraction Xcalibur E diffractometer | 2633 independent reflections |
| Radiation source: fine-focus sealed tube | 2197 reflections with I > 2σ(I) |
| graphite | Rint = 0.032 |
| ω scans | θmax = 29.0°, θmin = 3.2° |
| Absorption correction: analytical (CrysAlis PRO; Oxford Diffraction, 2009) | h = −32→18 |
| Tmin = 0.444, Tmax = 1.000 | k = −6→7 |
| 5100 measured reflections | l = −19→21 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.032 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.068 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0213P)2] where P = (Fo2 + 2Fc2)/3 |
| 2633 reflections | (Δ/σ)max < 0.001 |
| 151 parameters | Δρmax = 0.55 e Å−3 |
| 0 restraints | Δρmin = −0.60 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.418223 (9) | −0.36757 (5) | 0.230618 (14) | 0.01800 (9) | |
| O1 | 0.33754 (6) | −0.2340 (4) | 0.04409 (11) | 0.0205 (4) | |
| O2 | 0.09326 (6) | 0.3711 (4) | 0.06453 (11) | 0.0219 (4) | |
| O3 | 0.12126 (7) | 0.6341 (3) | 0.15486 (11) | 0.0224 (4) | |
| N1 | 0.34163 (8) | 0.0629 (4) | 0.13870 (13) | 0.0177 (5) | |
| N2 | 0.12933 (8) | 0.4612 (4) | 0.11152 (12) | 0.0175 (5) | |
| C1 | 0.44903 (10) | −0.3344 (5) | 0.05532 (15) | 0.0205 (6) | |
| H1A | 0.4520 | −0.2507 | 0.0008 | 0.031* | |
| H1B | 0.4251 | −0.4681 | 0.0461 | 0.031* | |
| H1C | 0.4860 | −0.3844 | 0.0756 | 0.031* | |
| C2 | 0.42421 (9) | −0.1793 (5) | 0.12266 (14) | 0.0156 (5) | |
| C3 | 0.46064 (9) | 0.0238 (5) | 0.14342 (16) | 0.0209 (6) | |
| H3A | 0.4986 | −0.0283 | 0.1564 | 0.031* | |
| H3B | 0.4465 | 0.1044 | 0.1937 | 0.031* | |
| H3C | 0.4605 | 0.1275 | 0.0937 | 0.031* | |
| C4 | 0.36323 (9) | −0.1220 (5) | 0.09765 (14) | 0.0143 (5) | |
| C5 | 0.28757 (9) | 0.1555 (5) | 0.12844 (14) | 0.0133 (5) | |
| C6 | 0.24391 (9) | 0.0415 (5) | 0.08499 (14) | 0.0156 (5) | |
| H6 | 0.2497 | −0.1040 | 0.0595 | 0.019* | |
| C7 | 0.19187 (9) | 0.1446 (5) | 0.07971 (14) | 0.0154 (5) | |
| H7 | 0.1618 | 0.0700 | 0.0503 | 0.018* | |
| C8 | 0.18422 (9) | 0.3543 (5) | 0.11722 (14) | 0.0135 (5) | |
| C9 | 0.22692 (9) | 0.4702 (5) | 0.16051 (14) | 0.0158 (5) | |
| H9 | 0.2208 | 0.6157 | 0.1857 | 0.019* | |
| C10 | 0.27856 (9) | 0.3680 (5) | 0.16594 (14) | 0.0150 (5) | |
| H10 | 0.3083 | 0.4438 | 0.1957 | 0.018* | |
| H1N | 0.3624 (11) | 0.132 (5) | 0.1742 (17) | 0.028 (8)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.01677 (13) | 0.01963 (16) | 0.01755 (13) | 0.00024 (10) | −0.00059 (9) | 0.00090 (10) |
| O1 | 0.0181 (8) | 0.0222 (12) | 0.0211 (9) | 0.0011 (8) | −0.0017 (7) | −0.0079 (8) |
| O2 | 0.0143 (8) | 0.0292 (13) | 0.0218 (9) | 0.0013 (8) | −0.0029 (7) | 0.0000 (8) |
| O3 | 0.0204 (9) | 0.0223 (12) | 0.0247 (9) | 0.0072 (8) | 0.0027 (7) | −0.0027 (9) |
| N1 | 0.0135 (10) | 0.0205 (14) | 0.0187 (11) | 0.0023 (9) | −0.0038 (8) | −0.0075 (10) |
| N2 | 0.0183 (10) | 0.0190 (14) | 0.0152 (10) | 0.0039 (9) | 0.0026 (8) | 0.0028 (9) |
| C1 | 0.0180 (12) | 0.0222 (17) | 0.0214 (12) | 0.0066 (11) | 0.0030 (10) | −0.0025 (11) |
| C2 | 0.0151 (11) | 0.0158 (15) | 0.0159 (11) | 0.0025 (10) | 0.0004 (9) | −0.0007 (10) |
| C3 | 0.0141 (11) | 0.0220 (17) | 0.0265 (13) | 0.0001 (11) | 0.0008 (10) | 0.0007 (12) |
| C4 | 0.0166 (11) | 0.0144 (14) | 0.0122 (11) | −0.0001 (10) | 0.0032 (9) | 0.0011 (10) |
| C5 | 0.0127 (11) | 0.0165 (15) | 0.0109 (10) | −0.0002 (10) | 0.0020 (8) | 0.0010 (10) |
| C6 | 0.0176 (11) | 0.0145 (15) | 0.0148 (11) | 0.0015 (10) | 0.0005 (9) | −0.0023 (10) |
| C7 | 0.0121 (11) | 0.0187 (16) | 0.0152 (11) | −0.0004 (10) | −0.0013 (9) | 0.0010 (10) |
| C8 | 0.0119 (11) | 0.0170 (15) | 0.0117 (11) | 0.0030 (10) | 0.0024 (8) | 0.0034 (10) |
| C9 | 0.0200 (12) | 0.0120 (14) | 0.0155 (11) | 0.0010 (10) | 0.0030 (9) | −0.0018 (10) |
| C10 | 0.0143 (11) | 0.0177 (15) | 0.0130 (11) | −0.0018 (10) | −0.0001 (8) | −0.0016 (10) |
Geometric parameters (Å, °)
| Br1—C2 | 2.010 (2) | C3—H3A | 0.9800 |
| O1—C4 | 1.211 (3) | C3—H3B | 0.9800 |
| O2—N2 | 1.234 (3) | C3—H3C | 0.9800 |
| O3—N2 | 1.232 (3) | C5—C10 | 1.392 (4) |
| N1—C4 | 1.366 (3) | C5—C6 | 1.400 (3) |
| N1—C5 | 1.416 (3) | C6—C7 | 1.393 (3) |
| N1—H1N | 0.84 (3) | C6—H6 | 0.9500 |
| N2—C8 | 1.465 (3) | C7—C8 | 1.373 (4) |
| C1—C2 | 1.519 (3) | C7—H7 | 0.9500 |
| C1—H1A | 0.9800 | C8—C9 | 1.387 (3) |
| C1—H1B | 0.9800 | C9—C10 | 1.382 (3) |
| C1—H1C | 0.9800 | C9—H9 | 0.9500 |
| C2—C3 | 1.506 (4) | C10—H10 | 0.9500 |
| C2—C4 | 1.546 (3) | ||
| C4—N1—C5 | 127.9 (2) | H3B—C3—H3C | 109.5 |
| C4—N1—H1N | 117 (2) | O1—C4—N1 | 123.5 (2) |
| C5—N1—H1N | 115 (2) | O1—C4—C2 | 121.0 (2) |
| O3—N2—O2 | 123.4 (2) | N1—C4—C2 | 115.4 (2) |
| O3—N2—C8 | 118.4 (2) | C10—C5—C6 | 120.1 (2) |
| O2—N2—C8 | 118.2 (2) | C10—C5—N1 | 116.8 (2) |
| C2—C1—H1A | 109.5 | C6—C5—N1 | 123.2 (2) |
| C2—C1—H1B | 109.5 | C7—C6—C5 | 119.0 (2) |
| H1A—C1—H1B | 109.5 | C7—C6—H6 | 120.5 |
| C2—C1—H1C | 109.5 | C5—C6—H6 | 120.5 |
| H1A—C1—H1C | 109.5 | C8—C7—C6 | 119.7 (2) |
| H1B—C1—H1C | 109.5 | C8—C7—H7 | 120.1 |
| C3—C2—C1 | 112.2 (2) | C6—C7—H7 | 120.1 |
| C3—C2—C4 | 115.2 (2) | C7—C8—C9 | 122.2 (2) |
| C1—C2—C4 | 110.53 (19) | C7—C8—N2 | 119.3 (2) |
| C3—C2—Br1 | 108.18 (15) | C9—C8—N2 | 118.5 (2) |
| C1—C2—Br1 | 106.45 (18) | C10—C9—C8 | 118.2 (2) |
| C4—C2—Br1 | 103.51 (14) | C10—C9—H9 | 120.9 |
| C2—C3—H3A | 109.5 | C8—C9—H9 | 120.9 |
| C2—C3—H3B | 109.5 | C9—C10—C5 | 120.9 (2) |
| H3A—C3—H3B | 109.5 | C9—C10—H10 | 119.6 |
| C2—C3—H3C | 109.5 | C5—C10—H10 | 119.6 |
| H3A—C3—H3C | 109.5 | ||
| C5—N1—C4—O1 | 0.7 (4) | C5—C6—C7—C8 | −0.3 (3) |
| C5—N1—C4—C2 | 179.5 (2) | C6—C7—C8—C9 | 0.3 (4) |
| C3—C2—C4—O1 | 145.0 (2) | C6—C7—C8—N2 | −179.9 (2) |
| C1—C2—C4—O1 | 16.6 (3) | O3—N2—C8—C7 | 170.6 (2) |
| Br1—C2—C4—O1 | −97.1 (2) | O2—N2—C8—C7 | −8.3 (3) |
| C3—C2—C4—N1 | −33.9 (3) | O3—N2—C8—C9 | −9.6 (3) |
| C1—C2—C4—N1 | −162.3 (2) | O2—N2—C8—C9 | 171.6 (2) |
| Br1—C2—C4—N1 | 84.0 (2) | C7—C8—C9—C10 | −0.4 (3) |
| C4—N1—C5—C10 | −168.9 (2) | N2—C8—C9—C10 | 179.8 (2) |
| C4—N1—C5—C6 | 12.7 (4) | C8—C9—C10—C5 | 0.5 (3) |
| C10—C5—C6—C7 | 0.4 (3) | C6—C5—C10—C9 | −0.5 (3) |
| N1—C5—C6—C7 | 178.7 (2) | N1—C5—C10—C9 | −179.0 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C6—H6···O1 | 0.95 | 2.27 | 2.862 (3) | 120 |
| C7—H7···O1i | 0.95 | 2.45 | 3.139 (3) | 129 |
| N1—H1n···O3ii | 0.84 (3) | 2.66 (3) | 3.316 (3) | 136 (2) |
| C10—H10···Br1iii | 0.95 | 2.91 | 3.812 (2) | 160 |
Symmetry codes: (i) −x+1/2, −y−1/2, −z; (ii) −x+1/2, y−1/2, −z+1/2; (iii) x, y+1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: TK2720).
References
- Etter, M. (1990). Acc. Chem. Res. 23, 120–126.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst. 39, 453–457.
- Matyjaszewski, K. & Xia, J. (2001). Chem. Rev. 101, 2921–2990. [DOI] [PubMed]
- Oxford Diffraction (2009). CrysAlis CCD and CrysAlis RED Oxford Diffraction Ltd, Yarnton, England.
- Pietrasik, J. & Tsarevsky, N. V. (2010). Eur. Polym. J. 46, 2333–2340.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811005320/tk2720sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811005320/tk2720Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report