Abstract
The crystal studied of the title compound, C19H13NO3S2, was found to be a non-merohedral twin with a domain ratio of 0.877 (3):0.123 (3). There are two independent molecules in the asymmetric unit. The dihedral angles between the mean plane of the indole ring system and the phenylsulfonyl ring are 71.67 (13) and 71.95 (13)° in the two molecules while the indole unit and the thiophene ring make dihedral angles of 54.91 (12) and 56.92 (13)° in the two molecules. The crystal packing is stabilized by weak C—H⋯π interactions.
Related literature
For biological activity of chromenopyrrole, see: Ma et al. (2001 ▶); Zhao et al. (2002 ▶); Zhou et al. (2006 ▶); Rajeswaran et al. (1999 ▶); For related structures, see: Chakkaravarthi et al. (2007 ▶); Gunasekaran et al. (2009 ▶); Saravanan et al. (2010 ▶).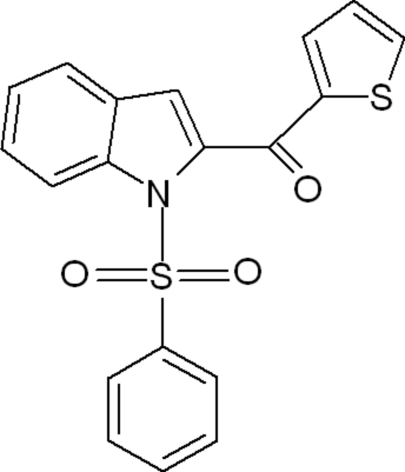
Experimental
Crystal data
C19H13NO3S2
M r = 367.42
Triclinic,

a = 9.3605 (5) Å
b = 10.8455 (5) Å
c = 17.5482 (9) Å
α = 88.716 (3)°
β = 80.425 (2)°
γ = 71.467 (2)°
V = 1664.68 (15) Å3
Z = 4
Mo Kα radiation
μ = 0.34 mm−1
T = 295 K
0.35 × 0.25 × 0.20 mm
Data collection
Bruker Kappa APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.924, T max = 0.951
36289 measured reflections
8039 independent reflections
6195 reflections with I > 2σ(I)
R int = 0.040
Refinement
R[F 2 > 2σ(F 2)] = 0.077
wR(F 2) = 0.245
S = 1.07
8039 reflections
453 parameters
H-atom parameters constrained
Δρmax = 0.55 e Å−3
Δρmin = −0.37 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: SAINT (Bruker, 2004 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811005666/bt5452sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811005666/bt5452Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg is the centroid of the C20–C25 ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C17—H17⋯Cg8i | 0.93 | 2.88 | 3.693 (6) | 147 |
Symmetry code: (i)  .
.
Acknowledgments
CK thanks AMET University management for their kind support.
supplementary crystallographic information
Comment
Indole derivatives are found to possess anticancer, antimalarial and antihypertensive activities (Ma et al., 2001; Zhou et al., 2006; Zhao et al., 2002). In addition, Indoles have been proved to display high aldose reductase inhibitory activity (Rajeswaran et al., 1999).
The geometric parameters of the title molecule (Fig. 1) agree well with reported similar structure(Chakkaravarthi et al., 2007; Gunasekaran et al., 2009; Saravanan et al., 2010). The compound is non-merohedrally twinned, the suggested transformation matrix is (-1 0 0, 0 - 1 0, -0.664 0.110 1). The dihedral angle between the nine membered indole moiety and the thiophene ring is 54.91 (12) ° for molecule (I) and 56.92 (13) ° for molecule (II) respectively. The torsion angles O1—S1—N1—C1 and O2—S1—N1—C8 in molecule (I), O4—S3—N2—C20 and O5—S3—N2—C27 in molecule (II) [-9.8 (4) ° and 27.7 (4) ° for molecule (I), 9.1 (4) ° and -27.2 (4) ° for molecule (II), respectively] indicates the syn conformation of the sulfonyl moiety.
The sum of bond angles around N1 and N2 are 358.9 (3) ° and 358.6 (3) ° respectively, indicates the sp2 hybridization state of atoms N1 and N2. The molecular structure is stabilized by weak intramolecular C—H···O interactions and the crystal packing is stabilized by weak C—H···π [C17—H17···Cg8(1 - x, 1 - y, 1 - z) distance of 3.693 (6)Å (Cg8 is the centroid of the ring defined by the atoms C20—C25)] interactions.
Experimental
To a solution of N-(2-Formylphenyl)benzenesulfonamide (0.5 g, 1.91 mmol) in dry CH3CN (20 ml), K2CO3 (0.8 g, 5.79 mmol), 2-bromo-1-(thiophen-2-yl) ethanone (0.5 g, 2.43 mmol) were added. The reaction mixture was stirred at room temperature for 6 h under N2 atmosphere. The solvent was removed and the residue was quenched with ice-water (50 ml), extracted with chloroform (3 x 10 ml) and dried (Na2SO4). Removal of solvent followed by the residue was dissolved in CH3CN (20 ml), Conc.HCl (3 ml) was added. The reaction mixture was then refluxed for 2 h. It was then poured over ice-water (50 ml), extracted with CHCl3 (3 x 10 ml) and dried (Na2SO4). Removal of solvent followed by crystallization from methanol afforded the compound as a colorless crystal.
Refinement
H atoms were positioned geometrically and refined using riding model with C—H = 0.93Å and Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
The molecular structure of the title compound, with atom labels and 30% probability displacement ellipsoids for non-H atoms.
Crystal data
| C19H13NO3S2 | Z = 4 |
| Mr = 367.42 | F(000) = 760 |
| Triclinic, P1 | Dx = 1.466 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.3605 (5) Å | Cell parameters from 6464 reflections |
| b = 10.8455 (5) Å | θ = 2.4–27.8° |
| c = 17.5482 (9) Å | µ = 0.34 mm−1 |
| α = 88.716 (3)° | T = 295 K |
| β = 80.425 (2)° | Block, colourless |
| γ = 71.467 (2)° | 0.35 × 0.25 × 0.20 mm |
| V = 1664.68 (15) Å3 |
Data collection
| Bruker Kappa APEXII CCD diffractometer | 8039 independent reflections |
| Radiation source: fine-focus sealed tube | 6195 reflections with I > 2σ(I) |
| graphite | Rint = 0.040 |
| Detector resolution: 0 pixels mm-1 | θmax = 28.0°, θmin = 1.2° |
| ω and φ scans | h = −12→12 |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | k = −14→14 |
| Tmin = 0.924, Tmax = 0.951 | l = −23→23 |
| 36289 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.077 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.245 | H-atom parameters constrained |
| S = 1.07 | w = 1/[σ2(Fo2) + (0.092P)2 + 4.8598P] where P = (Fo2 + 2Fc2)/3 |
| 8039 reflections | (Δ/σ)max < 0.001 |
| 453 parameters | Δρmax = 0.55 e Å−3 |
| 0 restraints | Δρmin = −0.37 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.4481 (5) | 0.1880 (4) | 0.3523 (2) | 0.0356 (8) | |
| C2 | 0.3808 (6) | 0.3165 (4) | 0.3334 (3) | 0.0465 (10) | |
| H2 | 0.4309 | 0.3781 | 0.3332 | 0.056* | |
| C3 | 0.2370 (6) | 0.3476 (5) | 0.3150 (3) | 0.0538 (12) | |
| H3 | 0.1889 | 0.4326 | 0.3021 | 0.065* | |
| C4 | 0.1597 (6) | 0.2562 (5) | 0.3149 (3) | 0.0565 (13) | |
| H4 | 0.0614 | 0.2816 | 0.3031 | 0.068* | |
| C5 | 0.2277 (5) | 0.1307 (5) | 0.3320 (3) | 0.0504 (11) | |
| H5 | 0.1768 | 0.0698 | 0.3314 | 0.061* | |
| C6 | 0.3763 (5) | 0.0933 (4) | 0.3507 (3) | 0.0404 (9) | |
| C7 | 0.4734 (5) | −0.0266 (4) | 0.3714 (3) | 0.0431 (10) | |
| H7 | 0.4527 | −0.1051 | 0.3736 | 0.052* | |
| C8 | 0.6014 (5) | −0.0084 (4) | 0.3877 (3) | 0.0376 (9) | |
| C9 | 0.7171 (5) | −0.0981 (4) | 0.4277 (3) | 0.0380 (9) | |
| C10 | 0.7561 (5) | −0.2365 (4) | 0.4090 (2) | 0.0366 (8) | |
| C11 | 0.7462 (5) | −0.2983 (4) | 0.3425 (3) | 0.0408 (9) | |
| H11 | 0.7074 | −0.2553 | 0.3003 | 0.049* | |
| C12 | 0.8028 (6) | −0.4352 (5) | 0.3473 (3) | 0.0530 (12) | |
| H12 | 0.8071 | −0.4930 | 0.3080 | 0.064* | |
| C13 | 0.8501 (6) | −0.4731 (5) | 0.4156 (3) | 0.0561 (13) | |
| H13 | 0.8880 | −0.5597 | 0.4285 | 0.067* | |
| C14 | 0.7289 (5) | 0.2305 (4) | 0.4700 (3) | 0.0383 (9) | |
| C15 | 0.8555 (5) | 0.1793 (5) | 0.5050 (3) | 0.0493 (11) | |
| H15 | 0.9434 | 0.1191 | 0.4785 | 0.059* | |
| C16 | 0.8498 (6) | 0.2187 (5) | 0.5801 (3) | 0.0552 (12) | |
| H16 | 0.9335 | 0.1830 | 0.6049 | 0.066* | |
| C17 | 0.7217 (6) | 0.3102 (5) | 0.6184 (3) | 0.0537 (12) | |
| H17 | 0.7190 | 0.3373 | 0.6687 | 0.064* | |
| C18 | 0.5971 (6) | 0.3618 (5) | 0.5823 (3) | 0.0493 (11) | |
| H18 | 0.5104 | 0.4238 | 0.6086 | 0.059* | |
| C19 | 0.5985 (5) | 0.3231 (4) | 0.5072 (3) | 0.0442 (10) | |
| H19 | 0.5143 | 0.3583 | 0.4828 | 0.053* | |
| N1 | 0.5908 (4) | 0.1243 (3) | 0.3762 (2) | 0.0371 (7) | |
| O2 | 0.8724 (4) | 0.0828 (4) | 0.3479 (2) | 0.0562 (9) | |
| O1 | 0.6943 (5) | 0.3007 (4) | 0.3310 (2) | 0.0583 (9) | |
| O3 | 0.7730 (4) | −0.0590 (3) | 0.4761 (2) | 0.0539 (9) | |
| S1 | 0.73502 (13) | 0.18488 (11) | 0.37382 (7) | 0.0413 (3) | |
| S2 | 0.83327 (16) | −0.34647 (13) | 0.47492 (8) | 0.0544 (3) | |
| C20 | 0.3630 (5) | 0.6980 (4) | 0.1576 (2) | 0.0421 (9) | |
| C21 | 0.2888 (7) | 0.8279 (5) | 0.1809 (3) | 0.0569 (13) | |
| H21 | 0.3425 | 0.8867 | 0.1815 | 0.068* | |
| C22 | 0.1326 (7) | 0.8653 (5) | 0.2032 (3) | 0.0645 (15) | |
| H22 | 0.0801 | 0.9519 | 0.2180 | 0.077* | |
| C23 | 0.0509 (6) | 0.7792 (6) | 0.2043 (3) | 0.0642 (15) | |
| H23 | −0.0542 | 0.8078 | 0.2212 | 0.077* | |
| C24 | 0.1237 (6) | 0.6514 (6) | 0.1808 (3) | 0.0575 (13) | |
| H24 | 0.0684 | 0.5938 | 0.1807 | 0.069* | |
| C25 | 0.2827 (5) | 0.6092 (5) | 0.1569 (3) | 0.0448 (10) | |
| C26 | 0.3888 (5) | 0.4867 (4) | 0.1316 (3) | 0.0440 (10) | |
| H26 | 0.3660 | 0.4097 | 0.1281 | 0.053* | |
| C27 | 0.5280 (5) | 0.5000 (4) | 0.1135 (3) | 0.0408 (9) | |
| C28 | 0.6673 (5) | 0.4060 (4) | 0.0691 (3) | 0.0418 (9) | |
| C29 | 0.6939 (5) | 0.2679 (4) | 0.0865 (3) | 0.0408 (9) | |
| C30 | 0.6482 (6) | 0.2140 (5) | 0.1540 (3) | 0.0525 (12) | |
| H30 | 0.5864 | 0.2620 | 0.1974 | 0.063* | |
| C31 | 0.7070 (8) | 0.0763 (6) | 0.1495 (4) | 0.0739 (17) | |
| H31 | 0.6879 | 0.0228 | 0.1894 | 0.089* | |
| C32 | 0.7944 (7) | 0.0321 (5) | 0.0798 (5) | 0.0729 (18) | |
| H32 | 0.8415 | −0.0558 | 0.0666 | 0.087* | |
| C33 | 0.7163 (5) | 0.7268 (4) | 0.0347 (3) | 0.0405 (9) | |
| C34 | 0.8649 (6) | 0.6794 (5) | −0.0023 (3) | 0.0543 (12) | |
| H34 | 0.9382 | 0.6208 | 0.0221 | 0.065* | |
| C35 | 0.9052 (7) | 0.7184 (6) | −0.0750 (4) | 0.0673 (15) | |
| H35 | 1.0055 | 0.6852 | −0.1006 | 0.081* | |
| C36 | 0.7982 (8) | 0.8063 (6) | −0.1101 (3) | 0.0643 (15) | |
| H36 | 0.8264 | 0.8334 | −0.1594 | 0.077* | |
| C37 | 0.6495 (7) | 0.8549 (5) | −0.0735 (3) | 0.0593 (13) | |
| H37 | 0.5772 | 0.9144 | −0.0979 | 0.071* | |
| C38 | 0.6077 (6) | 0.8155 (5) | −0.0007 (3) | 0.0500 (11) | |
| H38 | 0.5071 | 0.8483 | 0.0245 | 0.060* | |
| N2 | 0.5175 (4) | 0.6304 (3) | 0.1284 (2) | 0.0415 (8) | |
| O4 | 0.6061 (5) | 0.8001 (4) | 0.1772 (2) | 0.0609 (10) | |
| O5 | 0.7862 (4) | 0.5782 (4) | 0.1510 (2) | 0.0586 (9) | |
| O6 | 0.7506 (4) | 0.4410 (3) | 0.0187 (2) | 0.0563 (9) | |
| S3 | 0.66525 (14) | 0.68399 (12) | 0.13007 (7) | 0.0450 (3) | |
| S4 | 0.80927 (17) | 0.15141 (14) | 0.01948 (9) | 0.0630 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.038 (2) | 0.033 (2) | 0.034 (2) | −0.0084 (16) | −0.0079 (16) | 0.0043 (15) |
| C2 | 0.052 (3) | 0.034 (2) | 0.048 (3) | −0.0044 (18) | −0.009 (2) | 0.0024 (18) |
| C3 | 0.054 (3) | 0.043 (3) | 0.051 (3) | 0.004 (2) | −0.012 (2) | 0.006 (2) |
| C4 | 0.043 (3) | 0.065 (3) | 0.053 (3) | −0.001 (2) | −0.016 (2) | −0.005 (2) |
| C5 | 0.037 (2) | 0.055 (3) | 0.060 (3) | −0.012 (2) | −0.015 (2) | 0.000 (2) |
| C6 | 0.038 (2) | 0.038 (2) | 0.046 (2) | −0.0112 (17) | −0.0076 (17) | −0.0050 (18) |
| C7 | 0.043 (2) | 0.034 (2) | 0.056 (3) | −0.0142 (18) | −0.0142 (19) | 0.0015 (19) |
| C8 | 0.037 (2) | 0.0271 (19) | 0.048 (2) | −0.0079 (15) | −0.0099 (17) | 0.0018 (16) |
| C9 | 0.035 (2) | 0.036 (2) | 0.042 (2) | −0.0094 (16) | −0.0083 (16) | 0.0012 (17) |
| C10 | 0.038 (2) | 0.0288 (19) | 0.043 (2) | −0.0084 (15) | −0.0103 (17) | 0.0036 (16) |
| C11 | 0.043 (2) | 0.030 (2) | 0.048 (2) | −0.0081 (17) | −0.0082 (18) | 0.0000 (17) |
| C12 | 0.053 (3) | 0.035 (2) | 0.067 (3) | −0.007 (2) | −0.012 (2) | −0.006 (2) |
| C13 | 0.051 (3) | 0.034 (2) | 0.080 (4) | −0.009 (2) | −0.012 (2) | 0.013 (2) |
| C14 | 0.040 (2) | 0.032 (2) | 0.046 (2) | −0.0149 (17) | −0.0091 (17) | −0.0033 (17) |
| C15 | 0.037 (2) | 0.055 (3) | 0.057 (3) | −0.0115 (19) | −0.012 (2) | −0.005 (2) |
| C16 | 0.053 (3) | 0.057 (3) | 0.062 (3) | −0.018 (2) | −0.025 (2) | −0.002 (2) |
| C17 | 0.067 (3) | 0.053 (3) | 0.050 (3) | −0.029 (2) | −0.014 (2) | −0.002 (2) |
| C18 | 0.048 (3) | 0.042 (2) | 0.055 (3) | −0.012 (2) | −0.004 (2) | −0.012 (2) |
| C19 | 0.038 (2) | 0.037 (2) | 0.056 (3) | −0.0097 (17) | −0.0105 (19) | −0.0022 (19) |
| N1 | 0.0380 (17) | 0.0296 (16) | 0.046 (2) | −0.0119 (14) | −0.0123 (15) | 0.0019 (14) |
| O2 | 0.0428 (18) | 0.064 (2) | 0.058 (2) | −0.0176 (16) | 0.0042 (15) | −0.0177 (17) |
| O1 | 0.075 (2) | 0.056 (2) | 0.059 (2) | −0.0395 (19) | −0.0159 (18) | 0.0106 (17) |
| O3 | 0.060 (2) | 0.0457 (18) | 0.059 (2) | −0.0120 (16) | −0.0263 (17) | −0.0055 (15) |
| S1 | 0.0414 (6) | 0.0411 (6) | 0.0455 (6) | −0.0193 (4) | −0.0053 (4) | −0.0028 (4) |
| S2 | 0.0567 (7) | 0.0483 (7) | 0.0547 (7) | −0.0088 (5) | −0.0171 (6) | 0.0126 (5) |
| C20 | 0.048 (2) | 0.040 (2) | 0.033 (2) | −0.0095 (18) | 0.0001 (17) | 0.0041 (17) |
| C21 | 0.064 (3) | 0.039 (2) | 0.055 (3) | −0.009 (2) | 0.008 (2) | −0.003 (2) |
| C22 | 0.062 (3) | 0.045 (3) | 0.064 (3) | 0.005 (2) | 0.010 (3) | 0.000 (2) |
| C23 | 0.047 (3) | 0.063 (3) | 0.063 (3) | 0.000 (2) | 0.009 (2) | 0.010 (3) |
| C24 | 0.041 (3) | 0.064 (3) | 0.064 (3) | −0.016 (2) | −0.002 (2) | 0.010 (3) |
| C25 | 0.042 (2) | 0.045 (2) | 0.047 (2) | −0.0137 (19) | −0.0083 (19) | 0.0121 (19) |
| C26 | 0.048 (2) | 0.037 (2) | 0.047 (2) | −0.0145 (19) | −0.0047 (19) | 0.0015 (18) |
| C27 | 0.043 (2) | 0.036 (2) | 0.042 (2) | −0.0111 (17) | −0.0076 (18) | 0.0010 (17) |
| C28 | 0.040 (2) | 0.043 (2) | 0.041 (2) | −0.0105 (18) | −0.0063 (17) | −0.0031 (18) |
| C29 | 0.040 (2) | 0.033 (2) | 0.045 (2) | −0.0063 (17) | −0.0058 (17) | −0.0075 (17) |
| C30 | 0.061 (3) | 0.040 (2) | 0.049 (3) | −0.006 (2) | −0.006 (2) | 0.001 (2) |
| C31 | 0.073 (4) | 0.044 (3) | 0.097 (5) | −0.010 (3) | −0.012 (3) | 0.012 (3) |
| C32 | 0.058 (3) | 0.039 (3) | 0.115 (5) | −0.006 (2) | −0.013 (3) | −0.015 (3) |
| C33 | 0.043 (2) | 0.040 (2) | 0.043 (2) | −0.0207 (18) | −0.0083 (18) | 0.0027 (18) |
| C34 | 0.043 (2) | 0.059 (3) | 0.059 (3) | −0.015 (2) | −0.005 (2) | 0.001 (2) |
| C35 | 0.059 (3) | 0.074 (4) | 0.067 (4) | −0.027 (3) | 0.009 (3) | −0.001 (3) |
| C36 | 0.085 (4) | 0.066 (4) | 0.048 (3) | −0.037 (3) | −0.002 (3) | 0.006 (3) |
| C37 | 0.071 (4) | 0.053 (3) | 0.060 (3) | −0.021 (3) | −0.027 (3) | 0.016 (2) |
| C38 | 0.046 (2) | 0.052 (3) | 0.054 (3) | −0.016 (2) | −0.011 (2) | 0.005 (2) |
| N2 | 0.0423 (19) | 0.0351 (18) | 0.044 (2) | −0.0114 (15) | 0.0004 (15) | −0.0031 (15) |
| O4 | 0.076 (3) | 0.058 (2) | 0.055 (2) | −0.0302 (19) | −0.0089 (18) | −0.0104 (17) |
| O5 | 0.055 (2) | 0.064 (2) | 0.061 (2) | −0.0171 (17) | −0.0240 (17) | 0.0103 (18) |
| O6 | 0.055 (2) | 0.051 (2) | 0.054 (2) | −0.0133 (16) | 0.0062 (16) | 0.0019 (16) |
| S3 | 0.0491 (6) | 0.0461 (6) | 0.0439 (6) | −0.0193 (5) | −0.0107 (5) | −0.0003 (5) |
| S4 | 0.0559 (8) | 0.0545 (8) | 0.0683 (9) | −0.0081 (6) | 0.0023 (6) | −0.0222 (7) |
Geometric parameters (Å, °)
| C1—C2 | 1.393 (6) | C20—C21 | 1.394 (6) |
| C1—C6 | 1.398 (6) | C20—C25 | 1.399 (7) |
| C1—N1 | 1.423 (5) | C20—N2 | 1.410 (6) |
| C2—C3 | 1.371 (7) | C21—C22 | 1.377 (8) |
| C2—H2 | 0.9300 | C21—H21 | 0.9300 |
| C3—C4 | 1.401 (8) | C22—C23 | 1.381 (9) |
| C3—H3 | 0.9300 | C22—H22 | 0.9300 |
| C4—C5 | 1.357 (7) | C23—C24 | 1.373 (8) |
| C4—H4 | 0.9300 | C23—H23 | 0.9300 |
| C5—C6 | 1.411 (6) | C24—C25 | 1.403 (7) |
| C5—H5 | 0.9300 | C24—H24 | 0.9300 |
| C6—C7 | 1.409 (6) | C25—C26 | 1.410 (6) |
| C7—C8 | 1.350 (6) | C26—C27 | 1.342 (6) |
| C7—H7 | 0.9300 | C26—H26 | 0.9300 |
| C8—N1 | 1.422 (5) | C27—N2 | 1.414 (6) |
| C8—C9 | 1.472 (6) | C27—C28 | 1.483 (6) |
| C9—O3 | 1.216 (5) | C28—O6 | 1.216 (6) |
| C9—C10 | 1.458 (6) | C28—C29 | 1.472 (6) |
| C10—C11 | 1.390 (6) | C29—C30 | 1.373 (7) |
| C10—S2 | 1.717 (4) | C29—S4 | 1.710 (4) |
| C11—C12 | 1.415 (6) | C30—C31 | 1.417 (7) |
| C11—H11 | 0.9300 | C30—H30 | 0.9300 |
| C12—C13 | 1.357 (8) | C31—C32 | 1.356 (10) |
| C12—H12 | 0.9300 | C31—H31 | 0.9300 |
| C13—S2 | 1.693 (6) | C32—S4 | 1.678 (7) |
| C13—H13 | 0.9300 | C32—H32 | 0.9300 |
| C14—C15 | 1.378 (6) | C33—C34 | 1.371 (7) |
| C14—C19 | 1.382 (6) | C33—C38 | 1.381 (7) |
| C14—S1 | 1.757 (4) | C33—S3 | 1.757 (5) |
| C15—C16 | 1.382 (7) | C34—C35 | 1.367 (8) |
| C15—H15 | 0.9300 | C34—H34 | 0.9300 |
| C16—C17 | 1.371 (8) | C35—C36 | 1.365 (9) |
| C16—H16 | 0.9300 | C35—H35 | 0.9300 |
| C17—C18 | 1.375 (7) | C36—C37 | 1.372 (9) |
| C17—H17 | 0.9300 | C36—H36 | 0.9300 |
| C18—C19 | 1.388 (7) | C37—C38 | 1.371 (8) |
| C18—H18 | 0.9300 | C37—H37 | 0.9300 |
| C19—H19 | 0.9300 | C38—H38 | 0.9300 |
| N1—S1 | 1.674 (3) | N2—S3 | 1.666 (4) |
| O2—S1 | 1.417 (4) | O4—S3 | 1.423 (4) |
| O1—S1 | 1.427 (4) | O5—S3 | 1.424 (4) |
| C2—C1—C6 | 122.4 (4) | C21—C20—C25 | 121.7 (5) |
| C2—C1—N1 | 131.5 (4) | C21—C20—N2 | 131.5 (5) |
| C6—C1—N1 | 106.1 (3) | C25—C20—N2 | 106.8 (4) |
| C3—C2—C1 | 116.7 (5) | C22—C21—C20 | 117.1 (5) |
| C3—C2—H2 | 121.7 | C22—C21—H21 | 121.5 |
| C1—C2—H2 | 121.7 | C20—C21—H21 | 121.5 |
| C2—C3—C4 | 122.5 (5) | C21—C22—C23 | 122.4 (5) |
| C2—C3—H3 | 118.7 | C21—C22—H22 | 118.8 |
| C4—C3—H3 | 118.7 | C23—C22—H22 | 118.8 |
| C5—C4—C3 | 120.2 (5) | C24—C23—C22 | 120.5 (5) |
| C5—C4—H4 | 119.9 | C24—C23—H23 | 119.8 |
| C3—C4—H4 | 119.9 | C22—C23—H23 | 119.8 |
| C4—C5—C6 | 119.5 (5) | C23—C24—C25 | 119.0 (5) |
| C4—C5—H5 | 120.2 | C23—C24—H24 | 120.5 |
| C6—C5—H5 | 120.2 | C25—C24—H24 | 120.5 |
| C1—C6—C7 | 109.0 (4) | C20—C25—C24 | 119.3 (5) |
| C1—C6—C5 | 118.6 (4) | C20—C25—C26 | 108.2 (4) |
| C7—C6—C5 | 132.3 (4) | C24—C25—C26 | 132.5 (5) |
| C8—C7—C6 | 108.6 (4) | C27—C26—C25 | 108.4 (4) |
| C8—C7—H7 | 125.7 | C27—C26—H26 | 125.8 |
| C6—C7—H7 | 125.7 | C25—C26—H26 | 125.8 |
| C7—C8—N1 | 108.6 (4) | C26—C27—N2 | 109.3 (4) |
| C7—C8—C9 | 126.8 (4) | C26—C27—C28 | 127.3 (4) |
| N1—C8—C9 | 123.1 (4) | N2—C27—C28 | 122.2 (4) |
| O3—C9—C10 | 121.8 (4) | O6—C28—C29 | 122.1 (4) |
| O3—C9—C8 | 121.5 (4) | O6—C28—C27 | 121.6 (4) |
| C10—C9—C8 | 116.7 (4) | C29—C28—C27 | 116.2 (4) |
| C11—C10—C9 | 129.9 (4) | C30—C29—C28 | 129.0 (4) |
| C11—C10—S2 | 111.6 (3) | C30—C29—S4 | 111.7 (3) |
| C9—C10—S2 | 118.4 (3) | C28—C29—S4 | 119.1 (3) |
| C10—C11—C12 | 111.2 (4) | C29—C30—C31 | 111.7 (5) |
| C10—C11—H11 | 124.4 | C29—C30—H30 | 124.1 |
| C12—C11—H11 | 124.4 | C31—C30—H30 | 124.1 |
| C13—C12—C11 | 112.6 (5) | C32—C31—C30 | 111.7 (6) |
| C13—C12—H12 | 123.7 | C32—C31—H31 | 124.2 |
| C11—C12—H12 | 123.7 | C30—C31—H31 | 124.2 |
| C12—C13—S2 | 113.1 (4) | C31—C32—S4 | 113.5 (4) |
| C12—C13—H13 | 123.5 | C31—C32—H32 | 123.3 |
| S2—C13—H13 | 123.5 | S4—C32—H32 | 123.3 |
| C15—C14—C19 | 121.7 (4) | C34—C33—C38 | 120.3 (5) |
| C15—C14—S1 | 119.8 (4) | C34—C33—S3 | 120.2 (4) |
| C19—C14—S1 | 118.4 (3) | C38—C33—S3 | 119.3 (4) |
| C14—C15—C16 | 119.0 (5) | C35—C34—C33 | 119.9 (5) |
| C14—C15—H15 | 120.5 | C35—C34—H34 | 120.1 |
| C16—C15—H15 | 120.5 | C33—C34—H34 | 120.1 |
| C17—C16—C15 | 120.5 (5) | C36—C35—C34 | 120.0 (5) |
| C17—C16—H16 | 119.8 | C36—C35—H35 | 120.0 |
| C15—C16—H16 | 119.8 | C34—C35—H35 | 120.0 |
| C16—C17—C18 | 119.9 (5) | C35—C36—C37 | 120.6 (5) |
| C16—C17—H17 | 120.1 | C35—C36—H36 | 119.7 |
| C18—C17—H17 | 120.1 | C37—C36—H36 | 119.7 |
| C17—C18—C19 | 121.1 (5) | C38—C37—C36 | 119.9 (5) |
| C17—C18—H18 | 119.4 | C38—C37—H37 | 120.1 |
| C19—C18—H18 | 119.4 | C36—C37—H37 | 120.1 |
| C14—C19—C18 | 117.9 (4) | C37—C38—C33 | 119.4 (5) |
| C14—C19—H19 | 121.1 | C37—C38—H38 | 120.3 |
| C18—C19—H19 | 121.1 | C33—C38—H38 | 120.3 |
| C8—N1—C1 | 107.6 (3) | C20—N2—C27 | 107.2 (4) |
| C8—N1—S1 | 125.1 (3) | C20—N2—S3 | 126.1 (3) |
| C1—N1—S1 | 126.2 (3) | C27—N2—S3 | 125.3 (3) |
| O2—S1—O1 | 120.2 (2) | O4—S3—O5 | 119.6 (2) |
| O2—S1—N1 | 107.27 (19) | O4—S3—N2 | 105.7 (2) |
| O1—S1—N1 | 105.2 (2) | O5—S3—N2 | 107.4 (2) |
| O2—S1—C14 | 109.9 (2) | O4—S3—C33 | 108.0 (2) |
| O1—S1—C14 | 107.8 (2) | O5—S3—C33 | 109.9 (2) |
| N1—S1—C14 | 105.41 (19) | N2—S3—C33 | 105.2 (2) |
| C13—S2—C10 | 91.4 (2) | C32—S4—C29 | 91.5 (3) |
| C6—C1—C2—C3 | 1.9 (7) | C25—C20—C21—C22 | −0.3 (8) |
| N1—C1—C2—C3 | −177.6 (4) | N2—C20—C21—C22 | 176.6 (5) |
| C1—C2—C3—C4 | 0.0 (7) | C20—C21—C22—C23 | 1.4 (9) |
| C2—C3—C4—C5 | −1.3 (8) | C21—C22—C23—C24 | −1.9 (10) |
| C3—C4—C5—C6 | 0.7 (8) | C22—C23—C24—C25 | 1.2 (9) |
| C2—C1—C6—C7 | 179.1 (4) | C21—C20—C25—C24 | −0.4 (7) |
| N1—C1—C6—C7 | −1.2 (5) | N2—C20—C25—C24 | −177.9 (4) |
| C2—C1—C6—C5 | −2.5 (7) | C21—C20—C25—C26 | −179.3 (5) |
| N1—C1—C6—C5 | 177.2 (4) | N2—C20—C25—C26 | 3.2 (5) |
| C4—C5—C6—C1 | 1.2 (7) | C23—C24—C25—C20 | −0.1 (8) |
| C4—C5—C6—C7 | 179.1 (5) | C23—C24—C25—C26 | 178.5 (5) |
| C1—C6—C7—C8 | 1.4 (5) | C20—C25—C26—C27 | −3.0 (5) |
| C5—C6—C7—C8 | −176.6 (5) | C24—C25—C26—C27 | 178.3 (5) |
| C6—C7—C8—N1 | −1.1 (5) | C25—C26—C27—N2 | 1.5 (5) |
| C6—C7—C8—C9 | 165.5 (4) | C25—C26—C27—C28 | −165.7 (4) |
| C7—C8—C9—O3 | −138.0 (5) | C26—C27—C28—O6 | 135.6 (5) |
| N1—C8—C9—O3 | 26.8 (7) | N2—C27—C28—O6 | −30.2 (7) |
| C7—C8—C9—C10 | 39.3 (7) | C26—C27—C28—C29 | −41.2 (7) |
| N1—C8—C9—C10 | −155.9 (4) | N2—C27—C28—C29 | 153.0 (4) |
| O3—C9—C10—C11 | −157.1 (5) | O6—C28—C29—C30 | 155.8 (5) |
| C8—C9—C10—C11 | 25.6 (7) | C27—C28—C29—C30 | −27.5 (7) |
| O3—C9—C10—S2 | 20.2 (6) | O6—C28—C29—S4 | −18.3 (6) |
| C8—C9—C10—S2 | −157.1 (3) | C27—C28—C29—S4 | 158.4 (3) |
| C9—C10—C11—C12 | 177.3 (4) | C28—C29—C30—C31 | −175.4 (5) |
| S2—C10—C11—C12 | −0.1 (5) | S4—C29—C30—C31 | −0.9 (6) |
| C10—C11—C12—C13 | 1.1 (6) | C29—C30—C31—C32 | 0.4 (8) |
| C11—C12—C13—S2 | −1.7 (6) | C30—C31—C32—S4 | 0.4 (8) |
| C19—C14—C15—C16 | 2.0 (7) | C38—C33—C34—C35 | −1.1 (8) |
| S1—C14—C15—C16 | 178.4 (4) | S3—C33—C34—C35 | −176.6 (4) |
| C14—C15—C16—C17 | −1.8 (8) | C33—C34—C35—C36 | 1.2 (9) |
| C15—C16—C17—C18 | 0.9 (8) | C34—C35—C36—C37 | −0.8 (9) |
| C16—C17—C18—C19 | −0.1 (8) | C35—C36—C37—C38 | 0.3 (9) |
| C15—C14—C19—C18 | −1.2 (7) | C36—C37—C38—C33 | −0.2 (8) |
| S1—C14—C19—C18 | −177.7 (4) | C34—C33—C38—C37 | 0.5 (7) |
| C17—C18—C19—C14 | 0.3 (7) | S3—C33—C38—C37 | 176.2 (4) |
| C7—C8—N1—C1 | 0.3 (5) | C21—C20—N2—C27 | −179.5 (5) |
| C9—C8—N1—C1 | −166.9 (4) | C25—C20—N2—C27 | −2.3 (5) |
| C7—C8—N1—S1 | −168.3 (3) | C21—C20—N2—S3 | 13.4 (7) |
| C9—C8—N1—S1 | 24.5 (6) | C25—C20—N2—S3 | −169.5 (3) |
| C2—C1—N1—C8 | −179.8 (5) | C26—C27—N2—C20 | 0.5 (5) |
| C6—C1—N1—C8 | 0.6 (5) | C28—C27—N2—C20 | 168.5 (4) |
| C2—C1—N1—S1 | −11.4 (7) | C26—C27—N2—S3 | 167.8 (3) |
| C6—C1—N1—S1 | 169.0 (3) | C28—C27—N2—S3 | −24.2 (6) |
| C8—N1—S1—O2 | 27.6 (4) | C20—N2—S3—O4 | 9.0 (4) |
| C1—N1—S1—O2 | −138.9 (4) | C27—N2—S3—O4 | −156.0 (4) |
| C8—N1—S1—O1 | 156.7 (4) | C20—N2—S3—O5 | 137.7 (4) |
| C1—N1—S1—O1 | −9.8 (4) | C27—N2—S3—O5 | −27.2 (4) |
| C8—N1—S1—C14 | −89.5 (4) | C20—N2—S3—C33 | −105.2 (4) |
| C1—N1—S1—C14 | 104.0 (4) | C27—N2—S3—C33 | 89.9 (4) |
| C15—C14—S1—O2 | 7.8 (4) | C34—C33—S3—O4 | 119.1 (4) |
| C19—C14—S1—O2 | −175.7 (3) | C38—C33—S3—O4 | −56.5 (4) |
| C15—C14—S1—O1 | −125.0 (4) | C34—C33—S3—O5 | −13.0 (5) |
| C19—C14—S1—O1 | 51.6 (4) | C38—C33—S3—O5 | 171.3 (4) |
| C15—C14—S1—N1 | 123.1 (4) | C34—C33—S3—N2 | −128.4 (4) |
| C19—C14—S1—N1 | −60.4 (4) | C38—C33—S3—N2 | 56.0 (4) |
| C12—C13—S2—C10 | 1.4 (4) | C31—C32—S4—C29 | −0.8 (5) |
| C11—C10—S2—C13 | −0.7 (4) | C30—C29—S4—C32 | 1.0 (4) |
| C9—C10—S2—C13 | −178.5 (4) | C28—C29—S4—C32 | 176.1 (4) |
Hydrogen-bond geometry (Å, °)
| Cg is the centroid of the C20–C25 ring. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2—H2···O1 | 0.93 | 2.33 | 2.879 (6) | 117 |
| C15—H15···O2 | 0.93 | 2.56 | 2.932 (6) | 104 |
| C21—H21···O4 | 0.93 | 2.33 | 2.878 (7) | 117 |
| C34—H34···O5 | 0.93 | 2.58 | 2.950 (7) | 104 |
| C17—H17···Cg8i | 0.93 | 2.88 | 3.693 (6) | 147 |
Symmetry codes: (i) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BT5452).
References
- Bruker (2004). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Chakkaravarthi, G., Dhayalan, V., Mohanakrishnan, A. K. & Manivannan, V. (2007). Acta Cryst. E63, o3673.
- Gunasekaran, B., Sureshbabu, R., Mohanakrishnan, A. K., Chakkaravarthi, G. & Manivannan, V. (2009). Acta Cryst. E65, o1856. [DOI] [PMC free article] [PubMed]
- Ma, C., Liu, X., Li, X., Flippen-Anderson, J., Yu, S. & Cook, J. M. (2001). J. Org. Chem. 66, 4525–4542. [DOI] [PubMed]
- Rajeswaran, W. G., Labroo, R. B., Cohen, L. A. & King, M. M. (1999). J. Org. Chem. 64, 1369–1371.
- Saravanan, B., Dhayalan, V., Mohanakrishnan, A. K., Chakkaravarthi, G. & Manivannan, V. (2010). Acta Cryst. E66, o1509. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Zhao, S., Liao, X. & Cook, J. M. (2002). Org. Lett. 4, 687–690. [DOI] [PubMed]
- Zhou, H., Liao, X., Yin, W., Ma, J. & Cook, J. M. (2006). J. Org. Chem. 71, 251–259. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811005666/bt5452sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811005666/bt5452Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



