Abstract
In the title compound, [Cu(C14H17N5O3)2(H2O)2](C14H9O5)2, the Cu2+ atom, located on an inversion centre, exhibits a distorted octahedral geometry, coordinated by four O atoms from two pipemidic acid ligands in equatorial positions and two water molecules in axial positions. The pipemidic acid ligand acts a bidentate ligand and the single deprotonated 4,4′-oxydibenzoic acid acts as an anion. Classical N—H⋯O and O—H⋯O hydrogen bonds are present in the crystal structure.
Related literature
For general background to the use of quinolones in the treatment of infections, see: Mizuki et al. (1996 ▶).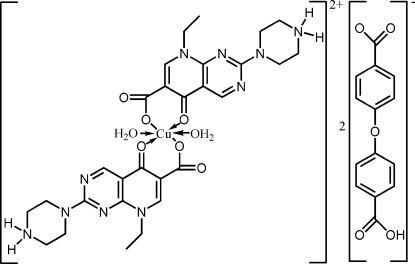
Experimental
Crystal data
[Cu(C14H17N5O3)2(H2O)2](C14H9O5)2
M r = 1220.66
Triclinic,
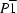
a = 8.611 (8) Å
b = 12.555 (12) Å
c = 13.436 (12) Å
α = 76.222 (10)°
β = 73.299 (10)°
γ = 81.015 (10)°
V = 1345 (2) Å3
Z = 1
Mo Kα radiation
μ = 0.49 mm−1
T = 295 K
0.47 × 0.41 × 0.33 mm
Data collection
Bruker SMART CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.801, T max = 0.854
11977 measured reflections
5790 independent reflections
4801 reflections with I > 2σ(I)
R int = 0.086
Refinement
R[F 2 > 2σ(F 2)] = 0.049
wR(F 2) = 0.140
S = 1.04
5790 reflections
397 parameters
3 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.56 e Å−3
Δρmin = −1.01 e Å−3
Data collection: SMART (Bruker, 2001 ▶); cell refinement: SAINT (Bruker, 2001 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811006672/rk2260sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811006672/rk2260Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| OW1—HW1A⋯O4i | 0.85 (1) | 2.08 (1) | 2.921 (3) | 169 (2) |
| OW1—HW1B⋯O7ii | 0.85 (1) | 2.08 (3) | 2.892 (3) | 161 (2) |
| O6—H6A⋯O5iii | 0.81 (3) | 1.81 (3) | 2.582 (3) | 160 (3) |
| N1—H1A⋯O1ii | 0.90 | 1.91 | 2.783 (3) | 162 |
| N1—H1B⋯O4iv | 0.90 | 1.73 | 2.604 (3) | 164 |
Symmetry codes: (i) 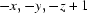 ; (ii)
; (ii) 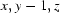 ; (iii)
; (iii) 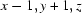 ; (iv)
; (iv) 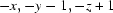 .
.
Acknowledgments
This work was supported by the Science and Technology Foundation of Southwest University (SWUB2007035).
supplementary crystallographic information
Comment
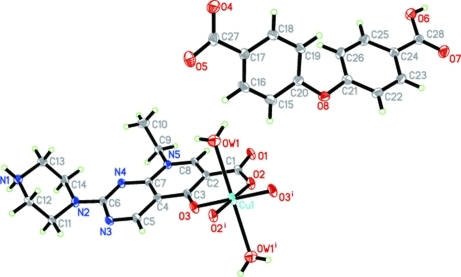
Pipemidic acid (Hppa, C14H17N5O3, 8-ethyl-5,8-dihydro-5- oxo-2-(1-piperazinyl)-pyrido(2,3-d)-pyrimidine-6-carboxylic acid) is member of a class of quinolones used to treat infections (Mizuki et al., 1996). H2oba (4,4'-oxybisbenzoic acid) acts a anion in this complex. The metal complexes of the Hppa and H2oba have not been reported till; the title copper(II) complex, I, is presented here (Fig. 1).
The Cu atom exhibits an approximate square environment with atoms O2, O3, O2i, O3i (see Fig. 1 for symmetry code) of two Hppa ligands (two O, O-bidentate). The Cu—O bond distances arising from the two carbonyl oxygen atoms O3 are longer (1.9605 (19)Å) than those arising from the carboxylate oxygen atoms O2 (1.932 (2)Å). The bond angles O2—Cu1—O3i and O2—Cu1—O3 open up slightly from 88.51 (9)° to 91.49 (9)°. The CuII atom at each short edge of the rectangle are bridged by an water molecule, which also interacts weakly (Cu1···OW1 = 2.642 (10)Å) with the central metal, resulting in a distortion octahedral geometry. Classical N—H···O and O—H···O hydrogen bonds are present in the crystal structure (Table 1).
Experimental
A mixture of CuI (0.095 g, 0.5 mmol), Hppa (0.089 g, 0.25 mmol), H2oba (0.0645 g, 0.25 mmol) and water (8 ml) was stirred for 30 min in air. The mixture was then transferred to a 18 ml teflon-lined hydrothermal bomb. The bomb was kept at 393 K for 120 h under autogenous pressure. Upon cooling, blue block of I were obtained from the reaction mixture.
Refinement
The H atoms bonded to C atoms were positioned geometrically and refined using a riding model approximation [aromatic C—H = 0.93Å, aliphatic C—H = 0.96Å–0.97Å], with Uiso(H) = 1.2–1.5Ueq(C). The H atoms based on N atoms were located in a difference Fourier map and were refined with a distance restraint of N—H = 0.90Å and with Uiso(H) = 1.2Ueq(N). The H atoms bonded to O atoms were located in a difference Fourier maps and refined with O—H distance restraints of 0.81 (3)Å and with Uiso(H) = 1.3Ueq(O). The H atoms bonded to OW atoms were located in a difference Fourier maps and refined with OW—H = 0.845Å–0.855Å and Uiso(H) = 1–1.2Ueq(OW).
Figures
Fig. 1.
Asymmetric unit of title compound with the atom numbering scheme. Displacement ellipsoids are drawn at 50% probability level. H atoms are presented as a small spheres of arbitrary radius. Symmetry code: (i) -x, -y, -z.
Crystal data
| [Cu(C14H17N5O3)2(H2O)2](C14H9O5)2 | Z = 1 |
| Mr = 1220.66 | F(000) = 635 |
| Triclinic, P1 | Dx = 1.507 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.611 (8) Å | Cell parameters from 11977 reflections |
| b = 12.555 (12) Å | θ = 2.5–27.0° |
| c = 13.436 (12) Å | µ = 0.49 mm−1 |
| α = 76.222 (10)° | T = 295 K |
| β = 73.299 (10)° | Block, blue |
| γ = 81.015 (10)° | 0.47 × 0.41 × 0.33 mm |
| V = 1345 (2) Å3 |
Data collection
| Bruker SMART CCD diffractometer | 5790 independent reflections |
| Radiation source: fine-focus sealed tube | 4801 reflections with I > 2σ(I) |
| graphite | Rint = 0.086 |
| φ and ω scans | θmax = 27.0°, θmin = 2.5° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −10→10 |
| Tmin = 0.801, Tmax = 0.854 | k = −15→16 |
| 11977 measured reflections | l = −17→17 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.049 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.140 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.080P)2] where P = (Fo2 + 2Fc2)/3 |
| 5790 reflections | (Δ/σ)max < 0.001 |
| 397 parameters | Δρmax = 0.56 e Å−3 |
| 3 restraints | Δρmin = −1.01 e Å−3 |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cu1 | 0.0000 | 0.0000 | 0.0000 | 0.03389 (14) | |
| O1 | −0.47101 (18) | 0.10445 (10) | 0.11352 (13) | 0.0351 (3) | |
| OW1 | −0.0750 (3) | −0.05617 (16) | 0.20934 (15) | 0.0526 (5) | |
| HW1A | −0.118 (3) | −0.0156 (16) | 0.2544 (16) | 0.049 (8)* | |
| HW1B | −0.129 (3) | −0.1115 (15) | 0.229 (2) | 0.064 (10)* | |
| O2 | −0.21239 (17) | 0.08163 (10) | 0.02336 (13) | 0.0348 (3) | |
| O3 | −0.09237 (18) | −0.13240 (11) | −0.00203 (13) | 0.0379 (4) | |
| O4 | 0.2629 (2) | −0.06638 (13) | 0.62834 (14) | 0.0541 (5) | |
| O5 | 0.3377 (2) | −0.15031 (13) | 0.49121 (16) | 0.0545 (5) | |
| O6 | −0.4437 (2) | 0.68639 (12) | 0.46026 (13) | 0.0425 (4) | |
| H6A | −0.494 (4) | 0.745 (2) | 0.470 (2) | 0.056 (8)* | |
| O7 | −0.3132 (2) | 0.78539 (12) | 0.30621 (13) | 0.0491 (4) | |
| O8 | 0.05881 (19) | 0.31270 (11) | 0.26786 (11) | 0.0360 (4) | |
| N1 | −0.3807 (2) | −0.77991 (12) | 0.23764 (13) | 0.0296 (4) | |
| H1A | −0.4293 | −0.8192 | 0.2091 | 0.036* | |
| H1B | −0.3499 | −0.8254 | 0.2928 | 0.036* | |
| N2 | −0.4064 (2) | −0.57091 (12) | 0.10300 (14) | 0.0302 (4) | |
| N3 | −0.2207 (2) | −0.44274 (12) | 0.01642 (13) | 0.0286 (4) | |
| N4 | −0.48747 (19) | −0.39169 (12) | 0.12450 (13) | 0.0245 (3) | |
| N5 | −0.56161 (19) | −0.21098 (12) | 0.14742 (13) | 0.0244 (3) | |
| C1 | −0.3500 (2) | 0.04524 (15) | 0.07391 (16) | 0.0277 (4) | |
| C2 | −0.3694 (2) | −0.07372 (14) | 0.08278 (15) | 0.0248 (4) | |
| C3 | −0.2405 (2) | −0.15063 (14) | 0.04021 (15) | 0.0252 (4) | |
| C4 | −0.2879 (2) | −0.25929 (14) | 0.05000 (14) | 0.0236 (4) | |
| C5 | −0.1829 (2) | −0.34213 (15) | 0.00345 (16) | 0.0284 (4) | |
| H5A | −0.0805 | −0.3245 | −0.0391 | 0.034* | |
| C6 | −0.3712 (2) | −0.46483 (14) | 0.08216 (15) | 0.0240 (4) | |
| C7 | −0.4444 (2) | −0.28933 (14) | 0.10703 (14) | 0.0224 (4) | |
| C8 | −0.5211 (2) | −0.10795 (14) | 0.13354 (15) | 0.0255 (4) | |
| H8A | −0.6022 | −0.0562 | 0.1604 | 0.031* | |
| C9 | −0.7306 (2) | −0.23875 (16) | 0.20318 (16) | 0.0302 (4) | |
| H9A | −0.7622 | −0.2865 | 0.1668 | 0.036* | |
| H9B | −0.8038 | −0.1717 | 0.1998 | 0.036* | |
| C10 | −0.7491 (3) | −0.2952 (2) | 0.31814 (18) | 0.0485 (6) | |
| H10A | −0.8601 | −0.3112 | 0.3504 | 0.073* | |
| H10B | −0.7202 | −0.2477 | 0.3550 | 0.073* | |
| H10C | −0.6789 | −0.3626 | 0.3220 | 0.073* | |
| C11 | −0.2860 (3) | −0.65883 (14) | 0.06486 (16) | 0.0295 (4) | |
| H11A | −0.1919 | −0.6267 | 0.0138 | 0.035* | |
| H11B | −0.3332 | −0.6995 | 0.0296 | 0.035* | |
| C12 | −0.2338 (2) | −0.73607 (15) | 0.15641 (16) | 0.0301 (4) | |
| H12A | −0.1598 | −0.7964 | 0.1314 | 0.036* | |
| H12B | −0.1773 | −0.6972 | 0.1877 | 0.036* | |
| C13 | −0.4992 (3) | −0.68931 (16) | 0.27633 (16) | 0.0324 (4) | |
| H13A | −0.4496 | −0.6494 | 0.3109 | 0.039* | |
| H13B | −0.5941 | −0.7199 | 0.3278 | 0.039* | |
| C14 | −0.5504 (2) | −0.61120 (15) | 0.18377 (18) | 0.0332 (5) | |
| H14A | −0.6098 | −0.6492 | 0.1536 | 0.040* | |
| H14B | −0.6218 | −0.5496 | 0.2083 | 0.040* | |
| C15 | 0.1449 (3) | 0.12351 (18) | 0.30272 (19) | 0.0443 (6) | |
| H15A | 0.1362 | 0.1210 | 0.2359 | 0.053* | |
| C16 | 0.2015 (3) | 0.03110 (18) | 0.36527 (19) | 0.0476 (6) | |
| H16A | 0.2319 | −0.0340 | 0.3400 | 0.057* | |
| C17 | 0.2144 (3) | 0.03297 (15) | 0.46562 (16) | 0.0312 (4) | |
| C18 | 0.1694 (3) | 0.13108 (16) | 0.50118 (17) | 0.0350 (5) | |
| H18A | 0.1772 | 0.1336 | 0.5682 | 0.042* | |
| C19 | 0.1130 (3) | 0.22545 (16) | 0.43918 (16) | 0.0328 (5) | |
| H19A | 0.0838 | 0.2909 | 0.4638 | 0.039* | |
| C20 | 0.1008 (2) | 0.22064 (15) | 0.34007 (16) | 0.0283 (4) | |
| C21 | −0.0382 (2) | 0.40359 (15) | 0.30085 (16) | 0.0284 (4) | |
| C22 | −0.0043 (3) | 0.50373 (17) | 0.23318 (17) | 0.0351 (5) | |
| H22A | 0.0823 | 0.5074 | 0.1726 | 0.042* | |
| C23 | −0.1005 (3) | 0.59877 (16) | 0.25639 (17) | 0.0333 (5) | |
| H23A | −0.0785 | 0.6662 | 0.2107 | 0.040* | |
| C24 | −0.2295 (2) | 0.59424 (15) | 0.34728 (16) | 0.0288 (4) | |
| C25 | −0.2633 (3) | 0.49235 (17) | 0.41362 (17) | 0.0365 (5) | |
| H25A | −0.3501 | 0.4883 | 0.4741 | 0.044* | |
| C26 | −0.1687 (3) | 0.39667 (16) | 0.39035 (18) | 0.0354 (5) | |
| H26A | −0.1928 | 0.3287 | 0.4344 | 0.043* | |
| C27 | 0.2781 (3) | −0.06920 (16) | 0.53302 (19) | 0.0375 (5) | |
| C28 | −0.3324 (3) | 0.69794 (16) | 0.36871 (17) | 0.0318 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cu1 | 0.0228 (2) | 0.02319 (19) | 0.0572 (3) | −0.00498 (13) | −0.00532 (17) | −0.01570 (16) |
| O1 | 0.0281 (8) | 0.0259 (7) | 0.0542 (10) | 0.0003 (5) | −0.0070 (7) | −0.0204 (6) |
| OW1 | 0.0558 (12) | 0.0515 (11) | 0.0439 (10) | −0.0037 (9) | −0.0031 (9) | −0.0101 (8) |
| O2 | 0.0277 (8) | 0.0232 (7) | 0.0524 (9) | −0.0036 (5) | −0.0055 (7) | −0.0109 (6) |
| O3 | 0.0254 (8) | 0.0276 (7) | 0.0602 (10) | −0.0082 (6) | 0.0024 (7) | −0.0212 (6) |
| O4 | 0.0749 (14) | 0.0403 (9) | 0.0428 (10) | 0.0150 (8) | −0.0237 (9) | −0.0033 (7) |
| O5 | 0.0603 (12) | 0.0321 (8) | 0.0657 (12) | 0.0172 (8) | −0.0160 (9) | −0.0140 (8) |
| O6 | 0.0433 (10) | 0.0307 (8) | 0.0410 (9) | 0.0085 (7) | 0.0017 (7) | −0.0068 (6) |
| O7 | 0.0612 (12) | 0.0294 (8) | 0.0451 (10) | 0.0032 (7) | −0.0046 (8) | −0.0017 (7) |
| O8 | 0.0431 (9) | 0.0331 (7) | 0.0270 (7) | 0.0107 (6) | −0.0080 (6) | −0.0073 (6) |
| N1 | 0.0354 (10) | 0.0264 (8) | 0.0285 (9) | −0.0012 (7) | −0.0120 (7) | −0.0049 (6) |
| N2 | 0.0260 (9) | 0.0195 (7) | 0.0405 (10) | −0.0045 (6) | −0.0013 (7) | −0.0052 (7) |
| N3 | 0.0262 (9) | 0.0249 (8) | 0.0325 (9) | −0.0048 (6) | 0.0014 (7) | −0.0111 (6) |
| N4 | 0.0223 (8) | 0.0214 (7) | 0.0305 (8) | −0.0047 (6) | −0.0057 (7) | −0.0064 (6) |
| N5 | 0.0206 (8) | 0.0229 (7) | 0.0284 (8) | −0.0028 (6) | −0.0022 (6) | −0.0075 (6) |
| C1 | 0.0299 (11) | 0.0244 (9) | 0.0333 (10) | −0.0025 (7) | −0.0125 (8) | −0.0094 (7) |
| C2 | 0.0261 (10) | 0.0206 (8) | 0.0286 (10) | −0.0028 (7) | −0.0065 (8) | −0.0073 (7) |
| C3 | 0.0222 (10) | 0.0242 (9) | 0.0305 (10) | −0.0056 (7) | −0.0038 (8) | −0.0095 (7) |
| C4 | 0.0217 (9) | 0.0233 (8) | 0.0268 (10) | −0.0038 (7) | −0.0046 (7) | −0.0081 (7) |
| C5 | 0.0231 (10) | 0.0275 (9) | 0.0336 (10) | −0.0059 (7) | −0.0013 (8) | −0.0099 (8) |
| C6 | 0.0258 (10) | 0.0223 (8) | 0.0254 (9) | −0.0024 (7) | −0.0084 (8) | −0.0054 (7) |
| C7 | 0.0226 (10) | 0.0224 (8) | 0.0236 (9) | −0.0028 (7) | −0.0071 (7) | −0.0057 (7) |
| C8 | 0.0246 (10) | 0.0232 (8) | 0.0288 (10) | −0.0004 (7) | −0.0043 (8) | −0.0103 (7) |
| C9 | 0.0194 (10) | 0.0322 (10) | 0.0376 (11) | −0.0053 (7) | 0.0010 (8) | −0.0128 (8) |
| C10 | 0.0492 (15) | 0.0577 (14) | 0.0351 (13) | −0.0167 (11) | 0.0006 (11) | −0.0099 (11) |
| C11 | 0.0342 (11) | 0.0226 (9) | 0.0317 (10) | −0.0034 (8) | −0.0045 (8) | −0.0100 (7) |
| C12 | 0.0269 (11) | 0.0298 (9) | 0.0366 (11) | 0.0013 (7) | −0.0079 (9) | −0.0156 (8) |
| C13 | 0.0292 (11) | 0.0329 (10) | 0.0332 (11) | −0.0052 (8) | −0.0018 (9) | −0.0099 (8) |
| C14 | 0.0221 (10) | 0.0242 (9) | 0.0490 (13) | −0.0046 (7) | −0.0026 (9) | −0.0060 (8) |
| C15 | 0.0632 (17) | 0.0390 (12) | 0.0325 (12) | 0.0083 (11) | −0.0155 (11) | −0.0156 (9) |
| C16 | 0.0695 (18) | 0.0336 (11) | 0.0399 (13) | 0.0162 (11) | −0.0149 (12) | −0.0205 (10) |
| C17 | 0.0306 (11) | 0.0259 (9) | 0.0324 (11) | 0.0042 (8) | −0.0044 (8) | −0.0062 (8) |
| C18 | 0.0450 (13) | 0.0317 (10) | 0.0303 (11) | 0.0020 (9) | −0.0133 (9) | −0.0093 (8) |
| C19 | 0.0404 (12) | 0.0262 (9) | 0.0315 (11) | 0.0047 (8) | −0.0084 (9) | −0.0116 (8) |
| C20 | 0.0242 (10) | 0.0294 (9) | 0.0289 (10) | 0.0016 (7) | −0.0052 (8) | −0.0061 (7) |
| C21 | 0.0275 (11) | 0.0298 (9) | 0.0292 (10) | 0.0026 (8) | −0.0108 (8) | −0.0077 (8) |
| C22 | 0.0331 (12) | 0.0378 (11) | 0.0262 (10) | 0.0014 (9) | −0.0009 (9) | −0.0023 (8) |
| C23 | 0.0351 (12) | 0.0288 (10) | 0.0304 (11) | −0.0028 (8) | −0.0052 (9) | 0.0004 (8) |
| C24 | 0.0291 (11) | 0.0296 (9) | 0.0286 (10) | −0.0003 (8) | −0.0092 (8) | −0.0072 (8) |
| C25 | 0.0306 (12) | 0.0339 (10) | 0.0348 (12) | 0.0008 (8) | 0.0018 (9) | −0.0027 (9) |
| C26 | 0.0335 (12) | 0.0270 (10) | 0.0370 (12) | −0.0007 (8) | −0.0024 (9) | 0.0004 (8) |
| C27 | 0.0362 (12) | 0.0276 (10) | 0.0445 (13) | 0.0034 (8) | −0.0104 (10) | −0.0036 (9) |
| C28 | 0.0333 (12) | 0.0303 (10) | 0.0327 (11) | 0.0004 (8) | −0.0106 (9) | −0.0081 (8) |
Geometric parameters (Å, °)
| Cu1—O2 | 1.932 (2) | C9—C10 | 1.512 (3) |
| Cu1—O2i | 1.932 (2) | C9—H9A | 0.9700 |
| Cu1—O3i | 1.9605 (19) | C9—H9B | 0.9700 |
| Cu1—O3 | 1.9605 (19) | C10—H10A | 0.9600 |
| O1—C1 | 1.245 (3) | C10—H10B | 0.9600 |
| OW1—HW1A | 0.850 (10) | C10—H10C | 0.9600 |
| OW1—HW1B | 0.845 (10) | C11—C12 | 1.506 (3) |
| O2—C1 | 1.278 (3) | C11—H11A | 0.9700 |
| O3—C3 | 1.271 (2) | C11—H11B | 0.9700 |
| O4—C27 | 1.258 (3) | C12—H12A | 0.9700 |
| O5—C27 | 1.249 (3) | C12—H12B | 0.9700 |
| O6—C28 | 1.317 (3) | C13—C14 | 1.517 (3) |
| O6—H6A | 0.81 (3) | C13—H13A | 0.9700 |
| O7—C28 | 1.215 (3) | C13—H13B | 0.9700 |
| O8—C21 | 1.388 (2) | C14—H14A | 0.9700 |
| O8—C20 | 1.395 (2) | C14—H14B | 0.9700 |
| N1—C13 | 1.490 (3) | C15—C16 | 1.373 (3) |
| N1—C12 | 1.494 (3) | C15—C20 | 1.386 (3) |
| N1—H1A | 0.9000 | C15—H15A | 0.9300 |
| N1—H1B | 0.9000 | C16—C17 | 1.390 (3) |
| N2—C6 | 1.358 (3) | C16—H16A | 0.9300 |
| N2—C14 | 1.461 (3) | C17—C18 | 1.386 (3) |
| N2—C11 | 1.470 (3) | C17—C27 | 1.510 (3) |
| N3—C5 | 1.312 (3) | C18—C19 | 1.384 (3) |
| N3—C6 | 1.370 (3) | C18—H18A | 0.9300 |
| N4—C7 | 1.341 (3) | C19—C20 | 1.381 (3) |
| N4—C6 | 1.343 (2) | C19—H19A | 0.9300 |
| N5—C8 | 1.348 (3) | C21—C22 | 1.382 (3) |
| N5—C7 | 1.382 (2) | C21—C26 | 1.386 (3) |
| N5—C9 | 1.486 (3) | C22—C23 | 1.388 (3) |
| C1—C2 | 1.501 (3) | C22—H22A | 0.9300 |
| C2—C8 | 1.371 (3) | C23—C24 | 1.391 (3) |
| C2—C3 | 1.434 (3) | C23—H23A | 0.9300 |
| C3—C4 | 1.450 (3) | C24—C25 | 1.393 (3) |
| C4—C7 | 1.405 (3) | C24—C28 | 1.495 (3) |
| C4—C5 | 1.408 (3) | C25—C26 | 1.390 (3) |
| C5—H5A | 0.9300 | C25—H25A | 0.9300 |
| C8—H8A | 0.9300 | C26—H26A | 0.9300 |
| O2—Cu1—O2i | 180.00 (9) | N2—C11—H11B | 109.6 |
| O2—Cu1—O3i | 88.51 (9) | C12—C11—H11B | 109.6 |
| O2i—Cu1—O3i | 91.49 (9) | H11A—C11—H11B | 108.1 |
| O2—Cu1—O3 | 91.49 (9) | N1—C12—C11 | 109.21 (17) |
| O2i—Cu1—O3 | 88.51 (9) | N1—C12—H12A | 109.8 |
| O3i—Cu1—O3 | 180.00 (9) | C11—C12—H12A | 109.8 |
| HW1A—OW1—HW1B | 106.0 (15) | N1—C12—H12B | 109.8 |
| C1—O2—Cu1 | 128.03 (13) | C11—C12—H12B | 109.8 |
| C3—O3—Cu1 | 124.09 (12) | H12A—C12—H12B | 108.3 |
| C28—O6—H6A | 111 (2) | N1—C13—C14 | 109.85 (18) |
| C21—O8—C20 | 121.82 (16) | N1—C13—H13A | 109.7 |
| C13—N1—C12 | 111.43 (15) | C14—C13—H13A | 109.7 |
| C13—N1—H1A | 109.3 | N1—C13—H13B | 109.7 |
| C12—N1—H1A | 109.3 | C14—C13—H13B | 109.7 |
| C13—N1—H1B | 109.3 | H13A—C13—H13B | 108.2 |
| C12—N1—H1B | 109.3 | N2—C14—C13 | 109.62 (17) |
| H1A—N1—H1B | 108.0 | N2—C14—H14A | 109.7 |
| C6—N2—C14 | 122.40 (16) | C13—C14—H14A | 109.7 |
| C6—N2—C11 | 122.31 (17) | N2—C14—H14B | 109.7 |
| C14—N2—C11 | 113.87 (15) | C13—C14—H14B | 109.7 |
| C5—N3—C6 | 115.40 (16) | H14A—C14—H14B | 108.2 |
| C7—N4—C6 | 115.62 (17) | C16—C15—C20 | 119.3 (2) |
| C8—N5—C7 | 118.91 (17) | C16—C15—H15A | 120.3 |
| C8—N5—C9 | 120.33 (15) | C20—C15—H15A | 120.3 |
| C7—N5—C9 | 120.74 (15) | C15—C16—C17 | 121.2 (2) |
| O1—C1—O2 | 123.21 (17) | C15—C16—H16A | 119.4 |
| O1—C1—C2 | 118.03 (19) | C17—C16—H16A | 119.4 |
| O2—C1—C2 | 118.72 (17) | C18—C17—C16 | 118.30 (18) |
| C8—C2—C3 | 119.53 (17) | C18—C17—C27 | 121.2 (2) |
| C8—C2—C1 | 116.70 (16) | C16—C17—C27 | 120.53 (19) |
| C3—C2—C1 | 123.76 (18) | C19—C18—C17 | 121.5 (2) |
| O3—C3—C2 | 126.19 (17) | C19—C18—H18A | 119.3 |
| O3—C3—C4 | 118.81 (16) | C17—C18—H18A | 119.3 |
| C2—C3—C4 | 114.99 (17) | C20—C19—C18 | 118.79 (18) |
| C7—C4—C5 | 114.89 (17) | C20—C19—H19A | 120.6 |
| C7—C4—C3 | 121.86 (16) | C18—C19—H19A | 120.6 |
| C5—C4—C3 | 123.25 (18) | C19—C20—C15 | 120.88 (18) |
| N3—C5—C4 | 124.03 (19) | C19—C20—O8 | 123.37 (18) |
| N3—C5—H5A | 118.0 | C15—C20—O8 | 115.51 (19) |
| C4—C5—H5A | 118.0 | C22—C21—C26 | 120.83 (18) |
| N4—C6—N2 | 117.63 (18) | C22—C21—O8 | 115.21 (19) |
| N4—C6—N3 | 126.45 (16) | C26—C21—O8 | 123.78 (18) |
| N2—C6—N3 | 115.89 (16) | C21—C22—C23 | 119.5 (2) |
| N4—C7—N5 | 117.44 (18) | C21—C22—H22A | 120.3 |
| N4—C7—C4 | 123.08 (16) | C23—C22—H22A | 120.3 |
| N5—C7—C4 | 119.48 (16) | C22—C23—C24 | 120.70 (19) |
| N5—C8—C2 | 124.93 (16) | C22—C23—H23A | 119.6 |
| N5—C8—H8A | 117.5 | C24—C23—H23A | 119.6 |
| C2—C8—H8A | 117.5 | C25—C24—C23 | 118.96 (18) |
| N5—C9—C10 | 112.82 (17) | C25—C24—C28 | 121.8 (2) |
| N5—C9—H9A | 109.0 | C23—C24—C28 | 119.23 (18) |
| C10—C9—H9A | 109.0 | C26—C25—C24 | 120.7 (2) |
| N5—C9—H9B | 109.0 | C26—C25—H25A | 119.7 |
| C10—C9—H9B | 109.0 | C24—C25—H25A | 119.7 |
| H9A—C9—H9B | 107.8 | C21—C26—C25 | 119.30 (19) |
| C9—C10—H10A | 109.5 | C21—C26—H26A | 120.4 |
| C9—C10—H10B | 109.5 | C25—C26—H26A | 120.4 |
| H10A—C10—H10B | 109.5 | O5—C27—O4 | 125.2 (2) |
| C9—C10—H10C | 109.5 | O5—C27—C17 | 118.0 (2) |
| H10A—C10—H10C | 109.5 | O4—C27—C17 | 116.79 (19) |
| H10B—C10—H10C | 109.5 | O7—C28—O6 | 123.32 (19) |
| N2—C11—C12 | 110.25 (17) | O7—C28—C24 | 122.1 (2) |
| N2—C11—H11A | 109.6 | O6—C28—C24 | 114.58 (17) |
| C12—C11—H11A | 109.6 | ||
| O3i—Cu1—O2—C1 | −148.18 (18) | C1—C2—C8—N5 | 178.93 (17) |
| O3—Cu1—O2—C1 | 31.82 (18) | C8—N5—C9—C10 | −99.3 (2) |
| O2—Cu1—O3—C3 | −22.22 (17) | C7—N5—C9—C10 | 82.1 (2) |
| O2i—Cu1—O3—C3 | 157.78 (17) | C6—N2—C11—C12 | 109.8 (2) |
| Cu1—O2—C1—O1 | 156.75 (15) | C14—N2—C11—C12 | −56.9 (2) |
| Cu1—O2—C1—C2 | −25.6 (3) | C13—N1—C12—C11 | −58.2 (2) |
| O1—C1—C2—C8 | 0.6 (3) | N2—C11—C12—N1 | 55.9 (2) |
| O2—C1—C2—C8 | −177.16 (17) | C12—N1—C13—C14 | 58.3 (2) |
| O1—C1—C2—C3 | 179.61 (18) | C6—N2—C14—C13 | −110.5 (2) |
| O2—C1—C2—C3 | 1.9 (3) | C11—N2—C14—C13 | 56.2 (2) |
| Cu1—O3—C3—C2 | 9.3 (3) | N1—C13—C14—N2 | −55.5 (2) |
| Cu1—O3—C3—C4 | −169.20 (13) | C20—C15—C16—C17 | 0.5 (4) |
| C8—C2—C3—O3 | −174.87 (19) | C15—C16—C17—C18 | −0.4 (4) |
| C1—C2—C3—O3 | 6.1 (3) | C15—C16—C17—C27 | −179.5 (2) |
| C8—C2—C3—C4 | 3.7 (3) | C16—C17—C18—C19 | 0.0 (4) |
| C1—C2—C3—C4 | −175.33 (17) | C27—C17—C18—C19 | 179.1 (2) |
| O3—C3—C4—C7 | 172.18 (17) | C17—C18—C19—C20 | 0.4 (3) |
| C2—C3—C4—C7 | −6.5 (3) | C18—C19—C20—C15 | −0.3 (3) |
| O3—C3—C4—C5 | −8.1 (3) | C18—C19—C20—O8 | −174.38 (19) |
| C2—C3—C4—C5 | 173.23 (18) | C16—C15—C20—C19 | −0.1 (4) |
| C6—N3—C5—C4 | −1.7 (3) | C16—C15—C20—O8 | 174.4 (2) |
| C7—C4—C5—N3 | −4.3 (3) | C21—O8—C20—C19 | −28.0 (3) |
| C3—C4—C5—N3 | 176.01 (19) | C21—O8—C20—C15 | 157.6 (2) |
| C7—N4—C6—N2 | 176.26 (17) | C20—O8—C21—C22 | 147.58 (19) |
| C7—N4—C6—N3 | −6.0 (3) | C20—O8—C21—C26 | −37.3 (3) |
| C14—N2—C6—N4 | −11.6 (3) | C26—C21—C22—C23 | 1.3 (3) |
| C11—N2—C6—N4 | −177.18 (17) | O8—C21—C22—C23 | 176.50 (19) |
| C14—N2—C6—N3 | 170.41 (17) | C21—C22—C23—C24 | 0.5 (3) |
| C11—N2—C6—N3 | 4.9 (3) | C22—C23—C24—C25 | −1.5 (3) |
| C5—N3—C6—N4 | 7.4 (3) | C22—C23—C24—C28 | −178.9 (2) |
| C5—N3—C6—N2 | −174.85 (18) | C23—C24—C25—C26 | 0.7 (3) |
| C6—N4—C7—N5 | 178.69 (15) | C28—C24—C25—C26 | 178.1 (2) |
| C6—N4—C7—C4 | −1.1 (3) | C22—C21—C26—C25 | −2.0 (3) |
| C8—N5—C7—N4 | 178.36 (17) | O8—C21—C26—C25 | −176.78 (19) |
| C9—N5—C7—N4 | −3.0 (2) | C24—C25—C26—C21 | 0.9 (3) |
| C8—N5—C7—C4 | −1.9 (3) | C18—C17—C27—O5 | −170.2 (2) |
| C9—N5—C7—C4 | 176.77 (16) | C16—C17—C27—O5 | 8.9 (3) |
| C5—C4—C7—N4 | 5.8 (3) | C18—C17—C27—O4 | 11.6 (3) |
| C3—C4—C7—N4 | −174.52 (17) | C16—C17—C27—O4 | −169.3 (2) |
| C5—C4—C7—N5 | −173.98 (16) | C25—C24—C28—O7 | −173.0 (2) |
| C3—C4—C7—N5 | 5.7 (3) | C23—C24—C28—O7 | 4.4 (3) |
| C7—N5—C8—C2 | −0.9 (3) | C25—C24—C28—O6 | 7.3 (3) |
| C9—N5—C8—C2 | −179.57 (18) | C23—C24—C28—O6 | −175.3 (2) |
| C3—C2—C8—N5 | −0.1 (3) |
Symmetry codes: (i) −x, −y, −z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| OW1—HW1A···O4ii | 0.85 (1) | 2.08 (1) | 2.921 (3) | 169 (2) |
| OW1—HW1B···O7iii | 0.85 (1) | 2.08 (3) | 2.892 (3) | 161 (2) |
| O6—H6A···O5iv | 0.81 (3) | 1.81 (3) | 2.582 (3) | 160 (3) |
| N1—H1A···O1iii | 0.90 | 1.91 | 2.783 (3) | 162 |
| N1—H1B···O4v | 0.90 | 1.73 | 2.604 (3) | 164 |
Symmetry codes: (ii) −x, −y, −z+1; (iii) x, y−1, z; (iv) x−1, y+1, z; (v) −x, −y−1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: RK2260).
References
- Bruker (2001). SMART and SAINT Bruker AXS Inc., Madison Wisconsion, USA.
- Mizuki, Y., Fujiwara, I. & Yamaguchi, T. (1996). J. Antimicrob. Chemother. Suppl. A, 37, 41–45. [DOI] [PubMed]
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811006672/rk2260sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811006672/rk2260Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report