Abstract
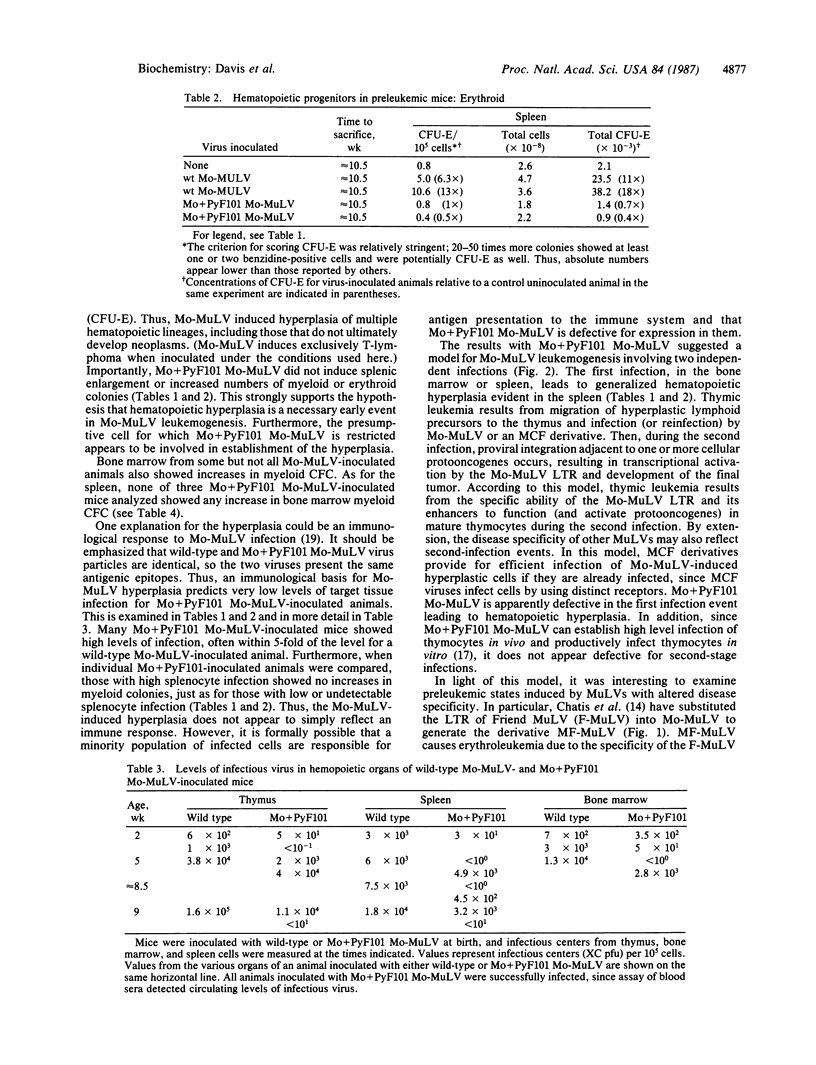
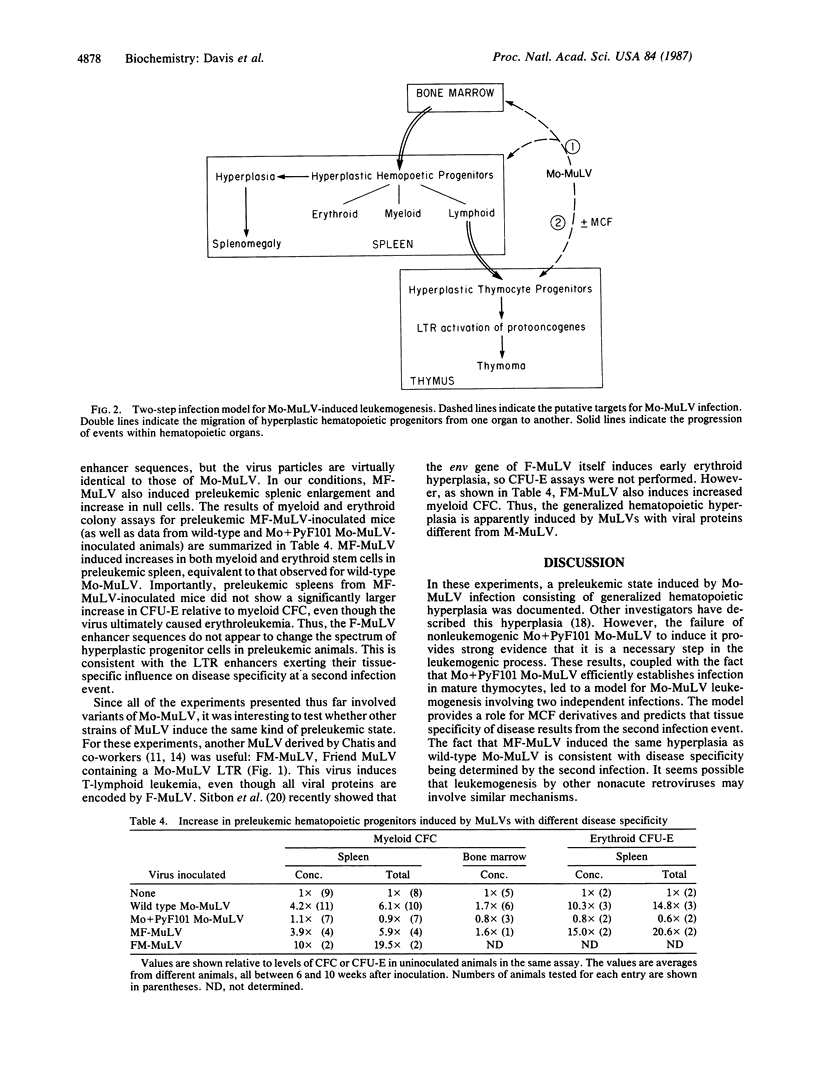
A preleukemic state in mice inoculated with Moloney murine leukemia virus (Mo-MuLV) was characterized. Six to 10 weeks after neonatal inoculation, animals developed mild splenomegaly and generalized hematopoietic hyperplasia. The hyperplasia was evident from myeloid and erythroid progenitor assays. A nonleukemogenic variant, Mo+PyF101 Mo-MuLV, did not induce the hyperplasia; this suggests that the hyperplasia is a necessary event in Mo-MuLV leukemogenesis. Another variant, MF-MuLV, which contains the long terminal repeat of Friend MuLV and causes erythroid leukemia instead of T-cell lymphoma, also induced the preleukemic hyperplasia. A model for Mo-MuLV leukemogenesis is presented in which two infection events are necessary: the first leads to generalized hematopoietic hyperplasia, and the second results in site-specific insertion and long terminal repeat activation of cellular protooncogenes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asjö B., Skoog L., Palminger I., Wiener F., Isaak D., Cerny J., Fenyö E. M. Influence of genotype and the organ of origin on the subtype of T-cell in Moloney lymphomas induced by transfer of preleukemic cells from athymic and thymus-bearing mice. Cancer Res. 1985 Mar;45(3):1040–1045. [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. Role for the 3' end of the genome in determining disease specificity of Friend and Moloney murine leukemia viruses. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4408–4411. doi: 10.1073/pnas.80.14.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Silver J. E., Frederickson T. N., Hopkins N., Hartley J. W. A 3' end fragment encompassing the transcriptional enhancers of nondefective Friend virus confers erythroleukemogenicity on Moloney leukemia virus. J Virol. 1984 Oct;52(1):248–254. doi: 10.1128/jvi.52.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers H. T., Selten G., Quint W., Zijlstra M., Maandag E. R., Boelens W., van Wezenbeek P., Melief C., Berns A. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1984 May;37(1):141–150. doi: 10.1016/0092-8674(84)90309-x. [DOI] [PubMed] [Google Scholar]
- Davis B. R., Chandy K. G., Brightman B. K., Gupta S., Fan H. Effects of nonleukemogenic and wild-type Moloney murine leukemia virus on lymphoid cells in vivo: identification of a preleukemic shift in thymocyte subpopulations. J Virol. 1986 Nov;60(2):423–430. doi: 10.1128/jvi.60.2.423-430.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B., Linney E., Fan H. Suppression of leukaemia virus pathogenicity by polyoma virus enhancers. Nature. 1985 Apr 11;314(6011):550–553. doi: 10.1038/314550a0. [DOI] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. Mapping the viral sequences conferring leukemogenicity and disease specificity in Moloney and amphotropic murine leukemia viruses. J Virol. 1984 Nov;52(2):448–456. doi: 10.1128/jvi.52.2.448-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger J. S., Shadduck R. K., Jaenisch R., Waheed A., Sakakeeny M. A. Effects of murine leukemia virus infection on long-term hematopoiesis in vitro emphasized by increased survival of bone marrow cultures derived from BALB/Mo mice. Cancer Res. 1981 Sep;41(9 Pt 1):3556–3565. [PubMed] [Google Scholar]
- Haran-Ghera N. Potential leukemic cells among bone marrow cells of young AKR/J mice. Proc Natl Acad Sci U S A. 1980 May;77(5):2923–2926. doi: 10.1073/pnas.77.5.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haran-Ghera N., Rubio N., Leef F., Goldstein G. Characteristics of preleukemia cells induced in mice. Cell Immunol. 1978 May;37(2):308–314. doi: 10.1016/0008-8749(78)90199-5. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Enjuanes L., Lee J. C., Keller J. The immune response to C-type viruses and its potential role in leukemogenesis. Curr Top Microbiol Immunol. 1982;101:31–49. doi: 10.1007/978-3-642-68654-2_2. [DOI] [PubMed] [Google Scholar]
- Linney E., Davis B., Overhauser J., Chao E., Fan H. Non-function of a Moloney murine leukaemia virus regulatory sequence in F9 embryonal carcinoma cells. 1984 Mar 29-Apr 4Nature. 308(5958):470–472. doi: 10.1038/308470a0. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. V., Fleissner E., Lonial H., Koehne C. F., Reicin A. Early clonality and high-frequency proviral integration into the c-myc locus in AKR leukemias. J Virol. 1985 Aug;55(2):500–503. doi: 10.1128/jvi.55.2.500-503.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Oliff I., Schmidt B., Famulari N. Isolation of immortal cell lines from the first stage of murine leukemia virus-induced leukemia. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5464–5467. doi: 10.1073/pnas.81.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Selten G., Cuypers H. T., Zijlstra M., Melief C., Berns A. Involvement of c-myc in MuLV-induced T cell lymphomas in mice: frequency and mechanisms of activation. EMBO J. 1984 Dec 20;3(13):3215–3222. doi: 10.1002/j.1460-2075.1984.tb02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitbon M., Evans L., Nishio J., Wehrly K., Chesebro B. Analysis of two strains of Friend murine leukemia viruses differing in ability to induce early splenomegaly: lack of relationship with generation of recombinant mink cell focus-forming viruses. J Virol. 1986 Jan;57(1):389–393. doi: 10.1128/jvi.57.1.389-393.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch T. G., Arnstein P., Manohar V., Leiserson W. M., Chused T. M. Proliferation of infected lymphoid precursors before Moloney murine leukemia virus-induced T-cell lymphoma. J Natl Cancer Inst. 1985 Jan;74(1):137–143. [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Hu L. F. A common region for proviral DNA integration in MoMuLV-induced rat thymic lymphomas. 1983 Mar 31-Apr 6Nature. 302(5907):445–449. doi: 10.1038/302445a0. [DOI] [PubMed] [Google Scholar]
- Villeneuve L., Rassart E., Jolicoeur P., Graham M., Adams J. M. Proviral integration site Mis-1 in rat thymomas corresponds to the pvt-1 translocation breakpoint in murine plasmacytomas. Mol Cell Biol. 1986 May;6(5):1834–1837. doi: 10.1128/mcb.6.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M. Properties of "mink cell focus-inducing" (MCF) virus isolated from spontaneous lymphoma lines of BALB/c mice carrying Moloney leukemia virus as an endogenous virus. Virology. 1979 Feb;93(1):226–236. doi: 10.1016/0042-6822(79)90290-3. [DOI] [PubMed] [Google Scholar]
- van der Putten H., Quint W., van Raaij J., Maandag E. R., Verma I. M., Berns A. M-MuLV-induced leukemogenesis: integration and structure of recombinant proviruses in tumors. Cell. 1981 Jun;24(3):729–739. doi: 10.1016/0092-8674(81)90099-4. [DOI] [PubMed] [Google Scholar]



