Summary
Background and objectives
Cognitive impairment is common among persons with chronic kidney disease, but the extent to which nontraditional vascular risk factors mediate this association is unclear.
Design, setting, participants, & measurements
We conducted cross-sectional analyses of baseline data collected from adults with chronic kidney disease participating in the Chronic Renal Insufficiency Cohort study. Cognitive impairment was defined as a Modified Mini-Mental State Exam score >1 SD below the mean score.
Results
Among 3591 participants, the mean age was 58.2 ± 11.0 years, and the mean estimated GFR (eGFR) was 43.4 ± 13.5 ml/min per 1.73 m2. Cognitive impairment was present in 13%. After adjustment for demographic characteristics, prevalent vascular disease (stroke, coronary artery disease, and peripheral arterial disease) and traditional vascular risk factors (diabetes, hypertension, smoking, and elevated cholesterol), an eGFR <30 ml/min per 1.73 m2 was associated with a 47% increased odds of cognitive impairment (odds ratio 1.47, 95% confidence interval 1.05, 2.05) relative to those with an eGFR 45 to 59 ml/min per 1.73 m2. This association was attenuated and no longer significant after adjustment for hemoglobin concentration. While other nontraditional vascular risk factors including C-reactive protein, homocysteine, serum albumin, and albuminuria were correlated with cognitive impairment in unadjusted analyses, they were not significantly associated with cognitive impairment after adjustment for eGFR and other confounders.
Conclusions
The prevalence of cognitive impairment was higher among those with lower eGFR, independent of traditional vascular risk factors. This association may be explained in part by anemia.
Introduction
Cognitive impairment has long been recognized as a complication of ESRD (1). Recent studies indicate that cognitive impairment is present at early stages of chronic kidney disease (CKD) (2,3), and several, although not all, suggest that the risk of cognitive decline is dependent on the severity of CKD (4–8). Most previously published studies were limited to the elderly and had limited racial/ethnic diversity or representation of advanced CKD. Thus, less is known about the recent epidemiology of cognitive impairment among young or middle-aged persons with CKD and among persons with advanced CKD who are approaching ESRD.
Persons with CKD have a large burden of clinical and subclinical cerebrovascular disease (9). In the general population traditional vascular risk factors such as diabetes mellitus, hypertension, dyslipidemia, and smoking are associated with a 20 to 40% increased risk for dementia, and these risks appear to be additive (10,11). However, in several studies these factors do not fully account for the high prevalence of cognitive impairment in persons with CKD (5,2,4). For example, in the Cardiovascular Health Study and in the Health, Aging and Body Composition Study, CKD was associated with a 32 to 143% increased risk for incident dementia and cognitive decline, respectively, after adjustment for prevalent vascular disease and traditional vascular risk factors (4,5). Nontraditional vascular risk factors such as inflammation, malnutrition, hyperhomocysteinemia, anemia, and albuminuria are common among persons with CKD and frequently speculated as contributing risk factors for death and cardiovascular disease in CKD, but their role as risk factors for cognitive impairment in persons with CKD has not been rigorously evaluated. We aimed to characterize the association between level of kidney function, traditional and nontraditional vascular risk factors, and cognitive impairment in a large, diverse sample of persons with CKD. We hypothesized that lower estimated GFR (eGFR) would be associated with cognitive impairment independent of traditional vascular risk factors but that the association would be attenuated after accounting for selected nontraditional vascular risk factors.
Materials and Methods
Study Design and Recruitment
The Chronic Renal Insufficiency Cohort (CRIC) Study is a prospective observational study designed to evaluate risk factors for progression of CKD and cardiovascular disease among adults with moderate to advanced CKD. The study design and methods have been previously described (12,13). Briefly, persons aged 21 to 74 years were recruited from seven clinical centers across the United States from July 1, 2003 through December 31, 2006. Participants met age-based eGFR criteria: 20 to 70 ml/min per 1.73 m2 for ages 21 to 44 years, 20 to 60 ml/min per 1.73 m2 for ages 45 to 64 years, and 20 to 50 ml/min per 1.73 m2 for ages 65 to 74 years. The target age distribution was approximately 25% of the sample being aged 21 to 44 years, 50% being aged 45 to 64 years, and 25% being aged 65 to 74 years; 50% were persons of color; and 50% had diabetes. Exclusion criteria included diagnosis of polycystic kidney disease, multiple myeloma, renal carcinoma, HIV infection, cirrhosis, New York Heart Association Class III or IV heart failure, pregnancy, recent immunosuppression for kidney disease, recent chemotherapy for systemic cancer, prior receipt of dialysis or organ transplant, or institutionalization. Institutional review boards at all clinical sites approved the study protocol, and all participants signed informed consent.
Traditional Vascular Risk Factors
At the baseline visit, participants completed questionnaires ascertaining sociodemographic information, medical and family history, medication use, and health behaviors. Height, weight, and BP were recorded by trained study personnel. Diabetes was defined as self-report of diabetes, use of medications for diabetes, or a fasting blood glucose of ≥126 mg/dl. Hypertension was defined as self-report of hypertension, use of medications for high BP, or a seated BP of ≥140/80 mmHg. History of elevated cholesterol was defined by self-report or use of medications for elevated cholesterol. Smoking was defined as current or previous history of smoking cigarettes. Coronary heart disease was defined as self-report of a myocardial infarction, angina, coronary artery bypass grafting, or percutaneous coronary intervention procedure. Cerebrovascular disease was defined as self-report of a stroke. Peripheral arterial disease was defined as self-report of claudication, amputation, or revascularization procedure of the extremities.
Kidney Function and Nontraditional Vascular Risk Factors
Blood was drawn at baseline in the fasting state for measurement of serum creatinine, glucose, lipoproteins, albumin, C-reactive protein (CRP), homocysteine, and a complete blood count. Serum creatinine was measured at a central study laboratory using the Jaffe rate method. Estimated GFR was calculated using the four-variable Modification of Diet in Renal Disease study equation (14). Total cholesterol was assayed on a Hitachi 912 analyzer using Roche reagents. High sensitivity C-reactive protein was assayed on the Siemens BNII Nephelometer. Homocysteine was assayed by fluorescence polarization on the Abbott Axsym using Abbott Reagent. Urine albumin and creatinine were measured on spot urine samples using a Siemens Immulite.
Assessment of Cognitive Function and Definition of Outcome
Cognitive function was assessed with the Modified Mini-Mental State Exam (3MS) (15). The 3MS is a test of global cognitive function with components for concentration, orientation, language, praxis, and memory. Scores on the 3MS range from 0 to 100, with higher scores denoting better cognitive function. Cognitive impairment was defined as a 3MS score >1 SD below the mean; this cut-point has a sensitivity of 92% and a specificity of 87% for a diagnosis of dementia in older individuals (16). In sensitivity analyses, we also defined cognitive impairment as a 3MS score <80 (5).
Statistical Analyses
The continuous variables were expressed as the means ± SD and compared using ANOVA. Categorical variables were expressed as proportions and compared using the chi-squared test. We first evaluated the distribution of 3MS scores and the association between eGFR and 3MS scores graphically. For subsequent analyses, eGFR was analyzed as a continuous variable and as a categorical variable using a modification of National Kidney Foundation strata (≥60, 45 to 59, 30 to 44, and <30 ml/min per 1.73 m2).
We used logistic regression to determine the association, expressed as an odds ratio (OR) and 95% confidence interval (CI), between eGFR and cognitive impairment, using individuals with an eGFR 45 to 59 ml/min per 1.73 m2 as the referent group because individuals in the eGFR ≥60 ml/min per 1.73 m2 group were substantially younger, as expected, due to age-based eGFR entry criteria. Because prevalent vascular disease may act as both a confounder and a mediator of cognitive impairment in persons with CKD, we tested the association of eGFR with cognitive impairment in (1) unadjusted models, (2) parsimonious models including demographic characteristics (age, sex, race, ethnicity, education, and CRIC clinical site) and stroke, and (3) fully adjusted multivariable models including variables in the parsimonious model plus traditional vascular risk factors (diabetes, hypertension, smoking, history of elevated cholesterol, coronary heart disease, and peripheral arterial disease). We also tested interaction terms for age, sex, race, ethnicity, diabetes status, and stroke by eGFR.
Next, we evaluated whether nontraditional vascular risk factors were independently associated with cognitive impairment and whether they helped to explain the association of eGFR with cognitive impairment. We first determined the association between nontraditional vascular risk factors and cognitive impairment in unadjusted models and in models adjusted for demographic characteristics and traditional vascular risk factors. Then, starting with the parsimonious eGFR model described above, we used stepwise forward logistic regression to test whether any traditional vascular risk factors remained significant in multivariable analyses. We then added nontraditional vascular risk factors to the resulting model using stepwise forward regression to determine whether any of these measures remained significant in multivariable analyses. Candidate variables in these analyses included hemoglobin, serum albumin, log (CRP), homocysteine (per 1 mg/dl increase), and albuminuria (<30, 30 to 299, 300 to 999, and ≥1000 mg/d), as well as measured BP and total cholesterol (the latter were evaluated in this model to avoid co-linearity with the dichotomous variables hypertension and history of elevated cholesterol). Analyses were conducted using SAS v9.1 (Cary, NC).
Results
Subject Characteristics
There were 3612 participants recruited into the CRIC cohort. Of these, 21 were missing 3MS scores; therefore the final analytic cohort consisted of 3591 participants. The mean age was 58.2 ± 11.0 years, and the mean eGFR was 43.4 ± 13.5 ml/min per 1.73 m2. Subject characteristics stratified by eGFR are shown in Table 1. Those with a lower eGFR were older, more likely to be women, and more likely to have vascular disease and traditional vascular risk factors. Those with a lower eGFR also had lower levels of hemoglobin and serum albumin and higher levels of CRP, homocysteine, and albuminuria.
Table 1.
Characteristics of CRIC participants, stratified by baseline eGFR (in ml/min per 1.73 m2)
| eGFR <30 (n = 665) | eGFR 30 to <45 (n = 1326) | eGFR 45 to <60 (n = 1200) | eGFR ≥60 (n = 400) | P | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| age (years) | 58.6 (11.3) | 59.7 (10.7) | 58.6 (10.4) | 51.5 (11.0) | <0.01 |
| male | 311 (46.8%) | 703 (53%) | 709 (59.1%) | 228 (57%) | <0.01 |
| non-white | 341 (51.3%) | 661 (49.8%) | 597 (49.8%) | 230 (57.5%) | 0.2 |
| Hispanic | 54 (8.1%) | 57 (4.3%) | 39 (3.3%) | 18 (4.5%) | <0.01 |
| education | <0.01 | ||||
| less than high school | 152 (22.9%) | 262 (19.8%) | 152 (12.7%) | 32 (8.0%) | |
| high school graduate | 143 (21.5%) | 279 (21.0%) | 200 (16.7%) | 70 (17.5%) | |
| some college | 197 (29.6%) | 395 (29.8%) | 369 (30.8%) | 139 (34.8%) | |
| college graduate | 173 (26.0%) | 390 (29.4%) | 479 (39.9%) | 158 (39.6%) | |
| Prevalent conditions | |||||
| diabetes | 349 (20.9%) | 691 (41.4%) | 500 (29.9%) | 131 (7.8%) | <0.01 |
| hypertension | 612 (92.0%) | 1201 (90.6%) | 996 (83.1%) | 268 (67.2%) | <0.01 |
| history of elevated cholesterol | 583 (87.7%) | 1114 (84%) | 957 (79.8%) | 271 (67.8%) | <0.01 |
| smoking (ever vs. never) | 405 (60.9%) | 758 (57.2%) | 655 (54.6%) | 191 (47.8%) | <0.01 |
| stroke | 78 (11.7%) | 147 (11.1%) | 120 (10.0%) | 18 (4.5%) | <0.01 |
| coronary heart disease | 169 (25.4%) | 324 (24.4%) | 257 (21.4%) | 52 (13.0%) | <0.01 |
| peripheral arterial disease | 74 (11.1%) | 103 (7.8%) | 60 (5.0%) | 11 (2.8%) | <0.01 |
| Anthropometric and laboratory measures | |||||
| eGFR (ml/min per 1.73 m2) | 25.0 (3.4) | 37.9 (4.2) | 51.7 (4.2) | 67.7 (7.6) | – |
| systolic blood pressure (mmHg) | 130.5 (23.6) | 129.0 (22.5) | 126.0 (20.6) | 123.5 (20.0) | <0.01 |
| diastolic blood pressure (mmHg) | 69.9 (13.3) | 70.8 (12.7) | 71.7 (12.5) | 74.8 (12.8) | <0.01 |
| total cholesterol (mg/dl) | 183.4 (51.7) | 182.9 (44.7) | 183.0 (41.8) | 183.52 (39.2) | 0.9 |
| hemoglobin (g/dl) | 11.9 (1.7) | 12.5 (1.7) | 13.1 (1.7) | 13.4 (1.6) | <0.01 |
| serum albumin (g/dl) | 3.9 (0.5) | 3.9 (0.5) | 4.0 (0.4) | 4.1 (0.4) | <0.01 |
| log C-reactive protein | 1.1 (1.3) | 1.1 (1.3) | 0.9 (1.2) | 0.6 (1.2) | <0.01 |
| total homocysteine (μmol/L) | 19.0 (6.9) | 15.9 (5.8) | 13.3 (5.0) | 10.9 (4.0) | <0.01 |
| albuminuria (mg/day) | <0.01 | ||||
| <30 mg/day | 123 (8.7%) | 450 (31.9%) | 599 (42.4%) | 240 (17%) | |
| 30 to 299 mg/day | 200 (32.2%) | 364 (28.7%) | 302 (26.4%) | 79 (21.4%) | |
| 300 to 999 mg/day | 121 (19.5%) | 227 (17.9%) | 141 (12.3%) | 30 (8.1%) | |
| ≥1000 mg/day | 178 (28.6%) | 226 (17.8%) | 101 (8.8%) | 20 (5.4%) |
The results are presented as the means (standard deviation) or frequency (N, %).
Association of eGFR with Cognitive Impairment
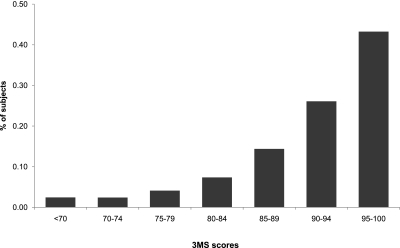
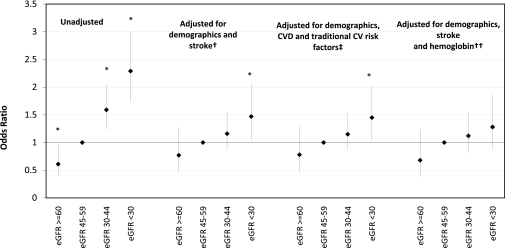
The distribution of 3MS scores among the 3591 CRIC participants is shown in Figure 1. The mean 3MS score was 92 ± 8. There were 343 participants (9.6%) who received a score of 100, the test maximum, and 453 participants (12.6%) who received a score more than 1 SD below the mean, meeting the definition for cognitive impairment. Compared with participants who had eGFR 45 to 59 ml/min per 1.73 m2, there were lower odds of cognitive impairment among participants with eGFR ≥60 ml/min per 1.73 m2 (OR 0.61, 95% CI 0.39, 0.97) and increased odds of cognitive impairment among participants with an eGFR 30 to 44 ml/min per 1.73 m2 (OR 1.59, 95% CI 1.24, 2.04) and participants with an eGFR <30 ml/min per 1.73 m2 (OR 2.29, 95% CI 1.74, 3.01) in unadjusted analyses (Figure 2). A test for linear trend across eGFR strata was significant (P < 0.001). These associations were attenuated but remained significant for participants with eGFR <30 ml/min per 1.73 m2 after adjustment for demographic characteristics and stroke (for eGFR <30 ml/min per 1.73 m2, OR 1.47, 95% CI 1.05, 2.05). Additional adjustment for diabetes, hypertension, smoking, elevated cholesterol, coronary heart disease, and peripheral arterial disease did not significantly affect the results compared with the demographics adjusted model (for eGFR <30 ml/min per 1.73 m2, OR 1.45, 95% CI 1.04, 2.03).
Figure 1.
Distribution of Modified Mini-Mental State Exam (3MS) scores among CRIC participants.
Figure 2.
Association of estimated GFR with cognitive impairment in unadjusted and adjusted models among 3591 CRIC participants. The bars indicate 95% confidence intervals. Referent category is eGFR 45 to 59 ml/min per 1.73 m2. An asterisk indicates P < 0.05. P for linear trend across eGFR strata = <0.0001, 0.059, 0.063, and 0.052 in the unadjusted, demographics adjusted, traditional vascular risk factor adjusted, and fully adjusted models, respectively. CVD, cardiovascular disease; CV, cardiovascular. †Model adjusted for eGFR, age, sex, race, ethnicity, education, stroke, and CRIC clinical site. ‡Model adjusted for eGFR, age, sex, race, ethnicity, education, stroke, diabetes, hypertension, smoking, history of elevated cholesterol, coronary heart disease, peripheral arterial disease, and CRIC clinical site. ††Model adjusted for eGFR, age, sex, race, ethnicity, education, stroke, hemoglobin, and CRIC clinical site.
When eGFR was analyzed as a continuous variable, each 10 ml/min per 1.73 m2 decrease in eGFR was associated with a 12% higher odds for cognitive impairment in the fully adjusted multivariable model (OR 1.12, 95% CI 1.01, 1.24). This association was not significantly modified by age, sex, race, ethnicity, diabetes status, and stroke (P for interaction terms was NS). In sensitivity analyses, there were 292 individuals with a 3MS <80 (8.1%). Using this cut-point, in the fully adjusted model each 10 ml/min per 1.73 m2 decrease in eGFR was associated with 10% higher odds for cognitive impairment (OR 1.10, 95% CI 0.97 to 1.23).
eGFR, Vascular Risk Factors, and Cognitive Impairment
In addition to eGFR, prevalent vascular disease and several traditional vascular risk factors were associated with higher odds of cognitive impairment in unadjusted analyses (Table 2). A number of nontraditional vascular risk factors were also associated with higher odds of cognitive impairment, including lower hemoglobin, lower serum albumin, higher log CRP, higher homocysteine, and higher levels of albuminuria; however, only hemoglobin remained associated with cognitive impairment after adjustment for other risk factors (OR 1.08, 95% CI 0.99, 1.17; Table 2). In a stepwise forward logistic regression model that included eGFR and demographic characteristics, stroke was the only traditional vascular risk factor that remained significantly associated with cognitive impairment. In a stepwise forward logistic regression model that included eGFR, demographic characteristics, stroke, and nontraditional vascular risk factors, hemoglobin was the only nontraditional risk factor that remained significantly correlated with cognitive impairment. Each 1 g/dl decrease in hemoglobin was associated with a 9% increased odds of cognitive impairment (OR 1.09, 95% CI 1.01, 1.18). Adjustment for hemoglobin attenuated the significant association of the lowest eGFR strata with cognitive impairment such that the association was no longer significant (Table 2), although a test for linear trend across eGFR categories remained of borderline significance (P = 0.05; Figure 2).
Table 2.
Association of eGFR and vascular risk factors with cognitive impairment (Modified Mini-Mental State Exam >1 SD below mean)
| Characteristic | Odds Ratio and 95% Confidence Interval |
||||
|---|---|---|---|---|---|
| Unadjusted | Adjusted for Demographics and Vascular Risk Factors | Adjusted for eGFR, Demographics, and Stroke | Adjusted for eGFR, Demographics, and Traditional Vascular Risk Factors | Fully Adjusted Model | |
| eGFR (ml/min per 1.73 m2) | |||||
| ≥60 | 0.61 (0.39, 0.97) | 0.77 (0.46, 1.28) | 0.78 (0.47, 1.30) | 0.68 (0.38, 1.24) | |
| 45 to 59 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | |
| 30 to 44 | 1.59 (1.24, 2.04) | 1.16 (0.87, 1.55) | 1.15 (0.86, 1.53) | 1.12 (0.82, 1.54) | |
| <30 | 2.29 (1.74, 3.01) | 1.47 (1.05, 2.05) | 1.46 (1.04, 2.04) | 1.28 (0.88, 1.86) | |
| Age (per 10 years) | 1.37 (1.24, 1.52) | 1.37 (1.19, 1.59) | 1.41 (1.24, 1.60) | 1.41 (1.24, 1.60) | 1.31 (1.14, 1.50) |
| Male (versus female) | 0.99 (0.81, 1.20) | 1.48 (1.12, 1.97) | 1.29 (1.02, 1.64) | 1.38 (1.08, 1.77) | 1.48 (1.13, 1.95) |
| Non-white (versus white) | 4.21 (3.32, 5.33) | 3.89 (2.76, 5.47) | 4.27 (3.12, 5.82) | 4.19 (3.06, 5.74) | 4.04 (2.88, 5.68) |
| Hispanic (versus non-Hispanic) | 4.41 (3.16, 6.14) | 4.38 (2.65, 7.26) | 5.05 (3.21, 7.94) | 4.63 (2.92, 7.34) | 4.76 (2.90, 7.82) |
| Education | |||||
| less than high school | 3.40 (2.62, 4.41) | 2.37 (1.72, 3.25) | 2.12 (1.59, 2.82) | 2.19 (1.65, 2.93) | 2.29 (1.67, 3.12) |
| high school graduate | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| some college | 0.37 (0.27, 0.51) | 0.39 (0.27, 0.56) | 0.38 (0.27, 0.53) | 0.38 (0.27, 0.53) | 0.39 (0.27, 0.56) |
| college graduate | 0.13 (0.09, 0.20) | 0.19 (0.12, 0.30) | 0.18 (0.12, 0.29) | 0.18 (0.11, 0.27) | 0.20 (0.12, 0.31) |
| Stroke | 2.29 (1.75, 2.99) | 1.91 (1.35, 2.70) | 1.64 (1.20, 2.24) | 1.62 (1.18, 2.22) | 1.97 (1.40, 2.78) |
| Diabetes | 1.83 (1.50, 2.24) | 1.01 (0.77, 1.34) | 1.09 (0.86, 1.39) | ||
| Hypertension | 3.62 (2.33, 5.61) | 1.07 (0.64, 1.80) | 1.16 (0.71, 1.91) | ||
| Elevated cholesterol | 1.33 (1.01, 1.75) | 1.15 (0.80, 1.65) | 1.09 (0.79, 1.52) | ||
| Smoking (ever versus never) | 1.07 (0.88, 1.31) | 0.68 (0.52, 0.89) | 0.65 (0.51, 0.84) | ||
| Coronary heart disease | 1.46 (1.17, 1.82) | 1.03 (0.76, 1.39) | 1.09 (0.83, 1.44) | ||
| Peripheral arterial disease | 2.00 (1.45, 2.76) | 1.06 (0.69, 1.64) | 1.04 (0.70, 1.54) | ||
| Systolic blood pressure (per 10 mmHg increase) | 1.23 (1.18, 1.28) | ||||
| Diastolic blood pressure (per 10 mmHg increase) | 1.00 (0.92, 1.08) | ||||
| Total cholesterol (per 10 mg/dl increase) | 0.99 (0.97, 1.01) | ||||
| Hemoglobin (per g/dl decrease) | 1.32 (1.23, 1.39) | 1.08 (0.99, 1.17) | 1.09 (1.01, 1.18) | ||
| Log C-reactive protein | 1.18 (1.09, 1.27) | 1.05 (0.95, 1.16) | |||
| Serum albumin (per g/dl decrease) | 1.79 (1.47, 2.22) | 1.13 (0.81, 1.56) | |||
| Total homocysteine (per μmol/L increase) | 1.04 (1.03, 1.06) | 1.00 (0.99, 1.02) | |||
| Albuminuria | |||||
| <30 mg/day | 1.00 (Referent) | 1.00 (Referent) | |||
| 30 to 299 mg/day | 1.67 (1.29, 2.17) | 1.25 (0.90, 1.74) | |||
| 300 to 999 mg/day | 2.01 (1.49, 2.72) | 1.22 (0.82, 1.79) | |||
| ≥1000 mg/day | 2.19 (1.63, 2.93) | 1.16 (0.76, 1.78) | |||
Note that adjusted models include CRIC clinical site in addition to the variables shown.
Discussion
Understanding the epidemiology of cognitive impairment in persons with CKD is important when considering the development and implementation of effective prevention and treatment strategies. Our findings demonstrate that among persons with CKD, the prevalence of cognitive impairment increases linearly as eGFR declines and that this association is consistent across age, sex, race, and other clinically important patient groups. The magnitude of the association, an increase in prevalence of approximately 12% for each 10 ml/min per 1.73 m2 decrease in eGFR, is comparable with or larger than that of other potentially modifiable risk factors for cognitive impairment, such as BP (17) or hyperglycemia in persons with diabetes (18). The association of eGFR with cognitive impairment was independent of prevalent known vascular disease and several traditional vascular risk factors; however, the association was attenuated and no longer significant after additional adjustment for hemoglobin, suggesting that anemia may be an important risk marker for cognitive impairment among individuals with CKD.
The findings from this study confirm and extend several previous reports indicating an association between CKD and the prevalence of cognitive impairment (3,2), the risk of cognitive decline (5,8), and the risk of incident dementia (4) in community samples of mostly older adults by demonstrating a robust association between eGFR and cognitive impairment in a racially and ethnically diverse sample with a broad age range and a wide spectrum of CKD severity. Thus, our results should be broadly generalizable to patients with CKD. In contrast to most previous studies, which had limited representation of individuals with stage 4 CKD, 19% of our study sample had an eGFR <30 ml/min per 1.73 m2; thus, we were able to assess whether there were nonlinear associations between eGFR and cognitive impairment. In contrast to our findings, a few studies have reported no association between eGFR and cognitive impairment. For example, in a study of 5529 community-dwelling elderly men, there was no association between eGFR and prevalent cognitive impairment or cognitive decline using the 3MS to assess cognitive function at baseline and follow-up (6). In a smaller study of frail elderly, albuminuria, but not eGFR, was associated with poorer performance on several cognitive measures (7). These negative findings may be explained by a low prevalence of CKD in these cohorts.
There is growing awareness that persons with CKD have a substantial burden of subclinical cerebrovascular disease, which may in turn contribute to cognitive impairment. Persons with CKD have more brain white matter lesions, lacunar infarcts, and subcortical atrophy, all markers of cerebral small vessel disease, even in the absence of clinical stroke (9,19,20). These lesions are strongly associated with risk for cognitive impairment and dementia in the general population (21,22). In this study, while a self-reported history of stroke was independently associated with higher odds of cognitive impairment, traditional vascular risk factors and other prevalent vascular diseases were not significantly associated with cognitive impairment after adjusting for demographic factors and eGFR. This may suggest that eGFR is a marker of brain vascular disease that reflects the severity of vascular injury. Alternatively, CKD may lead to cerebrovascular disease and impaired cognitive function through nontraditional vascular risk factors. We evaluated several nontraditional vascular risk markers in this study: hemoglobin, serum albumin, CRP, homocysteine, and albuminuria. Only hemoglobin remained significantly correlated with cognitive impairment after multivariable adjustment. The lack of a significant association between homocysteine and cognitive impairment in adjusted models is consistent with the results of a recent negative trial of homocysteine lowering in patients with CKD (23). The absence of a significant association between albuminuria and cognitive impairment in this study is in contrast to previous studies (7,24,25). This observation may indicate that, in persons with more advanced CKD, albuminuria does not add additional predictive information after accounting for eGFR.
Anemia has been linked with cognitive impairment in the general population (26) and in persons with ESRD (27,28). Furthermore, in uncontrolled studies of patients receiving hemodialysis, amelioration of severe anemia with erythropoietin is associated with improvement in cognitive function (29,30). In this study, low hemoglobin levels were significantly correlated with cognitive impairment and attenuated the association of eGFR with cognitive impairment. Taken together, these findings may suggest that anemia mediates, in part, the observed association of CKD with cognitive impairment. The mechanisms by which anemia might directly contribute to cognitive impairment have not been elucidated. Chronic anemia may cause subclinical cerebral ischemia, particularly in the setting of pre-existing cerebrovascular disease. Up-regulation of cellular mechanisms to maintain cerebral oxygen delivery may also have deleterious effects. For example, erythropoietin may promote vascular thrombosis, nitric-oxide synthase leads to vasodilation but may also increase reactive oxygen species, and vascular endothelial growth factor increases angiogenesis but may lead to disruption of the blood-brain barrier (31). To our knowledge, cognitive function was not assessed in recent trials of erythropoietin to correct anemia in persons with CKD (32,33); thus, it remains unclear whether anemia is a mediator or marker of other conditions, such as inflammation or nutritional deficiencies, which may contribute to cognitive impairment. In the absence of data from controlled studies, it is difficult to determine what effect recent calls for more restricted use of erythropoiesis-stimulating agents might have on the burden of cognitive impairment in this population (34).
Our study has several strengths, including a large, racially diverse sample with a wide age range and increased representation of young adults and persons with advanced CKD compared with previous studies and the systematic measurement of kidney function, cognitive function, and multiple nontraditional vascular risk factors. There are also several limitations. First, we used a single measure (i.e. 3MS) to evaluate cognitive function. While this test has been extensively validated, it does not assess executive function, which is more strongly linked with vascular causes of cognitive impairment. Second, individuals with normal screening eGFR were, by definition, excluded from CRIC. This may have led us to underestimate the magnitude of association between eGFR and cognitive impairment. Third, we lacked information on other factors such as vitamin D deficiency, which may also be associated with cognitive impairment (35,36). Finally, these analyses were cross-sectional and therefore causality cannot be determined. Ongoing longitudinal substudies within CRIC should further delineate the association between impaired kidney function and cognition and whether cognitive decline might be prevented by slowing the progression of CKD.
Conclusions
In sum, among a large diverse sample of adults with CKD, we demonstrated that lower eGFR is associated with cognitive impairment independent of traditional vascular risk factors and that this association is attenuated after adjustment for hemoglobin level. Given the burden of cognitive impairment in persons with CKD and its potential adverse effect on patient outcomes, intervention trials targeting CKD progression or correction of CKD-associated anemia should include careful evaluation of cognitive function as a primary or secondary end point.
Disclosures
Dr. Kurella Tamura has previously received grant support from Amgen.
Acknowledgments
The CRIC Study is supported by cooperative agreement project grants 5U01DK060990, 5U01DK060984, 5U01DK06102, 5U01DK061021, 5U01DK061028, 5U01DK60980, 5U01DK060963, and 5U01DK060902 from the National Institute of Diabetes and Digestive and Kidney Diseases and by grants UL1RR024134, UL1RR025005, M01RR16500, UL1RR024989, M01RR000042, UL1RR024986, UL1RR029879, RR05096, and UL1RR024131 from the National Institutes of Health. Dr. Kurella Tamura received support from the National Institute of Aging (grant K23AG028952). Dr. Yaffe was supported by R01DK069406, also from the National Institute of Diabetes and Digestive and Kidney Diseases. These results were presented in abstract form at the American Society of Nephrology Meeting.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Teschan PE: Electroencephalographic and other neurophysiological abnormalities in uremia. Kidney Int Suppl: 210–216, 1975 [PubMed] [Google Scholar]
- 2. Kurella Tamura M, Wadley V, Yaffe K, McClure LA, Howard G, Go R, Allman RM, Warnock DG, McClellan W: Kidney function and cognitive impairment in US adults: The reasons for geographic and racial differences in stroke (REGARDS) Study. Am J Kidney Dis 52: 227–234, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hailpern SM, Melamed ML, Cohen HW, Hostetter TH: Moderate Chronic Kidney Disease and Cognitive Function in Adults 20 to 59 Years of Age: Third National Health and Nutrition Examination Survey (NHANES III). J Am Soc Nephrol 18: 2205–2213, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Seliger SL, Siscovick DS, Stehman-Breen CO, Gillen DL, Fitzpatrick A, Bleyer A, Kuller LH: Moderate renal impairment and risk of dementia among older adults: The cardiovascular health cognition study. J Am Soc Nephrol 15: 1904–1911, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, Satterfield S, Ayonayon H, Yaffe K: Chronic kidney disease and cognitive impairment in the elderly: The health, aging, and body composition study. J Am Soc Nephrol 16: 2127–2133, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Slinin Y, Paudel ML, Ishani A, Taylor BC, Yaffe K, Murray AM, Fink HA, Orwoll ES, Cummings SR, Barrett-Connor E, Jassal S, Ensrud KE: Kidney function and cognitive performance and decline in older men. J Am Geriatr Soc 56: 2082–2088, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiner DE, Bartolomei K, Scott T, Price LL, Griffith JL, Rosenberg I, Levey AS, Folstein MF, Sarnak MJ: Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis 53: 438–447, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khatri M, Nickolas T, Moon YP, Paik MC, Rundek T, Elkind MS, Sacco RL, Wright CB: CKD associates with cognitive decline. J Am Soc Nephrol 20: 2427–2432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seliger SL, Longstreth WT, Jr., Katz R, Manolio T, Fried LF, Shlipak M, Stehman-Breen CO, Newman A, Sarnak M, Gillen DL, Bleyer A, Siscovick DS: Cystatin C and subclinical brain infarction. J Am Soc Nephrol 16: 3721–3727, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Elias MF, Sullivan LM, D'Agostino RB, Elias PK, Beiser A, Au R, Seshadri S, DeCarli C, Wolf PA: Framingham stroke risk profile and lowered cognitive performance. Stroke 35: 404–409, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K: Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64: 277–281, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT: The chronic renal insufficiency cohort (CRIC) study: Design and methods. J Am Soc Nephrol 14: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI: Chronic renal insufficiency cohort (CRIC) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation: Modification of diet in renal disease study group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Teng EL, Chui HC: The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 48: 314–318, 1987 [PubMed] [Google Scholar]
- 16. Hayden KM, Khachaturian AS, Tschanz JT, Corcoran C, Nortond M, Breitner JC: Characteristics of a two-stage screen for incident dementia. J Clin Epidemiol 56: 1038–1045, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, Chalmers J: Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 163: 1069–1075, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Cukierman-Yaffe T, Gerstein HC, Williamson JD, Lazar RM, Lovato L, Miller ME, Coker LH, Murray A, Sullivan MD, Marcovina SM, Launer LJ: Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: The action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care 32: 221–226, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ikram MA, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, Breteler MM: Kidney function is related to cerebral small vessel disease. Stroke 39: 55–61, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi S, Ikeda T, Moriya H, Ohtake T, Kumagai H: Asymptomatic cerebral lacunae in patients with chronic kidney disease. Am J Kidney Dis 44: 35–41, 2004 [DOI] [PubMed] [Google Scholar]
- 21. DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Carmelli D: Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute twin study. Arch Neurol 58: 643–647, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, Fitzpatrick A, Fried L, Haan MN: Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology 22: 13–22, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Brady CB, Gaziano JM, Cxypoliski RA, Guarino PD, Kaufman JS, Warren SR, Hartigan P, Goldfarb DS, Jamison RL: Homocysteine lowering and cognition in CKD: The Veterans Affairs homocysteine study. Am J Kidney Dis 54: 440–449, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barzilay JI, Fitzpatrick AL, Luchsinger J, Yasar S, Bernick C, Jenny NS, Kuller LH: Albuminuria and dementia in the elderly: A community study. Am J Kidney Dis 52: 216–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vupputuri S, Shoham DA, Hogan SL, Kshirsagar AV: Microalbuminuria, peripheral artery disease, and cognitive function. Kidney Int 73: 341–346, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Denny SD, Kuchibhatla MN, Cohen HJ: Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med 119: 327–334, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, Smith GE, Hochhalter AK, Collins AJ, Kane RL: Cognitive impairment in hemodialysis patients is common. Neurology 67: 216–223, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Kurella M, Mapes DL, Port FK, Chertow GM: Correlates and outcomes of dementia among dialysis patients: The dialysis outcomes and practice patterns study. Nephrol Dial Transplant 21: 2543–2548, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Grimm G, Stockenhuber F, Schneeweiss B, Madl C, Zeitlhofer J, Schneider B: Improvement of brain function in hemodialysis patients treated with erythropoietin. Kidney Int 38: 480–486, 1990 [DOI] [PubMed] [Google Scholar]
- 30. Marsh JT, Brown WS, Wolcott D, Carr CR, Harper R, Schweitzer SV, Nissenson AR: rHuEPO treatment improves brain and cognitive function of anemic dialysis patients. Kidney Int 39: 155–163, 1991 [DOI] [PubMed] [Google Scholar]
- 31. Hare GM, Tsui AK, McLaren AT, Ragoonanan TE, Yu J, Mazer CD: Anemia and cerebral outcomes: Many questions, fewer answers. Anesth Analg 107: 1356–1370, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R: A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Unger EF, Thompson AM, Blank MJ, Temple R: Erythropoiesis-stimulating agents: Time for a reevaluation. N Engl J Med 362: 189–192, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Slinin Y, Paudel ML, Taylor BC, Fink HA, Ishani A, Canales MT, Yaffe K, Barrett-Connor E, Orwoll ES, Shikany JM, Leblanc ES, Cauley JA, Ensrud KE: 25-Hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology 74: 33–41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Annweiler C, Schott AM, Allali G, Bridenbaugh SA, Kressig RW, Allain P, Herrmann FR, Beauchet O: Association of vitamin D deficiency with cognitive impairment in older women: cross-sectional study. Neurology 74: 27–32, 2010 [DOI] [PubMed] [Google Scholar]