Summary
Background and Objectives
Obesity and metabolic syndrome (MS) increase the risk of cardiovascular disease (CVD), chronic kidney disease (CKD), and all-cause mortality. Serum cystatin C (S-CysC), a marker of GFR, has been shown to be associated with CVD and CKD. This study was designed to elucidate the association of urinary CysC (U-CysC), a marker of renal tubular dysfunction, with CVD and CKD risk factors in patients with obesity and MS.
Design, setting, participants, & measurements
The U-CysC-creatinine ratio (UCCR) was examined in 343 Japanese obese outpatients enrolled in the multi-centered Japan Obesity and Metabolic Syndrome Study.
Results
UCCR was positively correlated with urine albumin-creatinine ratio (UACR) and S-CysC and negatively correlated with estimated GFR (eGFR). Among obese patients, UCCR was significantly higher in MS patients than in non-MS patients. UCCR had significant correlations with the number of components of MS and arterial stiffness, all of which are CVD predictors, similarly to UACR (P < 0.05). Interestingly, diet- and exercise-induced weight reduction for 3 months significantly decreased only UCCR among all of the renal markers examined (P < 0.01), in parallel with the decrease in BMI, HbA1c, and arterial stiffness, suggesting the beneficial effect of weight reduction on renal tubular dysfunction.
Conclusions
This study demonstrates that UCCR is significantly associated with renal dysfunction, the severity of MS, arterial stiffness, and weight change in obese patients. The data of this study suggest that U-CysC could serve as a CVD and CKD risk factor in patients with obesity and MS.
Introduction
Obesity and metabolic syndrome (MS), a cluster of multiple risk factors for atherosclerosis such as obesity, elevated BP, elevated glucose, and atherogenic dyslipidemia, increase the risk of all-cause mortality and cardiovascular morbidity and mortality (1,2). Chronic kidney disease (CKD), which is defined as renal damage or GFR < 60 ml/min per 1.73 m2 for at least 3 months (3), is also known to be an independent risk for cardiovascular diseases (CVD) (4,5). Recent epidemiologic studies revealed a close association of MS and obesity with CKD (6–8). It is speculated that CVD and CKD share common pathophysiologic bases involving metabolic abnormalities, endothelial dysfunction, oxidative stress, and chronic inflammation (9). However, conventional CVD risk factors underestimate the risk of CVD in patients with CKD, implying novel mechanisms linking CVD and CKD (10). It is, therefore, important to identify new biomarkers to evaluate the progression of CVD and CKD in patients with obesity and MS.
Microalbuminuria is an established biomarker that reflects the decline in GFR and a predictive and independent biomarker for all-cause mortality and CVD events (11,12). Cystatin C (CysC), a 13-kD endogenous cysteine proteinase inhibitor, is ubiquitously expressed, filtrated freely by the glomeruli, and reabsorbed by the proximal tubules (13,14). Serum CysC (S-CysC) is also a sensitive marker for detecting reduced GFR and is a stronger predictor of the risk of death and cardiovascular events in elderly persons than serum creatinine (14–16). On the other hand, the level of urinary CysC level (U-CysC) has been recognized as a marker of renal tubular dysfunction (17–19). Recently, several studies have provided evidence for the involvement of renal tubular dysfunction as well as glomerulopathy in the CKD progression in diabetic patients with macrovascular diseases (20,21). However, it is unknown whether renal tubular dysfunction is associated with the development of renal damage and CKD in obesity and MS. Moreover, whether U-CysC is associated with the conventional risk factors of CVD and CKD in patients with obesity and MS has never been addressed.
The Japan Obesity and Metabolic Syndrome Study (JOMS) is a prospective and cross-sectional multi-center study that involves several National Hospital Organization hospitals in Japan. In a series of previous studies, we demonstrated that as a new atherogenic index, cardio-ankle vascular index (CAVI) and serum amyloid A-oxidized LDL are useful for the evaluation and management of the CVD risks in patients with obesity and MS (22,23). We also reported that the markers are sensitive for detecting improvement of CVD risks during weight reduction. Thus, JOMS would provide unique opportunities to investigate the pathophysiology of obesity-related renal dysfunction by cross-sectional and prospective designs.
In this study, we examined the relationships of U-CysC with CVD and CKD risk factors and the significance of renal tubular dysfunction in the progression of CVD and CKD in obese patients. Among the obese patients, we compared the U-CysC levels by classifying the patients as either having MS (MS group) or not (non-MS group) or by stratifying according to the number of MS traits. Because weight reduction improves glomerular hemodynamics and reduces urine albumin excretion (24–26), we also studied the effect of weight-reduction therapy on U-CysC. Here, we show a significant correlation of U-CysC with CVD and CKD risk factors in obese patients. Interestingly, diet- and exercise-induced weight reduction effectively suppressed the increased U-CysC in obese patients. The data of this study suggest that U-CysC may serve as a potential biomarker that reflects the progression of CVD and CKD in patients with obesity and MS.
Materials and Methods
Subjects
A total of 343 Japanese obese outpatients (152 men and 191 women; mean age, 52.1 years) were consecutively enrolled in a multi-center study (JOMS), which involved five National Hospital Organization hospitals (Kyoto, Tokyo, Nagoya, and Kokura Medical Centers and Mie Hospital) and the Oishi Clinic in Japan as part of a study conducted by the Policy Based Medical Service Network for Endocrine and Metabolic Diseases during the period from October 2005 to March 2007 (22,23). Each institution's ethical committee approved this study, and all of the patients gave their written informed consent. The JOMS study has been registered in the University Hospital Medical Information Network system (study identification UMIN000000559), which is open to the public. It was designed to assess the characteristics of MS and the effectiveness of weight-reduction therapy at preventing CVD in Japanese obese subjects.
We recruited obese subjects with a body mass index (BMI) of ≥25 kg/m2. The exclusion criteria were no previous history of CVD, other vascular diseases, apparent renal disease, or severe liver dysfunction. The patients were classified as having MS (MS group) or not (non-MS group) according to the criteria proposed by the United States National Cholesterol Education Program-Adult Treatment Panel III with a slight modification on waist circumference, as a representative criteria of this syndrome (27). The criteria for waist circumference was set at ≥85 cm for men and ≥90 cm for women, on the basis of the criteria of the National Metabolic Syndrome Criteria Study Group of Japan (28).
Data Collection and Laboratory Measurements
At the beginning of and 3 months after weight-reduction therapy, we measured MS-related parameters (BMI, waist circumference, systolic [SBP], and diastolic BP [DBP]); blood parameters; and CAVI, an index of arterial stiffness (22). Fasting blood samples were drawn in the absence of prescription drugs, and fasting plasma glucose (FPG), hemoglobin A1c (HbA1c), immunoreactive insulin (IRI), LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), and triglycerides (TG) were determined according to the standard procedures (22). Serum levels of C-reactive protein (CRP), an inflammatory marker, and leptin and adiponectin, representative adipocytokines, were also determined (22). Urinary albumin (U-Alb) and urinary β-2 microglobulin (U-B2MG) were assessed quantitatively by the latex particle enhanced immunoturbidimetric method (Eiken, Japan). S-CysC was determined by a latex particle enhanced immunoturbidimetric assay (Ikagaku, Kyoto, Japan) (17). U-CysC was measured with an ELISA as described (17). The urine albumin-creatinine ratio (UACR) and urine U-CysC-creatinine ratio (UCCR) were calculated (12,17). Urinary N-acetyl-β-d-glucosaminidase (U-NAG) was measured using a colorimetric assay (Shionogi, Japan). Estimated GFR (eGFR) was calculated with the new Japanese coefficient for the abbreviated Modification of Diet in Renal Disease Study equation, including a correction factor of 0.739 for women (29). According to the United States National Kidney Foundation guidelines, CKD was defined as an eGFR <60 ml/min per 1.73 m2 and was classified into five stages according to e-GFR levels (3). CAVI, a newly developed indicator of arterial stiffness that is independent of BP, was determined using a Vasera VS-1000 vascular screening system (Fukuda Denshi, Tokyo, Japan), as described previously (22).
Weight-Reduction Therapy
Of those enrolled in this study, 182 obese patients (81 men and 101 women; mean age, 52.5 years; mean BMI, 31.1) undertook weight-reduction therapy through lifestyle modifications to reduce energy intake and increase physical activity for 3 months as described previously (22,23). We defined those who were able to reduce their body weight by 5% or more as successful in weight reduction and those who reduced their body weight by less than 5% as unsuccessful (22).
Statistical Analyses
The data are presented as the means ± SEM, and P < 0.05 was considered statistically significant. The t test and the χ2 test were used as appropriate for assessing intergroup differences. Analysis of covariance (ANCOVA) adjusted for gender and age was performed to assess differences in the mean levels of renal parameters stratified according to the number of MS components and the five categories of eGFR levels. A two-way repeated measured ANOVA was performed to compare changes in parameters over 3 months between patients with successful and unsuccessful in weight reduction. Pearson's correlation coefficient test was used to observe the correlations of renal parameters with metabolic parameters in all patients and of the changes in renal parameters with those in metabolic parameters during weight reduction. A stepwise multivariate regression analysis was performed to explore the factors related to the baseline levels and the changes in UCCR in all of the patients. All of the statistical analyses were performed using StatView version 5.0 for Windows (SAS Institute Inc.).
Results
Baseline Clinical Characteristics
Table 1 summarizes the characteristics of the study cohort and compares the patients with and without MS. The prevalence of MS among the obese patients was 73.2%. As expected, age, waist circumference, SBP, DBP, FPG, HbA1c, IRI, and TG were significantly higher and HDL-C level was significantly lower in the MS group than in the non-MS group (P < 0.05). In this study, BMI, LDL-C, leptin, and adiponectin levels did not differ significantly between the two groups. Moreover, CAVI was significantly higher in the MS group than in the non-MS group (P < 0.001), which is consistent with our previous report (22). Although eGFR did not differ significantly between the MS and non-MS groups, UCCR was significantly higher in the MS group than in the non-MS group (P < 0.05). S-CysC, UACR, U-B2MG, and U-NAG were also significantly higher in the MS group than the non-MS group (P < 0.05) (Table 1). In this study, the prevalence of hypertension, diabetes, dyslipidemia, and CKD was higher in the MS group than the non-MS group (proportion of CKD: non-MS group, 5.4%; MS group, 19.5%).
Table 1.
Baseline characteristics of the study obese patients stratified by the presence of MS diagnosed according to the National Cholesterol Education Program-Adult Treatment Panel III criteria
| Total | Non-MS | MS | |
|---|---|---|---|
| Male/female | 152/191 | 33/59 | 119/132 |
| Age (years) | 52.1 ± 0.8 | 48.8 ± 1.5 | 53.2 ± 0.9a |
| BMI (kg/m2) | 31.2 ± 0.3 | 30.7 ± 0.7 | 31.4 ± 0.4 |
| Waist circumference (cm) | 100 ± 0.8 | 97.6 ± 1.9 | 101 ± 0.8a |
| Systolic blood pressure (mmHg) | 139 ± 0.9 | 130 ± 1.5 | 143 ± 1.1b |
| Diastolic blood pressure (mmHg) | 82.5 ± 0.7 | 77.6 ± 1.2 | 84.3 ± 0.8b |
| Fasting plasma glucose (mg/dl) | 132 ± 2.7 | 108 ± 3.8 | 140 ± 3.3b |
| HbA1c (%) | 6.72 ± 0.1 | 5.99 ± 0.1 | 6.98 ± 0.1b |
| IRI (μU/ml) | 21.0 ± 1.7 | 15.4 ± 1.9 | 23.0 ± 2.1a |
| Triglyceride (mg/dl) | 173 ± 7.1 | 126 ± 5.6 | 190 ± 9.2b |
| HDL-C (mg/dl) | 53.4 ± 0.7 | 58.6 ± 1.3 | 51.5 ± 0.8b |
| LDL-C (mg/dl) | 124 ± 1.7 | 125 ± 2.9 | 124 ± 2.1 |
| Leptin (ng/ml) | 14.3 ± 0.7 | 13.4 ± 1.1 | 14.6 ± 0.8 |
| Adiponectin (μg/ml) | 6.48 ± 0.2 | 6.64 ± 0.3 | 6.43 ± 0.2 |
| CRP (μg/ml) | 1.41 ± 0.1 | 1.23 ± 0.1 | 1.47 ± 0.1 |
| eGFR | 82.6 ± 1.4 | 85.4 ± 2.2 | 81.7 ± 1.7 |
| S-CysC (mg/L) | 0.84 ± 0.02 | 0.78 ± 0.03 | 0.86 ± 0.02a |
| UACR (mg/g·Cr) | 99.2 ± 23 | 37.5 ± 9.7 | 122 ± 31a |
| UCCR | 0.77 ± 0.05 | 0.61 ± 0.02 | 0.82 ± 0.07a |
| U-B2MG (μg/L) | 186 ± 27 | 117 ± 20 | 212 ± 36a |
| U-NAG (U/L) | 4.17 ± 0.3 | 2.91 ± 0.3 | 4.63 ± 0.4a |
| CAVI | 7.85 ± 0.1 | 7.41 ± 0.2 | 8.02 ± 0.1b |
| Proportion of | |||
| hypertension (%) | 77.0 | 46.7 | 93.2b |
| diabetes (%) | 60.6 | 21.7 | 80.1b |
| dyslipidemia (%) | 62.2 | 23.9 | 81.3b |
| chronic kidney disease (%) | 15.7 | 5.4 | 19.5b |
| taking calcium antagonist (%) | 25.4 | 10.9 | 32.7b |
| taking ACE/ARB (%) | 31.2 | 13.1 | 40.2b |
| taking antidiabetic medications (%) | 45.1 | 19.6 | 57.8b |
| taking statins (%) | 25.0 | 6.5 | 34.3b |
The data are expressed as the means ± SEM. ACE, angiotensin-converting enzyme; ARB, angiotensin II type 1 receptor blocker.
P < 0.05 versus non-MS.
P < 0.001 versus non-MS.
Correlation between Renal Markers and Metabolic Parameters
Among the renal markers, logUCCR was significantly correlated with logUACR (P < 0.01). Interestingly, both logUACR and logUCCR had significant positive correlations with age, SBP, HbA1c, adiponectin, CAVI, and other renal markers (S-CysC, logU-B2MG, and logU-NAG) and had a negative correlation with eGFR (P < 0.05) (Table 2). Only logUCCR, but not logUACR, was significantly correlated with leptin (P < 0.05). On the other hand, only logUACR, but not logUCCR was significantly correlated with BMI, FPG, and IRI (P < 0.05). In this study, eGFR was negatively correlated with age, adiponectin, CAVI, and renal markers (S-CysC, logUACR, logUCCR, and logU-B2MG) and was positively correlated with BMI, waist circumference, and DBP (P < 0.05). S-CysC was positively correlated with age, waist circumference, leptin, and adiponectin and was negatively correlated with HDL-C and eGFR (P < 0.05). logU-B2MG was positively correlated with age, SBP, DBP, HbA1c, leptin, and adiponectin and negatively correlated with LDL-C. logU-NAG was positively correlated with BMI, waist circumference, FPG, HbA1c, leptin, and CRP and negatively correlated with HDL-C and adiponectin (P < 0.05). CAVI was positively correlated with S-CysC, logUACR, logUCCR, and logU-B2MG and negatively correlated with eGFR (P < 0.01) (Table 2). Because CAVI demonstrated a strong significant correlation with age, as previously reported (22), age-adjusted CAVI was analyzed with renal markers (data not shown). Importantly, only logUACR and logUCCR were significantly correlated with age-adjusted CAVI (logUACR, r = 0.205; logUCCR, r = 0.185, P < 0.001), whereas eGFR, S-CysC, logU-B2MG, and logU-NAG were not correlated with age-adjusted CAVI.
Table 2.
Baseline correlations of eGFR, S-CysC, logUACR, logUCCR, logU-B2MG, and logU-NAG with metabolic parameters
|
r |
||||||
|---|---|---|---|---|---|---|
| eGFR | S-CysC | logUACR | logUCCR | logU-B2MG | logU-NAG | |
| Age | −0.458a | 0.377a | 0.321a | 0.221a | 0.156b | −0.001 |
| BMI | 0.272a | 0.011 | −0.143b | 0.003 | 0.032 | 0.133c |
| Waist circumference | 0.123c | 0.111c | −0.102 | 0.028 | 0.038 | 0.131c |
| Systolic blood pressure | 0.008 | 0.086 | 0.198a | 0.115c | 0.147b | 0.062 |
| Diastolic blood pressure | 0.112c | −0.083 | 0.050 | −0.003 | 0.120c | 0.015 |
| Fasting plasma glucose | −0.025 | 0.018 | 0.257a | 0.083 | 0.058 | 0.214a |
| HbA1c | 0.065 | 0.049 | 0.245a | 0.167b | 0.138c | 0.348a |
| IRI | 0.007 | −0.010 | 0.140c | 0.098 | 0.046 | 0.085 |
| Triglyceride | −0.083 | 0.080 | 0.043 | 0.001 | −0.012 | −0.061 |
| HDL-C | 0.045 | −0.144b | 0.020 | 0.006 | −0.045 | −0.155b |
| LDL-C | 0.058 | −0.022 | 0.001 | −0.009 | −0.109c | −0.009 |
| Leptin | −0.029 | 0.282a | 0.106 | 0.282a | 0.203a | 0.108c |
| Adiponectin | −0.136c | 0.147b | 0.218a | 0.201a | 0.146b | −0.109c |
| CRP | 0.019 | 0.048 | 0.006 | −0.026 | 0.035 | 0.126c |
| eGFR | – | −0.573a | −0.208a | −0.151b | −0.119c | 0.062 |
| S-CysC | −0.573a | – | 0.312a | 0.376a | 0.292a | 0.109c |
| logUACR | −0.208a | 0.312a | – | 0.233a | 0.321a | 0.180b |
| logUCCR | −0.151b | 0.376a | 0.233a | – | 0.341a | 0.180b |
| CAVI | −0.419a | 0.328a | 0.373a | 0.200a | 0.178b | 0.031 |
The data were analyzed by Pearson's correlation coefficient test.
P < 0.001.
P < 0.01.
P < 0.05.
After adjusting for gender, age, BMI, SBP, FPG, IRI, TG, HDL-C, and LDL-C, which were selected as general CVD risk factors to avoid using variables that demonstrate high colinearity with each other, only logUCCR and logUACR had significant associations with age-adjusted CAVI (logUACR, β = 0.203; logUCCR, β = 0.173, P < 0.01) (Supplemental Table 1). S-CysC, logU-B2MG, and logU-NAG had no such associations with CAVI (Supplemental Table 1). A stepwise multivariate regression analysis revealed that age, HbA1c, and leptin are independently associated with logUCCR (P < 0.001). On the other hand, age, SBP, and HbA1c were independently associated with logUACR (P < 0.01) (Supplemental Table 2).
Renal Markers Stratified According to the Prognostic Stages of CKD and the Number of MS Traits
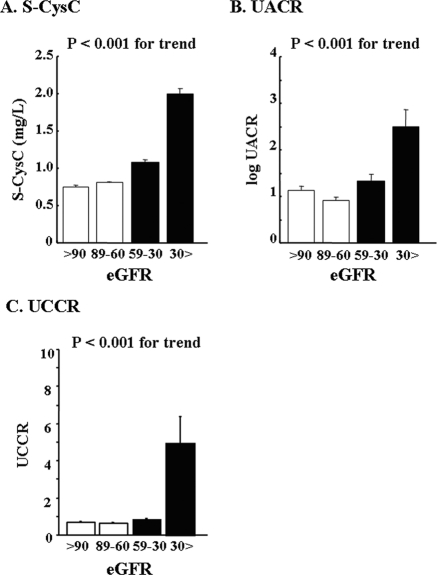
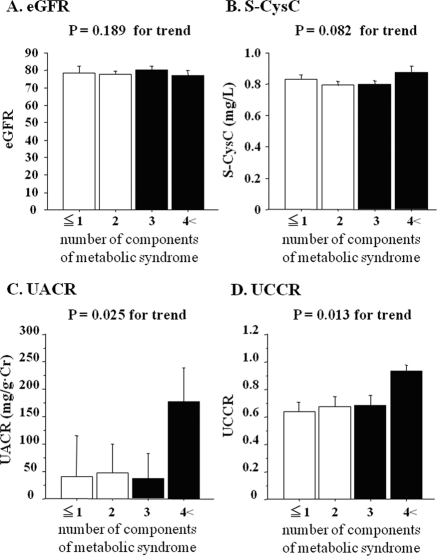
ANCOVA revealed that the mean levels of UCCR were increased in accordance with the prognostic stage of CKD in obese patients (P < 0.001) (Figure 1). There was also a significant increase in S-CysC and UACR in accordance with the prognostic stage of CKD (P < 0.001). We also examined the mean levels of eGFR, S-CysC, UACR, and UCCR, which were stratified according to the number of MS risk factors in all patients. When both gender and age were taken into account, UCCR was significantly associated with the number of MS risk factors as well as UACR (P < 0.05), whereas eGFR and S-CysC were not (Figure 2). Similarly, U-B2MG and U-NAG were significantly correlated with the number of MS risk factors (P < 0.05) (Supplemental Figure IC and ID). The mean levels of U-B2MG were increased in accordance with the prognostic stage of CKD (Supplemental Figure IA), whereas U-NAG was not significantly correlated with decreased eGFR (Supplemental Figure IB).
Figure 1.
The values of S-CysC (A), UACR (B), and UCCR (C) stratified according to the prognostic stages of CKD after gender and age adjustment. The data are shown as the means ± SEM. The differences between groups were assessed by ANCOVA. The open and closed bars indicate CKD and non-CKD according to the United States National Kidney Foundation guidelines, respectively.
Figure 2.
The values of eGFR (A), S-CysC (B), UACR (C), and UCCR (D) stratified according to the number of components of metabolic syndrome after gender and age adjustment. The data are shown as the means ± SEM. The differences between groups were assessed by ANCOVA. Nonmetabolic syndrome and metabolic syndrome are indicated by the open and closed bars, respectively.
Effect of Body Weight-reduction therapy on Renal Markers and Metabolic Parameters
Of the 182 patients, 54 (30%) successfully reduced their body weight by more than 5%. In those who were successful, BMI, waist circumference, SBP, FPG, HbA1c, LDL-C, and CAVI were significantly decreased, and adiponectin was significantly increased 3 months after the weight-reduction therapy (P < 0.05) (Table 3). Among all of the renal markers examined, only UCCR was decreased markedly after successful weight reduction (P < 0.01). There were no significant reductions in eGFR, S-CysC, UACR, U-B2MG, or U-NAG. Of those unsuccessful in body-weight reduction (n = 128), none of the above parameters changed throughout the study (Table 3). The decrease in logUCCR was strongly correlated with those in BMI, HbA1c, and CAVI (r = 0.221, 0.159, and 0.198, respectively; P < 0.05) (Supplemental Table 3). In this study, multivariate regression analysis revealed that the decrease in BMI was an independent determinant of the reduction in logUCCR during weight-reduction therapy (β = 0.221, P = 0.003) (Supplemental Table 3).
Table 3.
Effects of weight reduction in obese patients on metabolic variables and renal markers
| Group Unsuccessful in Weight Reduction |
Group Successful in Weight Reduction |
|||
|---|---|---|---|---|
| Before | 3 Months | Before | 3 Months | |
| Male/female | 56/72 | 25/29 | ||
| Age (years) | 53.4 ± 1.2 | 50.4 ± 2.1 | ||
| BMI (kg/m2) | 30.7 ± 0.5 | 30.7 ± 0.5 | 32.1 ± 0.7 | 29.7 ± 0.7a |
| Waist circumference (cm) | 99.5 ± 1.3 | 99.1 ± 1.2 | 102 ± 1.6 | 96.3 ± 2.2a |
| Systolic blood pressure (mmHg) | 140 ± 1.5 | 137 ± 1.4 | 137 ± 2.7 | 130 ± 2.1a |
| Diastolic blood pressure (mmHg) | 81.2 ± 1.1 | 80.7 ± 1.0 | 81.4 ± 2.5 | 76.3 ± 1.3 |
| Fasting plasma glucose (mg/dl) | 135 ± 4.4 | 134 ± 4.3 | 142 ± 7.6 | 129 ± 7.3b |
| HbA1c (%) | 6.85 ± 0.1 | 6.83 ± 0.1 | 6.98 ± 0.2 | 6.45 ± 0.2a |
| IRI (mU/ml) | 18.2 ± 1.8 | 20.2 ± 2.7 | 22.3 ± 5.7 | 21.6 ± 10 |
| Triglyceride (mg/dl) | 162 ± 8.2 | 161 ± 6.8 | 170 ± 12 | 153 ± 12 |
| HDL-C (mg/dl) | 55.1 ± 1.2 | 53.3 ± 1.0 | 54.0 ± 1.8 | 53.5 ± 1.7 |
| LDL-C (mg/dl) | 122 ± 3.0 | 123 ± 0.5 | 123 ± 4.3 | 113 ± 3.5a |
| Leptin (ng/ml) | 13.9 ± 1.1 | 14.6 ± 1.0 | 13.6 ± 2.0 | 12.2 ± 2.3 |
| Adiponectin (μg/ml) | 7.11 ± 0.4 | 7.73 ± 0.5 | 6.76 ± 0.5 | 7.98 ± 0.5b |
| CRP (μg/ml) | 1.39 ± 0.2 | 1.17 ± 0.1 | 1.32 ± 0.2 | 1.00 ± 0.1 |
| eGFR | 72.2 ± 2.7 | 70.6 ± 2.3 | 71.6 ± 3.5 | 72.1 ± 3.7 |
| S-CysC (mg/L) | 0.84 ± 0.03 | 0.85 ± 0.03 | 0.81 ± 0.04 | 0.84 ± 0.05 |
| UACR (mg/g·Cr) | 116 ± 45 | 132 ± 50 | 158 ± 72 | 130 ± 58 |
| UCCR | 0.78 ± 0.11 | 0.70 ± 0.08 | 0.83 ± 0.17 | 0.66 ± 0.16a |
| U-B2MG (μg/L) | 165 ± 36 | 229 ± 92 | 184 ± 68 | 129 ± 39 |
| U-NAG (U/L) | 4.70 ± 0.5 | 4.02 ± 0.6 | 4.08 ± 1.1 | 2.96 ± 0.4 |
| CAVI | 7.92 ± 0.1 | 7.89 ± 0.2 | 7.75 ± 0.3 | 7.42 ± 0.3a |
The data are expressed as the means ± SEM.
P < 0.01 versus before determined by a two-tailed paired t test.
P < 0.05 versus before determined by a two-tailed paired t test.
Discussion
Here we demonstrated for the first time that U-CysC is significantly correlated with the number of components of MS, the prognostic stages of CKD, and arterial stiffness. This study also revealed that among renal markers, only UCCR was significantly reduced, and its reduction was correlated with only BMI change during weight-reduction therapy, suggesting that U-CysC is closely associated with obesity itself. Because CysC is expressed ubiquitously, filtrated freely by the glomeruli, and reabsorbed by the proximal tubules, U-CysC has been used as a biomarker reflecting renal tubular dysfunction (17–19). Evidence has accumulated suggesting that not only renal glomeruli and but also renal tubules play an important role in the pathophysiology of diabetic and obesity-related nephropathies (30). Moreover, the rate of renal dysfunction is correlated with the degree of renal tubulointerstitial fibrosis (31). This study highlights the possible role of renal tubular dysfunction in obesity and MS (obesity traits in particular), thereby suggesting that U-CysC could serve as a potential risk marker that reflects the progression of CVD and CKD, similarly to U-Alb, in patients with obesity and MS.
However, from the present association results of UCCR in comparison with UACR, the superior effect of U-CysC on microalbuminuria is debatable. Microalbuminuria predicts GFR decline, cardiovascular events, and mortality in both the general population and patients with diabetes or hypertension (11,12). In this study, we found that UACR is negatively correlated with eGFR and positively correlated with MS traits and arterial stiffness. Similar to UACR, UCCR was also significantly correlated with several CKD and CVD risk factors, such as eGFR, MS traits, and arterial stiffness. In addition, both UACR and UCCR were significantly and independently correlated with age and HbA1c. During weight-reduction therapy, the reduction of UCCR was also significantly correlated with that of HbA1c, suggesting that increased UCCR associates with hyperglycemia, similarly to UACR, and serves as an index of diabetic nephropathy. On the other hand, only UACR, but not UCCR, was correlated with SBP. It is noteworthy that urinary excretion of CysC and albumin is increased via distinct mechanisms; U-CysC is regulated mainly by renal tubular dysfunction, and urinary albumin excretion is regulated mainly by glomerular leakage (11,17,18). The differential correlations of UCCR and UACR with clinical parameters may be attributed to the different mechanisms of their production. Leptin was found to be independently associated with UCCR on multivariate analysis, suggesting that UCCR may be more closely related to obesity itself and obesity-related nephropathy than UACR. It may be clinically beneficial to investigate the different implications and specificities of U-CysC and U-Alb and the merits of utilizing them in combination as an index of CVD or CKD in obesity. To clarify the involvement of U-Alb and U-CysC or glomerular and tubular disorders in the progression of CVD and CKD accompanied by obesity and metabolic diseases, the long-term observation of an increased number of patients is necessary.
Evidence has suggested that S-CysC is associated with GFR reduction and arterial stiffness in patients with conventional CVD risk factors (15,16,32,33). We also found that S-CysC is significantly correlated with the prognostic stages of CKD. However, there was no significant correlation between S-CysC and the number of MS components or age-adjusted CAVI in obesity. It is conceivable that increased U-CysC affects S-CysC in obesity, thereby decreasing the sensitivity for predicting CVD risk factors. Therefore, our data suggest that U-CysC might reflect the atherogenic CVD risk factors relative to S-CysC in obese patients. Further studies are needed to explore the homeostasis of CysC in obesity.
U-B2MG and U-NAG are established biomarkers for renal tubular dysfunction. Recent evidence has also suggested that U-B2MG and U-NAG are associated with CVD risks in type 2 diabetic patients (21,34). We also observed that U-B2MG and U-NAG are significantly correlated with MS traits and that U-B2MG is significantly correlated with eGFR decline. However, only U-CysC, but not U-B2MG and U-NAG, was significantly associated with age-adjusted CAVI, an index of arterial stiffness, in obese patients. These observations could be attributed to the following reasons: (1) U-CysC has different kinetics from U-B2MG and U-NAG, being more closely correlated with creatinine (17,19); (2) the major site of their production differs from one another (for instance, proximal renal tubules for NAG, lymphatic tissues for B2MG, and systemic nucleated cells for CysC) (35–37); and (3) each has distinct functional properties (for instance, NAG as a glycolytic enzyme and CysC as a cysteine proteinase inhibitor). Because cysteine proteinase inhibitors are involved in atherogenesis (35–37), it would be interesting to examine the role of CysC in the pathophysiology of atherosclerosis.
Weight reduction is essential for the management of obesity-related complications (24–26). It has been reported that moderate weight loss effectively ameliorates proteinuria and GFR decline in obesity and MS (24–26). The data of this study demonstrate that successful weight reduction reduces UCCR, suggesting that renal tubular damage in obese patients is reversibly improved by weight reduction. In this regard, Chagnac et al. (38) reported that weight reduction improves glomerular hyperfiltration and ameliorates obesity-related glumerulopathy. We and others previously demonstrated that weight reduction alleviates hyperglycemia, hypertension, hyperlipidemia, and arterial stiffness in patients with obesity and MS (22,23,39). Our data, therefore, newly support the concept that weight-reduction therapy is effective to improve obesity-related tubular dysfunction and subsequently CKD.
The dysregulation of adipocytokine production may contribute to the pathophysiology of metabolic syndrome (40,41). In this study, we demonstrated that leptin is positively correlated with UCCR in obese patients. Leptin, which is overproduced during the course of obesity, is reported to accelerate atherosclerotic lesions, glomerulosclerosis, and renal tubulointerstitial fibrosis in rodent models (42–45). It is conceivable that the production of pro-inflammatory adipocytokine accelerates renal tubular damage as well as arterial stiffness in obese patients. On the other hand, it has recently been reported that the plasma concentration of adiponectin, an anti-inflammatory adipocytokine (46), is elevated because of reduced clearance in chronic renal disorders (47), which might be related to our finding of a positive correlation between the adiponectin and UCCR. The pathophysiological significance of U-CysC in obesity-associated CKD onset and advancement may be clarified through further investigations of how adipocytokines are involved in CysC dynamics in obesity.
There are some study limitations to our study. The study had a cross-sectional design and a short-term intervention and did not examine CVD-related morbidity or mortality in this cohort. Although many prospective studies have shown CRP and arterial stiffness to be independent predictors of future CVD events (48,49), they cannot reflect the critical hard end points of CVD and/or CKD. Accordingly, to clarify the significance of U-CysC as a risk factor for CVD and CKD, it is necessary to conduct a long-term prospective cohort study to examine CVD- and CKD-related morbidity and mortality along with CRP and CAVI. We acknowledge that the correlations between U-CysC and various parameters were not strong, so repeated studies with larger sample sizes should be performed. Furthermore, this study examined the usefulness of U-CysC by classifying it according to the presence or absence of MS. Because there are various opinions on whether the concept of MS can fully contribute to CVD management (50), the clinical implications of these findings should be studied from various viewpoints in future.
In conclusion, this study is the first to demonstrate that increased UCCR is significantly associated with the severity of CKD and MS and arterial stiffness, CVD risk factors, in obese patients. Our data also suggest that U-CysC is helpful for assessing the effect of weight reduction on CVD and CKD risk factors in obesity. Therefore, U-CysC could serve as a potential biomarker for evaluating and managing the progression of CVD and CKD in patients with obesity and MS.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Shinichi Mashiba for his valuable discussion and Hajime Yamakage, Yousuke Sasaki, and Kazuya Muranaka for their excellent technical assistance. This work was supported in part by grants from the National Hospital Organization for collaborative clinical research, the Smoking Research Foundation, and the Kidney Foundation, Japan (JFK 09-2) (to N.S.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at http://www.cjasn.org.
References
- 1. Eckel RH, Grundy SM, Zimmet PZ: The metabolic syndrome. Lancet 365: 1415–1428, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L: Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24: 683–689, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Schiffrin EL, Lipman ML, Mann JF: Chronic kidney disease: Effects on the cardiovascular system. Circulation 116: 85–97, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Wahba IM, Mak RH: Obesity and obesity-initiated metabolic syndrome: Mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol 2: 550–562, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J: The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med 140: 167–174, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Tanaka H, Shiohira Y, Uezu Y, Higa A, Iseki K: Metabolic syndrome and chronic kidney disease in Okinawa, Japan. Kidney Int 69: 369–374, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Muntner P, He J, Astor BC, Folsom AR, Coresh J: Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: Results from the atherosclerosis risk in communities study. J Am Soc Nephrol 16: 529–538, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Dogra G, Irish A, Chan D, Watts G: Insulin resistance, inflammation, and blood pressure determine vascular dysfunction in CKD. Am J Kidney Dis 48: 926–934, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Weir MR: Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephrol 2: 581–590, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Arnlöv J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Benjamin EJ, D'Agostino RB, Vasan RS: Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: The Framingham Heart Study. Circulation 112: 969–975, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Dharnidharka VR, Kwon C, Stevens G: Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 40: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C: Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 352: 2049–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Madero M, Wassel CL, Peralta CA, Najjar SS, Sutton-Tyrrell K, Fried L, Canada R, Newman A, Shlipak MG, Sarnak MJ: Health ABC Study. Cystatin C associates with arterial stiffness in older adults. J Am Soc Nephrol 20: 1086–1093, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakamura K, Iizuka T, Takahashi M, Shimizu K, Mikamo H, Nakagami T, Suzuki M, Hirano K, Sugiyama Y, Tomaru T, Miyashita Y, Shirai K, Noike H: Association between cardio-ankle vascular index and serum cystatin C levels in patients with cardiovascular risk factor. J Atheroscler Thromb 16: 371–379, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Uchida K, Gotoh A: Measurement of cystatin-C and creatinine in urine. Clin Chim Acta 323: 121–128, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Conti M, Moutereau S, Zater M, Lallali K, Durrbach A, Manivet P, Eschwège P, Loric S: Urinary cystatin C as a specific marker of tubular dysfunction. Clin Chem Lab Med 44: 288–291, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Herget-Rosenthal S, van Wijk JA, Bröcker-Preuss M, Bökenkamp A: Increased urinary cystatin C reflects structural and functional renal tubular impairment independent of glomerular filtration rate. Clin Biochem 40: 946–951, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Singh DK, Winocour P, Farrington K: Mechanisms of disease: The hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol 4: 216–226, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Weitgasser R, Schnoell F, Gappmayer B, Kartnig I: Prospective evaluation of urinary N-acetyl-beta-D-glucosaminidase with respect to macrovascular disease in elderly type 2 diabetic patients. Diabetes Care 22: 1882–1886, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Satoh N, Shimatsu A, Kato Y, Araki R, Koyama K, Okajima T, Tanabe M, Ooishi M, Kotani K, Ogawa Y. Japan Obesity and Metabolic Syndrome Study (JOMS) Group: Evaluation of cardio-ankle vascular index: A new indicator of arterial stiffness independent of blood pressure, in obesity and metabolic syndrome. Hypertens Res 31: 1921–1930, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Kotani K, Satoh N, Kato Y, Araki R, Koyama K, Okajima T, Tanabe M, Oishi M, Yamakage H, Yamada K, Hattori M, Shimatsu A. Japan Obesity and Metabolic Syndrome Study Group: A novel oxidized low-density lipoprotein marker, serum amyloid A-LDL, is associated with obesity and the metabolic syndrome. Atherosclerosis 204: 526–531, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Navaneethan SD, Yehnert H, Moustarah F, Schreiber MJ, Schauer PR, Beddhu S: Weight loss interventions in chronic kidney disease: A systematic review and meta-analysis. Clin J Am Soc Nephrol 4: 1565–1574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Agrawal V, Shah A, Rice C, Franklin BA, McCullough PA: Impact of treating the metabolic syndrome on chronic kidney disease. Nat Rev Nephrol 5: 520–528, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Morales E, Valero MA, León M, Hernández E, Praga M: Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am J Kidney Dis 41: 319–327, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults: Executive summary of the third report of the National Cholesterol Education Program (y) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285: 2486–2497, 2001 [DOI] [PubMed] [Google Scholar]
- 28. The Examination Committee of Criteria for Metabolic syndrome: The Definition and criteria of metabolic syndrome. J Jpn Soc Int Med 94: 794–809, 2005 [Google Scholar]
- 29. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A: Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Phillips AO. The role of renal proximal tubular cells in diabetic nephropathy. Curr Diab Rep 3: 491–496, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Phillips AO, Steadman R: Diabetic nephropathy: The central role of renal proximal tubular cells in tubulointerstitial injury. Histol Histopathol 17: 247–252, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Taglieri N, Koenig W, Kaski JC: Cystatin C and cardiovascular risk. Clin Chem 55: 1932–1943, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Maahs DM, Ogden LG, Kretowski A, Snell-Bergeon JK, Kinney GL, Berl T, Rewers M: Serum cystatin C predicts progression of subclinical coronary atherosclerosis in individuals with type 1 diabetes. Diabetes 56: 2774–2779, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Yoshikawa R, Wada J, Seiki K, Matsuoka T, Miyamoto S, Takahashi K, Ota S, Taniai K, Hida K, Yamakado M, Shikata K, Uehara Y, Urade Y, Makino H: Urinary PGDS levels are associated with vascular injury in type 2 diabetes patients. Diabetes Res Clin Pract 76: 358–367, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P: Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest 102: 576–583, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP: Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol 24: 1359–1366, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Bengtsson E, To F, Hakansson K, Grubb A, Branen L, Nilsson J, Jovinge S: Lack of the cysteine protease inhibitor cystatin C promotes atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 25: 2151–2156, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y: The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 14: 1480–1486, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Dengel DR, Kelly AS, Olson TP, Kaiser DR, Dengel JL, Bank AJ: Effects of weight loss on insulin sensitivity and arterial stiffness in overweight adults. Metabolism 55: 907–911, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Matsuzawa Y: Therapy Insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Clin Pract Cardiovasc Med 3: 35–42, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Salmenniemi U, Ruotsalainen E, Pihlajamäki J, Vauhkonen I, Kainulainen S, Punnonen K, Vanninen E, Laakso M: Multiple abnormalities in glucose and energy metabolism and coordinated changes in levels of adiponectin, cytokines, and adhesion molecules in subjects with metabolic syndrome. Circulation 110: 3842–3848, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Koh KK, Park SM, Quon MJ: Leptin and cardiovascular disease: Response to therapeutic interventions. Circulation 117: 3238–3249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolf G, Hamann A, Han DC, Helmchen U, Thaiss F, Ziyadeh FN, Stahl RA: Leptin stimulates proliferation an TGF-beta expression in renal glomerular endothelial cells: Potential role in glomerulosclerosis. Kidney Int 56: 860–872, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Kümpers P, Gueler F, Rong S, Mengel M, Tossidou I, Peters I, Haller H, Schiffer M: Leptin is a coactivator of TGF-beta in unilateral ureteral obstructive kidney disease. Am J Physiol Renal Physiol 293: F1355–F1362, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Tanaka M, Suganami T, Sugita S, Shimoda Y, Kasahara M, Aoe S, Takeya M, Takeda S, Kamei Y, Ogawa Y: Role of central leptin signaling in renal macrophage infiltration. Endocr J 7: 61–72, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K: Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116: 1784–1792, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Komura N, Kihara S, Sonoda M, Maeda N, Tochino Y, Funahashi T, Shimomura I: Increment and impairment of adiponectin in renal failure. Cardiovasc Res 86: 471–477, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Ridker PM: Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: Implications for longevity. Nutr Rev 65: S253–S259, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A: Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37: 1236–1241, 2001 [DOI] [PubMed] [Google Scholar]
- 50. de Zeeuw D, Bakker SJ: Does the metabolic syndrome add to the diagnosis and treatment of cardiovascular disease? Nat Clin Pract Cardiovasc Med 5 [Suppl 1]: S10–S14, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.