Abstract
The Epithelial Na+ Channel (ENaC) is an apical heteromeric channel that mediates Na+ entry into epithelial cells from the luminal cell surface. ENaC is activated by proteases that interact with the channel during biosynthesis or at the extracellular surface. Meprins are cell surface and secreted metalloproteinases of the kidney and intestine. We discovered by affinity chromatography that meprins bind γ-ENaC, a subunit of the ENaC hetero-oligomer. The physical interaction involves NH2-terminal cytoplasmic residues 37–54 of γ-ENaC, containing a critical gating domain immediately before the first transmembrane domain, and the cytoplasmic COOH-terminal tail of meprin β (residues 679–704). This potential association was confirmed by co-expression and co-immunoprecipitation studies. Functional assays revealed that meprins stimulate ENaC expressed exogenously in Xenopus oocytes and endogenously in epithelial cells. Co-expression of ENaC subunits and meprin β or α/β in Xenopus oocytes increased amiloride-sensitive Na+ currents approximately two-fold. This increase was blocked by preincubation with an inhibitor of meprin activity, actinonin. The meprin-mediated increase in ENaC currents in oocytes and epithelial cell monolayers required meprin β, but not the α subunit. Meprin β promoted cleavage of α and γ-ENaC subunits at sites close to the second transmembrane domain in the extracellular domain of each channel subunit. Thus, meprin β regulates the activity of ENaC in a metalloprotease-dependent fashion.
Key words: epithelial sodium channel (ENaC), meprin, metalloprotease
The Epithelial Na+ Channel (ENaC)1 mediates the rate-limiting step in conductive Na+ absorption across epithelial-lined organs including airways, kidney and intestine. ENaC is a heteromeric channel consisting of α, β and γ subunits.1 Each subunit contains two transmembrane domains with an amino-terminal (N-terminal) cytosolic domain, a large extracellular domain and a cytosolic carboxyl-terminus (C-terminus).2 In the kidney and colon, ENaC helps maintain total body salt and volume homeostasis, while in the lung ENaC regulates the depth and composition of the airway surface liquid to maintain efficient mucociliary clearance.3 The functional importance of ENaC activity is highlighted by the fact that human mutations in ENaC and abnormalities in the regulation of ENaC, are involved in the pathogenesis of salt-sensitive hypertension, pseudo-hypoaldosteronism and cystic fibrosis.2,4
ENaC is regulated through signaling pathways that link channel localization and activity to distinct tissue-specific stimuli.2 The stimuli and signaling pathways that regulate ENaC are not completely understood, but all converge on cellular processes that determine the number of active channels at the apical membrane, the single channel conductance and/or the open probability of the channels on the membrane. Recent data indicate that proteases, such as channel activating proteases (CAPs) and furin, regulate ENaC activity, however the mechanisms of activation are poorly understood.5–9 The first protease shown to increase ENaC activity was Xenopus channel-activating protein (xCAP1), which was identified in a functional screen for novel regulators of ENaC.5 Mouse CAPs increase amiloride-sensitive Na+ currents when co-expressed with ENaC in the oocyte system.6,7 However, it is unclear whether CAPs cleave ENaC directly or function in an unidentified pathway to increase Na+ conductance via ENaC. There is some evidence that the α and γ-ENaC subunits are substrates of the preproconvertase furin.8,10 Although furin cycles from the trans-Golgi network to the cell surface,11 and is known to cleave substrates in multiple cellular compartments,12 furin is proposed to cleave ENaC as the channel traverses the secretory pathway.5–9 On the other hand, Caldwell demonstrated that extracellular proteases markedly increase the open probability of quiescent ENaC already present at the plasma membrane.13 Thus, proteases may affect ENaC activity by modulating the gating of channels trafficking in intracellular compartments or at the plasma membrane.
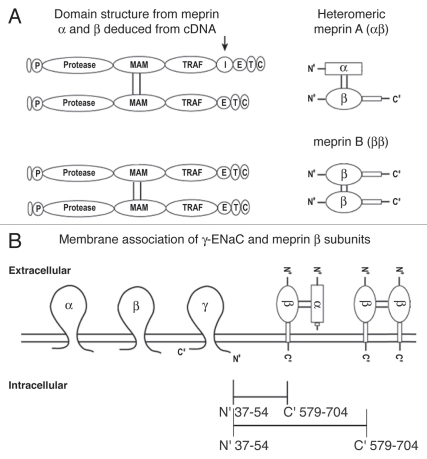
In a proteomic screen of mouse kidney to find ENaC-interacting proteins we identified the meprin α subunit among proteins that bind to the cytoplasmic N-terminus of γ-ENaC. Meprin α is one of two evolutionarily-related proteins (α and β) that form homo- and hetero-oligomeric complexes of meprin metalloproteinase (α2, β2 or αβ; Fig. 1).14 Meprins are abundantly expressed in epithelial cells of the kidney and intestine, and also found in skin, leukocytes and lung.15–18 They are multidomain zinc-containing proteinases that are members of the ‘astacin family’ and ‘metzincin superfamily’ of metalloproteinases.15 The meprin α and β subunits both have proteolytic activity, but are distinct in their substrate specificities.19 For example, meprin α has a preference for small or hydrophobic residues flanking the cleavage site, whereas meprin β has a preference for negatively charged residues.19 The meprin subunits are also processed differentially during biosynthesis. Thus, both are synthesized as type 1 membrane spanning proteins, however, the meprin α protein, but not β, is proteolytically cleaved in the endoplasmic reticulum consequently loosing its transmembrane and cytoplasmic domain.20,21 Thus, meprin β remains a transmembrane protein in the secretory pathway and at the cell surface, whereas the meprin α subunit is extracellular. The meprin subunits form disulfide-linked homo- and heterodimers.14 Thus, meprin β dimers (called meprin B) and meprin α/β dimers (heteromeric meprin A) are membrane-bound proteins, whereas meprin α homodimers (that tend to self-associate into larger oligomers of over 10 subunits) are secreted: meprin α is only at the cell surface when it is covalently (through disulfide bridges) associated with meprin β.22
Figure 1.
Diagrammatic representation of the heteromeric meprin α/β and homomeric meprin β/β dimers, interactions with ENaC at the plasma membrane. (A) Domain structures of the meprin α and β subunits, deduced from their cDNAs; the subunits are disulfide linked through the MAM domain forming heterodimers α/β and homodimers β/β. The arrow above the meprin α subunit indicates that there is a proteolytic cleavage in or near the I domain that removes the subunit from the membrane. P, prosequence; Protease, catalytic domain; MAM, meprin; A5, protein-tyrosine phosphatase µ; TRAF, tumor necrosis factor α receptor associated factor; I, inserted domain; E, epidermal growth factor-like; T, transmembrane; C, cytoplasmic domain. (B) Plasma membrane association of γ-ENaC subunits and the β subunits of heteromeric meprin A (α/β) and homomeric meprin B (β/β).
The aim of the studies herein was to determine: (1) molecular details on how ENaC and meprin subunits interact and (2) whether meprins affect the function of ENaC through proteolytic activity.
Results
Interactions of EN aC and meprin subunits.
The short (70–90 residues) cytosolic N-terminal tails of ENaC subunits contain a highly conserved domain essential for channel opening23 and multiple positively charged residues that have been implicated in regulation of the channel by lipid binding. We reasoned that proteins capable of interacting with ENaC N-termini could affect ENaC activity. Therefore, affinity chromatography and mass spectrometry were used to identify proteins that associate with the N-terminus of γ-ENaC. A biotinylated peptide encompassing residues 1–54 of rat γ-ENaC was immobilized on streptavidin beads and incubated with solubilized membrane proteins prepared from C57BL/6 mouse kidney. Several proteins were identified as γ-ENaC-interacting proteins using MALDI-TOF and electrospray tandem MS/MS including the α subunit of the metalloprotease meprin A. Immunoprecipitation techniques using biotinylated peptides derived from α, β and γ-ENaC with antibodies directed against meprin α were used to further characterize the ENaC-meprin interaction. Meprin α robustly associated with the N-terminus of γ-ENaC but did not associate with the N-terminus of α-ENaC (Fig. 2A). The meprin α migration pattern on a denatured SDS-gel (∼100 kDa) corresponded to that of the glycosylated latent precursor (14). Minor association of meprin α with the N-terminus of β-ENaC was evident in some experiments but it was not reproducible. Meprin β was not detected by mass spectrometry in the initial precipitation assays, even though meprin α would not be present in membrane fractions without meprin β. However, western blots confirmed that meprin β was also present in γ-ENaC precipitates (Fig. 2B). The antibody to meprin β identified two bands with molecular masses equivalent to the latent and active highly glycosylated forms of meprin β (∼110 and ∼75 kDa, respectively).14 These results demonstrate that meprin α and β subunits associate, directly or indirectly, with the N-terminus of γ-ENaC in vitro.
Figure 2.
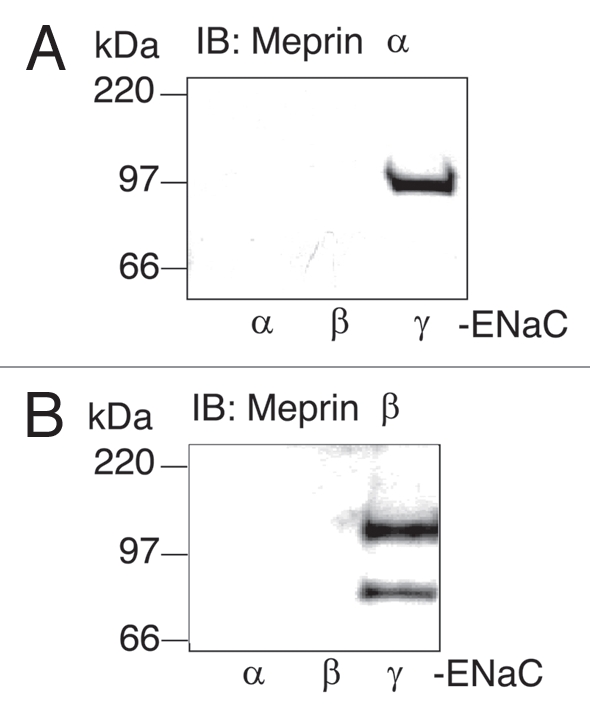
Meprins interact with the γ-ENaC N-terminal (NT) peptide. Affinity purification assays were done using the following biotinylated peptides; α-ENaC42–109, β-ENaC1–49 and γ-ENaC1–54. Kidney membrane preparations (500 µg) from C57BL/6 mice were incubated with each peptide (5 µg) and streptavidin beads for 2 hr at 4°C. The beads were subsequently washed three times with BB150 buffer and twice with PBS buffer. Laemmli sample buffer was added, the samples were boiled for 7 min and elutes were subjected to SDS-PA GE analysis on 4–20% gradient acrilamide gels. Proteins were transferred onto polyvinylidene difluoride membranes (PVDF) and western blot analysis was performed using a monoclonal antibody to meprin α (A) and a polyclonal antibody to meprin β (B). Representative immunoblots are shown (n = 3).
To determine whether meprins can affect the function of ENaC, amiloride-sensitive Na+ currents were measured in Xenopus oocytes expressing rat ENaC subunits alone or coinjected with human meprin α and β (Fig. 3). A significant increase in amiloride-sensitive Na+ currents was observed when ENaC was co-expressed with meprin α and β (8,413 ± 529 nA (N = 33)) compared to control oocytes expressing ENaC alone (4,259 ± 390 nA (N = 32)). Taken together, our data indicate that hetero-oligomeric meprin can associate with ENaC and can modulate ENaC function in a heterologous system.
Figure 3.
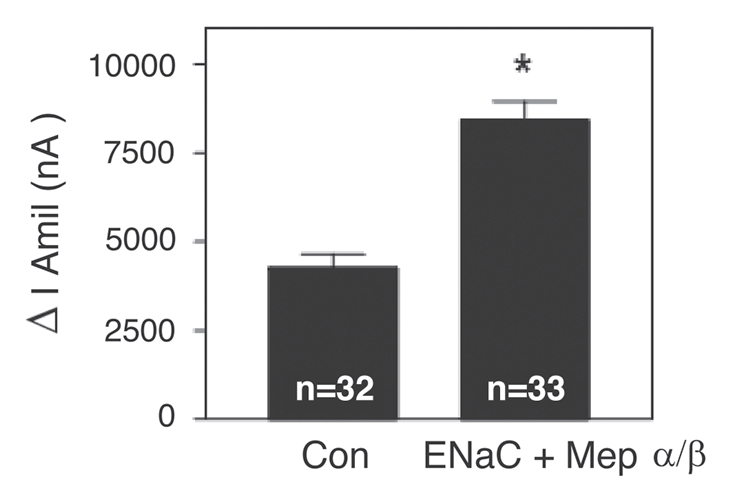
Increase in amiloride-sensitive Na+ currents by co-expression of αβγ-ENaC with meprin α and β subunits in Xenopus oocytes. Wildtype α, β and γ-ENaC cRNA and meprin α and β cRNA (0.5 ng) subunits were injected into oocytes. Twenty-four hr post-injection, two-electrode voltage clamp assays were conducted. Currents measured in the presence and absence of amiloride (10 µM), while clamping the membrane voltage to −100 mV, were digitized and recorded. Batches of oocytes were extracted from 3–5 different frogs. Results are expressed as the means ± S.E. Statistical significance was determined using an unpaired Student's t test. ENaC + Meprin α/β; *Significant p < 0.0001.
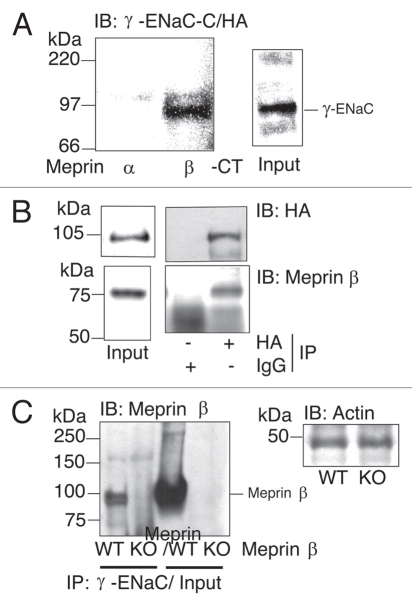
For membrane-associated forms of meprins, meprin β spans the plasma membrane, with its C-terminus located within the cell.15 To determine if the cytosolic N-terminus of γ-ENaC binds the C-terminus of meprin β, peptides derived from the meprin α and β cytosolic domains were incubated with membranes prepared from MDCK cells expressing HA-tagged γ-ENaC together with untagged α and β-ENaC subunits. MDCK cells express exogenous amiloride-sensitive Na+ channels on the cell surface and provide an excellent model system to study the regulation of ENaC by specific protein-protein interactions. The C-terminal peptide from the nascent meprin α subunit is cleaved from the subunit during biosynthesis in the endoplasmic reticulum, therefore there is no meprin α cytoplasmic tail in the cell. The biotinylated C-terminus of meprin β (βCT), but not meprin α (αCT), was found to bind γ-ENaC in vitro (Fig. 4A). These data indicate that the ENaC-meprin interaction involves the N-terminus of γ-ENaC and the C-terminus of meprin β. Thus meprin A (containing meprin α and β subunits) or meprin B (consisting exclusively of meprin β subunits) could associate with ENaC in cells.
Figure 4.
The meprin β679–704 cytosolic domain binds to γ-ENaC and meprin β co-immunoprecipitates with γ-ENaC. (A) Affinity purification assays were performed using biotinylated peptides that correspond to the C-termini (CT) cytosolic domain of mouse meprin α750–760 or meprin β679–704. Membranes from ENaC-MDCK cells transiently transfected with HA epitope-tagged γ-ENaC (γ-ENaC-C/HA) were incubated with meprin peptides. γ-ENaC-C/HA staining in the cell lysate is shown (Input). Samples were processed as specified above (Fig. 2A) and western blot analyses were done using a monoclonal anti-HA antibody. A representative experiment is shown (n = 3). (B) Co-IP assays were carried out with oocytes co-expressing α, β-ENaC untagged and γ-ENaC-C/HA tagged subunits with meprin β. γ-ENaC subunits were immunoprecipitated (IP) using an anti-HA antibody and analyzed by western blot using a polyclonal anti-meprin β (lower) or anti-HA antibodies (upper). Representative experiments are shown (n = 3). IB: immunoblot. (C) Co-IP assays were done with kidney lysates from wild type (wt) or meprin β knockout (βko) mice. γ-ENaC subunits were immunoprecipitated (IP) using a specific γ-ENaC monoclonal antibody. Immunoprecipitates (1–2 lanes) and inputs (3–4 lanes) were analyzed by western blot using a polyclonal anti-meprin β antibody (left part). Mice kidney lysates were analyzed by western blot using an anti-actin antibody as loading control (right part).
To test the prediction that full-length γ-ENaC and meprin β interact, C-terminal HA epitope tagged γ-ENaC was coexpressed with wild type α and β-ENaC and meprin β in Xenopus oocytes. HA antibodies were used to immunoprecipitate γ-ENaC/C-HA from solubilized membranes and antisera directed against meprin β subunits were used to probe the immunoprecipitates for meprin. The data showed that γ-ENaC associates with meprin β, but not with the IgG controls, in oocytes (Fig. 4B). These results are consistent with previous findings using MDCK-ENaC cells and meprin C-termini peptides (Fig. 4A). Taken together these data indicate that the γ-ENaC N-terminus and meprin β C-terminus can interact to form an ENaC-meprin multimeric complex.
To validate this ENaC-meprin protein interaction in a more physiological context, we next asked if γ-ENaC and meprin β could interact in mouse kidney tissue. γ-ENaC immunoprecipitates from wild-type and meprin β knockout mice kidney lysates were probed with a meprin β antibody. As expected, meprin β was found to coimmunoprecipitate with γ-ENaC subunits only from wild-type but not from β knockout kidney lysates (Fig. 4C). Thus γ-ENaC and the metalloprotease meprin β interact in the nephron where both proteins are known to be expressed abundantly.24,25
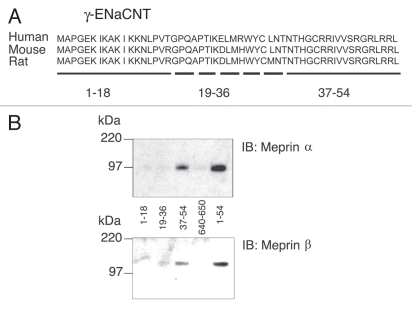
The N-terminus of γ-ENaC is highly conserved (Fig. 5A) and contains residues that are important for channel gating.26 Biotinylated peptides corresponding to amino acids 1–18, 19–36 and 37–54 of rat γ-ENaC were immobilized on streptavidin beads and incubated with membranes prepared from mouse kidney. After extensive washing, bound proteins were separated by SDS-PAGE and analyzed by western blot with meprin α or meprin β antibodies. Endogenous meprin did not bind γ-ENaC1–18, γ-ENaC19–36 or a peptide from the C-terminus of γ-ENaC640–650 used as an additional specificity control (Fig. 5B). In contrast, robust association of meprin α and β to γ-ENaC37–54 as well as the γ-ENaC1–54 peptide was detected. The association of meprin with the γ-ENaC1–54 peptide is stronger than that with a shorter γ-ENaC37–54 peptide, suggesting that other regions of the γ-ENaC N-terminus contribute to this interaction. Thus, meprin subunits associate with γ-ENaC between Asn37 and the beginning of the first transmembrane domain. Interestingly, this segment contains an absolutely conserved gating domain centered at His-Gly39–40,26 as well as multiple arginines that have been implicated in channel gating and in lipid binding.26,27
Figure 5.
Mapping of γ-ENaC-N-terminal and meprin interacting residues. (A) Human, mouse and rat γ-ENaC N-terminal amino acid sequences. (B) Affinity purification assays using biotinylated peptides; rat γ-ENaC1–18, 19–36, 37–54, 640–650 and 1–54. Solubilized mouse kidney membranes (500 µg) were incubated with each peptide (5 µg) and streptavidin beads for 2 hrs at 4°C. Meprin α (top part) and meprin β (bottom part) association to γ-ENaC N-terminus peptides were assayed as in Figure 2. Representative experiments are shown (n = 3).
Functional analysis of meprin's action.
To determine whether the catalytic activity of hetero-oligomeric meprin is required to increase ENaC currents in Xenopus oocytes, meprin activity was inhibited with actinonin, a selective inhibitor that interacts with zinc in the active site of both meprin subunits (IC50 values for mouse meprin A and B; 100 and 400 nM, respectively; reviewed in ref. 19). As before, amiloride-sensitive Na+ currents were significantly increased by ∼1.8 fold in vehicle-treated oocytes expressing ENaC + meprin α/β (Fig. 6A). In contrast, amiloride-sensitive Na+ currents in actinonin-treated oocytes co-expressing ENaC + meprin did not differ from Na+ currents recorded from oocytes expressing ENaC subunits alone (110.6 ± 13.06% of basal (n = 44)). Because actinonin had no effect when applied to oocytes expressing ENaC alone, it is concluded that catalytic activity of meprin is involved in the stimulation of ENaC-mediated Na+ currents in Xenopus oocytes.
Figure 6.
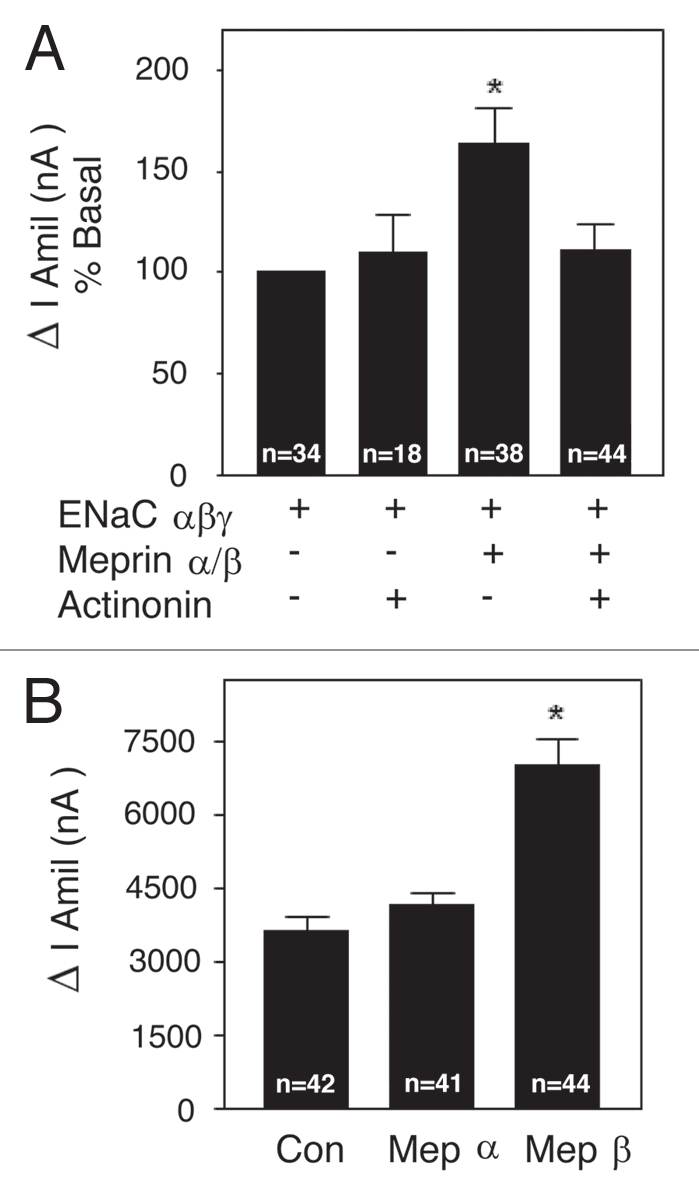
Meprin stimulation of ENaC currents is inhibited by actinonin and mediated by meprin β. (A) Wild-type α, β and γ-ENaC and meprin α and β cRNA (0.5 ng) subunits were injected into oocytes. Total injection volume was kept constant with H2O. ENaC only and ENaC + meprin subunit groups were preincubated with 1 µM of actinonin or vehicle overnight at 18°C. Twenty four h post-injection, two-electrode voltage clamp assays were conducted. Currents measured in the presence and absence of amiloride (10 µM), while clamping the membrane voltage to −100 mV, were digitized and recorded. Batches of oocytes were extracted from 3–5 different frogs. Results are expressed as percentage of basal amiloride-sensitive currents. Statistical significance was determined using an unpaired Student's t test. Meprin αβ + actinonin; no significant difference; Meprin αβ without actinonin; *Significant p = 0.0006. (B) Wild-type α, β and γ-ENaC RNAs were injected with either meprin α or with meprin β RNA (0.5 ng) subunits into oocytes. Twenty-four hr post-injection, two-electrode voltage clamp assays were conducted exactly as in (A). Results are expressed as the means ± S.E. Statistical significance was determined using an unpaired Student's t test. Meprin α; no significant difference, Meprin β; *Significant p < 0.0001.
To further investigate the role of each meprin subunit, changes in amiloride-sensitive Na+ currents from oocytes co-expressing ENaC with meprin α or β, were measured. When expressed alone, meprin α had no appreciable effect on amiloride-sensitive Na+ currents (meprin α: 4,154 ± 261 nA (N = 41) vs. control: 3,644 ± 274 nA (N = 42)). In contrast, meprin β significantly increased amiloride-sensitive current (7,027 ± 518 nA; (N = 44)) (Fig. 6B). The meprin β-induced increase in amiloride-sensitive Na+ currents was near the full expected increase if compared with ENaC currents recorded from oocytes expressing ENaC and meprin α + β subunits (Fig. 3). The data presented in Figure 6 indicate that the catalytic activity of meprin is required for meprin's effect on ENaC and that meprin β is responsible for activating ENaC in the Xenopus oocyte expression system.
Cleavage of ENaC by meprin β.
When Xenopus oocytes co-expressing ENaC + meprin (α + β) were pre-incubated with 1µM actinonin, meprin failed to increase ENaC-mediated Na+ currents, suggesting that the proteolysis of ENaC or an ENaC-associated regulatory protein is involved in the activation process (Fig. 6A). To distinguish between these two possibilities, we asked whether the application of soluble meprin α or meprin β was able to cleave ENaC subunits at the cell surface of intact Xenopus oocytes. Soluble meprins were activated with trypsin (1:20 µg/ml, trypsin:meprin) and excess soybean trypsin inhibitor (STI) was used to inactivate trypsin prior to the experiments. Activated meprin α or β was applied to oocytes expressing a combination of tagged and untagged ENaC subunits; proteolytic cleavage of individual ENaC subunits was assessed by SDS-PAGE and western blot analysis using monoclonal antibodies against the N-terminal HA or C-terminal V5 epitope tags. When oocytes expressing ENaC were treated with activated soluble homomeric meprin A (α/α) no differences in the migration pattern of any full-length ENaC subunit were detected (data not shown). These data indicate that meprin α does not cleave ENaC and are consistent with our previous results demonstrating that meprin α alone cannot activate ENaC-mediated currents in Xenopus oocytes (Fig. 6B). In contrast, proteolysis of α and γ, but not β-ENaC, was consistently observed when activated soluble meprin B (β/β) was added to intact oocytes (Fig. 7). In oocytes expressing untagged β and γ-ENaC together with double tagged α-ENaC C/HA + N/V5, fragments of ∼80 and ∼17 kDa were observed with antibodies directed against HA or V5 (Fig. 7A and B), respectively. The proteolysis of α-ENaC was abolished when 1 µM actinonin was included in the bath solution (Fig. 7C). These data indicate that meprin β (or a protease activated by the addition of meprin β) cleaves the extracellular domain of α-ENaC near the second transmembrane domain (TMII). γ-ENaC fragments of ∼76 and ∼17 kDa were detected when oocytes expressing γ-ENaC containing N-terminal V5 and C-terminal HA tags were treated with soluble activated meprin β (Fig. 7), indicating that γ-ENaC was also cleaved near the second transmembrane domain. Thus α and γ-ENaC are substrates for meprin β.
Figure 7.
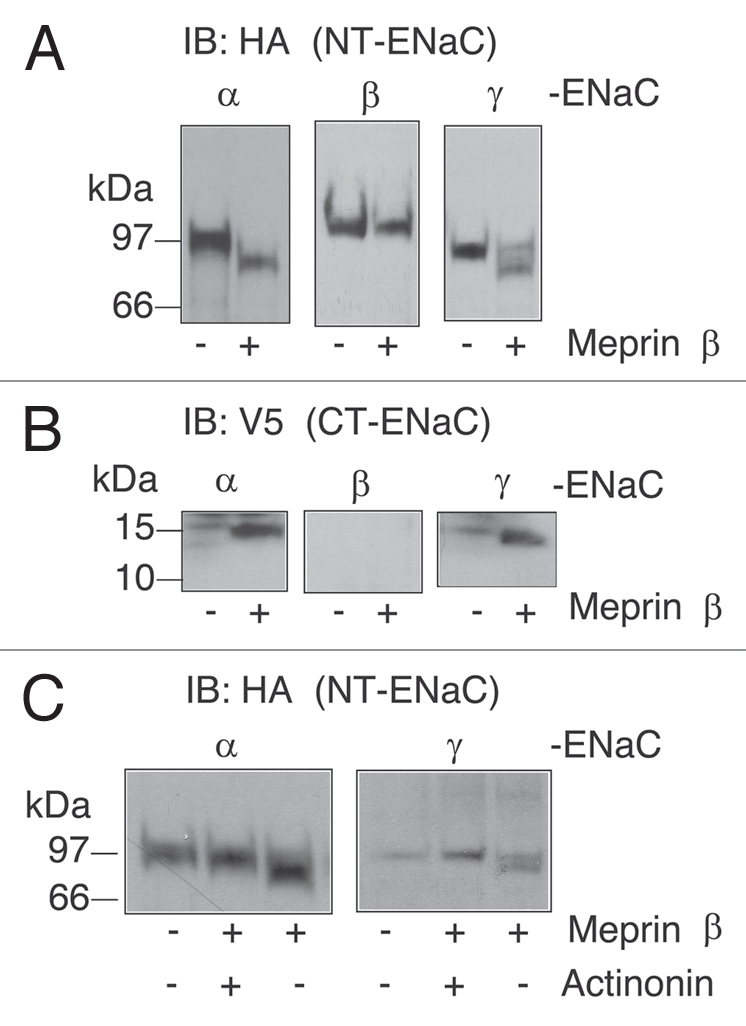
Cleavage of α and γ-ENaC subunits in intact oocytes by soluble activated meprin β. (A and B) Wild-type α, β and γ-ENaC double tagged (N-terminus HA and C-terminus V5 tags) and α, β or γ-ENaC untagged cRNA (1 ng) subunits were injected into oocytes. ENaC expressing oocytes were incubated with 10 µg/ml of soluble activated meprin β at 25°C for 30 min with or without 1 µM actinonin (C). Yolk-depleted lysates and total membrane fractions were prepared from oocytes and western blots analysis were conducted using anti-HA (A) and anti-V5 (B) monoclonal antibodies. Batches of oocytes were extracted from three different frogs. Representative experiments are shown (n = 3).
Meprin regulates ENaC in human bronchial epithelial cell monolayers.
To extend our studies from oocytes to epithelial cells, the effects of soluble recombinant meprin on ENaC currents were tested in16HBE14o- cells, a cultured human airway epithelial cell line endogenously expressing α-ENaC. These cells were stably transfected with cDNAs encoding human β and γ-ENaC to generate a cell line with 5–10 µAmps of amiloride-sensitive Na+ currents, when studied in Ussing chambers.28 Meprin expression is not detected in native respiratory epithelium.29,30 Soluble recombinant meprin β (10 µg/ml) applied in the luminal bath at steady state increased amiloride-sensitive current by 1.0 ± 0.2 µA (N = 6) (Fig. 8). This response required meprin β activity because it was blocked by actinonin (1 µM) and because inactive meprin β was ineffective. This effect was specific to meprin β because active meprin α did not affect ENaC currents in this human bronchial epithelial cell line (data not shown). These data show that the proteolytic activity of soluble meprin β stimulates Na+ transport in an epithelial monolayer.
Figure 8.
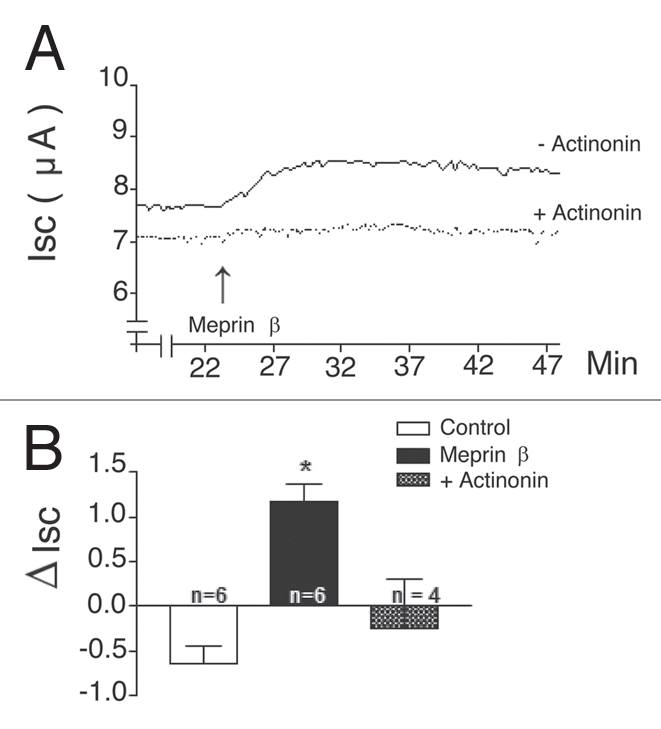
Meprin β stimulates ENaC in human bronchial epithelial monolayers. (A) Representative traces of Isc during application of soluble activated meprin β in the presence (+Act) or absence of actinonin (-Act). Human bronchial epithelial cells (16HBE14o-/hβγENaC) expressing human α, β and γ-ENaC subunits were cultured on permeable supports. Tissues were mounted in Ussing chambers in KBR; ISC, R and equivalent PD were recorded continuously for 30 min. Soluble activated forms of rat meprin α or β (10 µg/ml) proteins were applied to the luminal surface of cell monolayers pretreated or not with actinonin (1 µM); all tissues were treated with 10 µM amiloride at the end the experiment to assess the magnitude of the ISC that is due to ENaC. (B) Summary of change induced in ISC by meprin β in HBE monolayers. Results are expressed as the means ± S.E. Statistical significance was determined using an unpaired Student's t test. Meprin β; *Significant p < 0.0001. Summary of 4–6 experiments similar to (A). *different from Control.
Discussion
The studies herein demonstrate that the metalloprotease meprin β and γ-ENaC associate directly through cytoplasmic domains. In addition, meprin β is capable of cleaving the α and γ-ENaC subunits in extracellular portions of these proteins and activating the ion channel when the channel proteins and protease are coexpressed in Xenopus oocytes. Channel proteins were activated by membrane-bound meprin β (the homodimer meprin B or the hetero-oligomer meprin αβ) or by activated soluble meprin B added extracellularly to ENaC expressing cells. This activation was inhibited by the meprin inhibitor actinonin, indicating that meprin catalytic activity mediates the effect. Indeed, actinonin-sensitive cleavage of α and γ-ENaC was observed when activated soluble meprin B (β/β) (but not homomeric meprin A (α/α)) was applied to ENaC-expressing cells. Thus, the meprin β protease is capable of regulating ENaC activity in cells when the channel and protease are both expressed as membrane proteins or in channel expressing cells that are exposed to extracellular soluble meprin B (β/β). These findings are particularly relevant to kidney and intestinal tissues where ion channels and meprins are highly expressed on apical membranes and where ENaC functions critically in salt and volume homeostasis.24,25 Because the meprins are also secreted or shed into the lumen of these organs,31 they are capable of cleaving and activating channel proteins distal to their site of synthesis (e.g., in the kidney distal tubules) where there is a high concentration of ion channel proteins. In addition, meprins are expressed in leukocytes under certain conditions (e.g., in human and experimental models of inflammatory bowel diseases) and thus have the potential to affect cell surface proteins in inflammation.32
Several proteases have now been linked to the biosynthesis and activity of ENaC,4–9,33,34 but definitive mechanisms for the actions of proteases underlying these observations are not yet agreed upon. Most studies on protease regulation of ENaC have detected stimulation of ENaC by co-expressed proteases (CAPs, furin). In these studies, proteases are envisioned acting on ENaC either during biosynthesis (e.g., furin) or at the cell surface (e.g., CAPs). Recently, exogenously applied soluble proteases, including trypsin and elastase, have been shown to acutely increase the open probability of ENaC.13,28 Overall, these observations are consistent with a model in which proteases can (1) act during ENaC biosynthesis to increase the activity of channels prior to insertion into the membrane; or (2) act in the extracellular space on “near silent” ENaC channels that have been inserted to the membrane without the action of a protease during biosynthesis. Although some studies clearly demonstrate that ENaC is a protease substrate,8–10 proteases could regulate ENaC by indirect mechanisms that do not include cleavage of channel subunits.34 Our study is the first to show a biochemical interaction between an ENaC subunit and a protease that stimulates ENaC activity and the first to show a metalloproteinase affects activity.
The biochemical association of meprins with ENaC and cleavage and regulation of ENaC by meprins add to an increasingly complex model of regulation of ENaC by proteases. The essential feature of the model is that ENaC responds to proteases with increased activity. However, major unresolved questions include the number and identities of ENaC-regulating proteases in different tissues, the cellular vs extracellular site of protease-ENaC interaction and the substrates targeted by proteases that regulate ENaC. In addition, proteases commonly exist as inactive zymogens and the conversion to active protease provides an additional layer of complexity to ENaC regulation. For example, it was recently proposed that CAP1–3 could form part of a proteolytic cascade at the cell surface that regulated ENaC.7 Previous studies in mouse kidney,35 and the present studies indicate that meprin β is only partially activated in mouse kidney, as demonstrated by the immunoblots of Figure 2B, implying that activation of this protease could also be part of a regulated cascade of events at the cell surface.
We observed actinonin-sensitive cleavage of α and γ-ENaC by meprin β. Within the region of α and γ-ENaC where meprin cleaves there are several glutamate and aspartate residues and meprin β prefers to cleave peptide bonds containing these residues. Interestingly, a recently constructed database that predicts substrates for meprin β coded for by the mouse and human genomes identified several other ion channel proteins as excellent substrates, including for example, the ä subunit of the amiloride-sensitive sodium channel, amiloride-sensitive cation channel 4 isoform 1; acid-sensing ion channel 4, voltage-gated type I–IV sodium channel and voltage-dependent α1F and α1H calcium channel, (http://vbc.med.monash.edu.au/∼sboyd/Meprins/). Thus, ENaC may be a model for a group of proteins that interact, are cleaved and functionally altered my meprins at the cell surface.
The cytoplasmic tail of meprin β is also known to interact with OS-9, a ubiquitously expressed cytoplasmic protein thought to be important for the transport of the protease to the cell surface.36 Recently, it has been shown that OS-9 also interacts with HIF1α (hypoxia-inducible factor α), a master transcription factor that activates the hypoxic response elements in the promoters of several target genes and thereby regulates cellular metabolism in response to hypoxia.37,38 The cytoplasmic tail interactions may be important in a network of interactions that signal changes in transcription as well as events at the cell surface. In addition, polymorphisms in the cytoplasmic tail of meprin β have been associated with susceptibility of Pima Indians to diabetic nephropathy.39 Taken together the meprin β cytoplasmic tail has interactions with membrane-bound and cytosol proteins that have important implications for cellular functions and disease.
The studies of ENaC and meprins imply that the cytoplasmic tails position the proteins to enable cleavage and activation of ENaC. Since ENaC has been shown experimentally to respond to several diverse proteases, it is reasonable to suggest that close physical association of ENaC and proteases, such as shown for meprins, is a key determinant of tissue specificity and physiologic regulation.
Materials and Methods
Cell lines and plasmid preparation.
Madin-Darby Canine Kidney (MDCK) cells stably expressing all three rat ENaC subunits (MDCK-ENaC cells; reviewed in ref. 40) and cultured human airway epithelial cells (16HBE14o-) stably expressing human β and γ-ENaC (16HBE14o-/βγ) were maintained as described previously.28,41 16HBE14o- cells endogenously express α-ENaC that assembles efficiently with the transfected β and γ subunits to form functional channels.28
Rat ENaC subunits containing C-terminal HA tags (ENaC-C/HA) were generated by PCR and cloned into pcDNA3.1(+) using AscI and EcoRI restriction sites. These cDNAs were used to transiently transfect MDCK-ENaC cells using Lipofectamine 2000 (Invitrogen) and transfected cells were used for biochemical assays. For biochemical analyses of ENaC subunits proteolysis, cDNAs encoding rat β and γ-ENaC with N-terminal HA and C-terminal V5 epitope tags (N/HA + C/V5) were generated by PCR and cloned into pCR-BluntII-TOPO (Invitrogen), linearized (HindIII) and in vitro transcribed using T7 RNA polymerase. A PolyA tail was added after transcription (Ambion). Full-length cDNAs encoding human meprin α and β were excised from pcDNA3.1(+) and cloned into pSDE using BamHI/NotI or XbaI/NotI, respectively. The sequence of all plasmids was verified at the University of North Carolina sequencing facility.
Affinity precipitation of EN aC-interacting proteins.
Membranes from C57BL/6 mouse kidney were prepared by ultracentrifugation at 100,000× g for 30 min in 50 mM Tris pH 7.5, 150 mM NaCl containing 2 µg/ml leupeptin, 16 µg/ml benzimidine, 2 µg/ml PMSF (phenylmethanesulfonyl fluoride) and 2µg/ml trypsin inhibitor.42 Membrane proteins were solubilized in buffer containing 50 mM Tris pH 7.6, 150 mM NaCl, 1% Triton X-100, 10 mM EDTA and 10 mM EGTA and protease inhibitors. Soluble proteins were collected by centrifugation at 16,100 g for 10 min. Supernatant fractions were precleared by incubation with streptavidin beads (Sigma St. Louis, MO) and then incubated for 2 hr at 4°C with a rat γ-ENaC1–54 peptide covalently linked to a C-terminal biotin (Genemed synthesis Inc., San Francisco, CA). γ-ENaC-interacting proteins were collected by incubation with streptavidin-agarose beads for 1 hr at 4°C and, after extensive washing, bound proteins were analyzed by SDS-PAGE and visualized by silver staining (Invitrogen). Visible bands were excised and samples analyzed by MALDI/TOF-MS (Bruker Instruments Co., Bremen, Germany) and Nano-ESI-MS/MS on an API QSTAR-Pulsar (QSTAR, Applied Biosystems Div., Perkin-Elmer Corp., Foster City, CA) as described.43 Alternatively small-scale binding assays, using 500 µg of solubilized membrane proteins from MDCK-ENaC or mouse kidney fractions and 5 µg biotinylated peptide, were analyzed by western blot.
Affinity precipitation of meprin subunit interacting proteins.
The COOH-terminal peptides of mouse meprin α (amino acids 750–760) and β (amino acids 679–704) were synthesized and covalently linked to biotin at the C-terminus (Genemed synthesis Inc., San Francisco, CA). Kidney membrane preparations were incubated with the peptides and streptavidin beads as described for ENaC peptides.
Functional studies of Na+ channels in xenopus oocytes.
Xenopus oocytes were harvested as described previously44 and maintained in modified Barth's solution at 18°C. cRNAs encoding wild-type rat α, β and γ-ENaC (0.5 ng each) were expressed in oocytes with or without cRNA encoding meprin α and/or β. Twenty-four hr after injection, two-electrode voltage clamping was performed using a Genclamp amplifier (Axon Instruments) in a constant perfusion system. Currents measured in the presence and absence of 10 µM amiloride, while clamping the membrane voltage to −100 mV, were digitized and recorded using a Digidata 1200 A/D converter (Axon Instruments) and Axoscope software. For some analyses, amiloride-sensitive currents were normalized as a percentage of basal currents to deal with the customary variability between oocyte batches. All results are expressed as the mean ± S.E. and the data were analyzed using an unpaired Student's t test. Proteins extracted from control and injected oocytes were analyzed by western blots to verify expression of ENaC and meprin subunits.
Co-immunoprecipitation and western blot analysis.
Proteins were extracted from oocytes as described previously.44 Membrane fractions were solubilized in Laemmli sample buffer for western blots or solubilized in modified radio-immunoprecipitation assay (RIPA) buffer (50 mM Tris pH 7.5, 100 mM NaCl, 1% triton X-100, 0.2% NP-40, 0.1% SDS, 0.1% NaDeoxycholate, 20 mM NaF, 10 mM Na pyrophosphate, 10 mM EDTA + protease inhibitor cocktail) for immunoprecipitation. A modified RIPA buffer was used for co-mmunoprecipitations, (50 mM Tris pH 7.5, 100 mM NaCl, 1% triton X-100, 1% NP-40, 10 mM EDTA + protease inhibitor cocktail). Membrane proteins were incubated at 4°C overnight with 2 µg of anti-HA antibody and 40 µl of protein A beads. Samples were extensively washed with RIPA buffer and beads were aspirated to dryness. Laemmli sample buffer was added and samples were boiled for 7 min prior to loading onto 4–20% SDS-PAGE gels. western blots were performed with anti-HA (Covance), anti-meprin α or anti-meprin β antibodies as described previously.14,45
Co-Immunoprecipitations from Meprin beta WT and KO kidney lysates were done as described above. γ-ENaC subunits were enriched with a γ-ENaC monoclonal antibody generated at the UNC Immunology Core Facility. Membranes with γ-ENaC immunoprecipitates were probed for meprin beta with antimeprin β antibodies. Meprin beta KO mouse were generated as previously described46 on a mixed background (C57BL/6 × 129 × 1Sv) and then backcrossed for 13 generations on a C57BL/6 strain; C57BL/6 mice were used as the WT controls.
Treatment of intact oocytes with activated recombinant meprin proteins.
Various combinations of tagged and untagged rat ENaC subunits were expressed in Xenopus oocytes for twenty-four hr. Batches of twenty oocytes were incubated with 10 µg/ml of rat meprin α or β at 25°C with gentle rocking for 30–120 min in 20 mM Tris, 150 mM NaCl, pH 7.5. Oocytes were transferred to the same buffer containing 50 mM NaF, 10 mM β-glycerophosphate, 1 mM EDTA, 2 µg/ml PMSF (phenylmethanesulfonyl fluoride), 2 µg/ml leupeptin, 16 µg/ml benzimidine, 2 µg/ml trypsin inhibitor and 10 µM actinonin. Yolk-depleted lysates and membrane fractions were prepared as described above and proteins were fractionated by SDS-PAGE and analyzed by western blot using monoclonal antibodies directed against HA or V5 epitope tags.44
Ussing chamber experiments.
16HBE14o-/βγ cells were grown in Minimal Essential Medium with 10 µM amiloride and seeded onto Costar Snapwell culture inserts (Corning) for study, as reported previously.28 After 6–10 days when R >200 Ohm.cm and PD >1 mV (lumen negative), cells were induced for twenty-four hr with 1 mM Na-butyrate and 10−6 M dexamethasone. Induced preparations were mounted in Ussing chambers in Krebs-Bicarbonate-Ringer Buffer (KBR); basal Isc, R and equivalent PD were recorded continuously for 30 min. In these preparations, Isc represents active Na+ absorption, a process for which ENaC activity at the apical membrane is limiting. At 30 min active rat meprin α or β (10 µg/ml) were applied to the luminal bath in the presence or absence of 1 µM actinonin (Sigma St. Louis, MO); Isc was recorded for 45 min and 10 µM amiloride was applied. Some tissues were treated with 2 µg/ml trypsin (Sigma St. Louis, MO) before amiloride to assess the maximum proteolytic responsiveness.
Acknowledgements
This work was supported by National Institutes of Health Grants DK 19691 (to Judith S. Bond) and HL 060280-7 (to M. Jackson Stutts).
Abbreviations
- ENaC
epithelial sodium channel
- NT
amino terminus
- CT
carboxy terminus
- MDCK
madin-darby canine kidney
- HBE's
human bronchial epithelial
- IP
immunoprecipitate
- IB
immunoblot
References
- 1.Canessa CM, Schild L, Buell G, et al. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 2.Schild L. The epithelial sodium channel: from molecule to disease. Rev Physiol Biochem Pharmacol. 2004;151:93–107. doi: 10.1007/s10254-004-0023-7. [DOI] [PubMed] [Google Scholar]
- 3.Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 4.Adachi M, Kitamura K, Miyoshi T, et al. Activation of epithelial sodium channels by prostasin in Xenopus oocytes. J Am Soc Nephrol. 2001;12:1114–1121. doi: 10.1681/ASN.V1261114. [DOI] [PubMed] [Google Scholar]
- 5.Vallet V, Chraibi A, Gaeggeler HP, et al. An epithelial serine protease activates the amiloride-sensitive sodium channe. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- 6.Vuagniaux G, Vallet V, Jaeger NF, et al. Activation of the amiloride-sensitive epithelial sodium channel by the serine protease mCAP1 expressed in a mouse cortical collecting duct cell line. J Am Soc Nephrol. 2000;11:828–834. doi: 10.1681/ASN.V115828. [DOI] [PubMed] [Google Scholar]
- 7.Vuagniaux G, Vallet V, Jaeger NF, et al. Synergistic activation of ENaC by three membrane-bound channel-activating serine proteases (mCAP1, mCAP2 and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus Oocytes. J Gen Physiol. 2002;120:191–201. doi: 10.1085/jgp.20028598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughey RP, Mueller GM, Bruns JB, et al. Maturation of the epithelial Na+ channel involves proteolytic processing of the α- and γ-subunits. J Biol Chem. 2003;278:37073–37082. doi: 10.1074/jbc.M307003200. [DOI] [PubMed] [Google Scholar]
- 9.Hughey RP, Bruns JB, Kinlough CL, et al. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem. 2004;279:18111–18114. doi: 10.1074/jbc.C400080200. [DOI] [PubMed] [Google Scholar]
- 10.Masilamani S, Kim GH, Mitchell C, et al. Aldosteronemediated regulation of ENaC α, β and γ subunit proteins in rat kidney. J Clin Invest. 1999;104:19–23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jean F, Thomas L, Molloy SS, et al. A protein-based therapeutic for human cytomegalovirus infection. Proc Natl Acad Sci USA. 2000;97:2864–2869. doi: 10.1073/pnas.050504297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol. 2004;286:190–194. doi: 10.1152/ajpcell.00342.2003. [DOI] [PubMed] [Google Scholar]
- 14.Bertenshaw GP, Norcum MT, Bond JS. Structure of homo- and hetero-oligomeric meprin metalloproteases. Dimers, tetramers and high molecular mass multimers. J Biol Chem. 2003;278:2522–2532. doi: 10.1074/jbc.M208808200. [DOI] [PubMed] [Google Scholar]
- 15.Bond JS, Beynon RJ. The astacin family of metalloendopeptidases. Protein Sci. 1995;4:1247–1261. doi: 10.1002/pro.5560040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker-Pauly C, Howel M, Walker T, et al. The alpha and beta subunits of the metalloprotease meprin are expressed in separate layers of human epidermis, revealing different functions in keratinocyte proliferation and differentiation. J Invest Dermatol. 2007;127:1115–1125. doi: 10.1038/sj.jid.5700675. [DOI] [PubMed] [Google Scholar]
- 17.Sun Q, Jin HJ, Bond JS. Disruption of the meprin alpha and beta genes in mice alters homeostasis of monocytes and natural killer cells. Exp Hematol. 2009;37:346–356. doi: 10.1016/j.exphem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergin DA, Greene CM, Sterchi EE, et al. Activation of the epidermal growth factor receptor (EGFR) by a novel metalloprotease pathway. J Biol Chem. 2008;283:31736–31744. doi: 10.1074/jbc.M803732200. [DOI] [PubMed] [Google Scholar]
- 19.Bertenshaw GP, Turk BE, Hubbard SJ, et al. Marked differences between metalloproteases meprin A and B in substrate and peptide bond specificity. J Biol Chem. 2001;276:13248–13255. doi: 10.1074/jbc.M011414200. [DOI] [PubMed] [Google Scholar]
- 20.Hengst JA, Bond JS. Transport of meprin subunits through the secretory pathway: role of the transmembrane and cytoplasmic domains and oligomerization. J Biol Chem. 2004;279:34856–34864. doi: 10.1074/jbc.M405774200. [DOI] [PubMed] [Google Scholar]
- 21.Sterchi EE, Stocker W, Bond JS. Meprins, membranebound and secreted astacin metalloproteinases. Mol Aspects Med. 2008;29:309–328. doi: 10.1016/j.mam.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villa JP, Bertenshaw GP, Bylander JE, Bond JS. Saklatvala J, Nagase H, Salvesen G, editors. Handbook of Biochemical Society Symposium. Proteases and the Regulation of Biological Processes. 2003;70:53–63. [Google Scholar]
- 23.Schild L, Schneeberger E, Gautschi I, et al. Identification of amino acid residues in the alpha, beta and gamma subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. J Gen Physiol. 1997;109:15–26. doi: 10.1085/jgp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loffing J, Pietri L, Aregger F, et al. Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol Renal Physiol. 2000;279:252–258. doi: 10.1152/ajprenal.2000.279.2.F252. [DOI] [PubMed] [Google Scholar]
- 25.Amasheh S, Epple HJ, Mankertz J, et al. Differential regulation of ENaC by aldosterone in rat early and late distal colon. Ann NY Acad Sci. 2000;915:92–94. doi: 10.1111/j.1749-6632.2000.tb05227.x. [DOI] [PubMed] [Google Scholar]
- 26.Grunder S, Firsov D, Chang SS, et al. A mutation causing pseudohypoaldosteronism type 1 identifies a conserved glycine that is involved in the gating of the epithelial sodium channel. EMBO J. 1997;16:899–907. doi: 10.1093/emboj/16.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC) J Biol Chem. 2002;277:7641–7644. doi: 10.1074/jbc.C100737200. [DOI] [PubMed] [Google Scholar]
- 28.Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol. 2005;288:813–819. doi: 10.1152/ajplung.00435.2004. [DOI] [PubMed] [Google Scholar]
- 29.Gorbea CM, Marchand P, Jiang W, et al. Cloning, expression and chromosomal localization of the mouse meprin b subunit. J Biol Chem. 1993;268:21035–21043. [PubMed] [Google Scholar]
- 30.Jiang W, Sadler PM, Jenkins NA, et al. Tissue-specific expression and chromosomal localization of the alpha subunit of mouse meprin A. J Biol Chem. 1993;268:10380–10385. [PubMed] [Google Scholar]
- 31.Hahn D, Pischitzis A, Roesmann S, et al. Phorbol 12-myristate 13-acetate-induced ectodomain shedding and phosphorylation of the human meprinbeta metalloprotease. J Biol Chem. 2003;278:42829–42839. doi: 10.1074/jbc.M211169200. [DOI] [PubMed] [Google Scholar]
- 32.Crisman JM, Zhang B, Norman LP, et al. Deletion of the mouse meprin β metalloprotease gene diminishes the ability of leukocytes to disseminate through extracellular matrix. J Immunol. 2004;172:4510–4519. doi: 10.4049/jimmunol.172.7.4510. [DOI] [PubMed] [Google Scholar]
- 33.Guipponi M, Vuagniaux G, Wattenhofer M, et al. The transmembrane serine protease (TMPRSS3) mutated in deafness DFNB8/10 activates the epithelial sodium channel (ENaC) in vitro. Hum Mol Genet. 2002;11:2829–2836. doi: 10.1093/hmg/11.23.2829. [DOI] [PubMed] [Google Scholar]
- 34.Vallet V, Pfister C, Loffing J, et al. Cell-surface expression of the channel activating protease xCAP-1 is required for activation of ENaC in the Xenopus oocyte. J Am Soc Nephrol. 2002;13:588–594. doi: 10.1681/ASN.V133588. [DOI] [PubMed] [Google Scholar]
- 35.Kounnas MZ, Wolz RL, Gorbea CM, et al. Meprin-A and -B. Cell surface endopeptidases of the mouse kidney. J Biol Chem. 1991;266:17350–17357. [PubMed] [Google Scholar]
- 36.Litovchick L, Friedmann E, Shaltiel S. A selective interaction between OS-9 and the carboxyl-terminal tail of meprin beta. J Biol Chem. 2002;277:34413–34423. doi: 10.1074/jbc.M203986200. [DOI] [PubMed] [Google Scholar]
- 37.Baek JH, Mahon PC, Oh J, et al. OS-9 interacts with hypoxia-inducible factor 1α and prolyl hydroxylases to promote oxygen-dependent degradation of HIF-1α. Mol Cell. 2005;17:503–512. doi: 10.1016/j.molcel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Flashman E, McDonough MA, Schofield CJ. OS-9: another piece in the HIF complex story. Mol Cell. 2005;17:472–473. doi: 10.1016/j.molcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Red Eagle AR, Hanson RL, Jiang W, et al. Meprin beta metalloprotease gene polymorphisms associated with diabetic nephropathy in the Pima Indians. Hum Genet. 2005;118:12–22. doi: 10.1007/s00439-005-0019-7. [DOI] [PubMed] [Google Scholar]
- 40.Stutts MJ, Canessa CM, Olsen JC, et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 41.Kunzelmann K, Kathofer S, Hipper A, et al. Culture-dependent expression of Na+ conductances in airway epithelial cells. Pflugers Arch. 1996;431:578–586. doi: 10.1007/BF02191906. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Caballero A, Olivares-Reyes JA, Catt KJ, et al. Angiotensin AT(1) receptor phosphorylation and desensitization in a hepatic cell line. Roles of protein kinase c and phosphoinositide 3-kinase. Mol Pharmacol. 2001;59:576–585. doi: 10.1124/mol.59.3.576. [DOI] [PubMed] [Google Scholar]
- 43.Howell M, Borchers C, Milgram SL. Heterogeneous nuclear ribonuclear protein U associates with YAP and regulates its co-activation of Bax transcription. J Biol Chem. 2004;279:26300–26306. doi: 10.1074/jbc.M401070200. [DOI] [PubMed] [Google Scholar]
- 44.Donaldson SH, Hirsh A, Li DC, et al. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem. 2002;277:8338–8345. doi: 10.1074/jbc.M105044200. [DOI] [PubMed] [Google Scholar]
- 45.Ishmael FT, Norcum MT, Benkovic SJ, et al. Multimeric structure of the secreted meprin A metalloproteinase and characterization of the functional protomer. J Biol Chem. 2001;276:23207–23211. doi: 10.1074/jbc.M102654200. [DOI] [PubMed] [Google Scholar]
- 46.Norman LP, Jiang W, Han X, et al. Targeted disruption of the meprin beta gene in mice leads to underrepresentation of knockout mice and changes in renal gene expression profiles. Mol Cell Biol. 2003;23:1221–1230. doi: 10.1128/MCB.23.4.1221-1230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]