Summary
Background and objectives
Vascular calcification (VC) has a significant effect in cardiovascular diseases on dialysis patients. However, VC is assessed with x-ray-based techniques, which do not inform about calcium localization (intima, media, atherosclerosis-related). The aim of this work is to study VC and its related factors using arterial ultrasound to report the exact location of calcium.
Design, setting, participants, & measurements
This was an observational, cross-sectional, case-control study that included 232 patients in dialysis and 208 age- and sex-matched controls with normal kidney function. Demographic data and laboratory values were collated. Carotid, femoral, and brachial ultrasounds were performed to assess VC and atherosclerosis burden using a standardized protocol.
Results
Cardiovascular risk factors were predominantly found in controls, although the burden of atherosclerosis was higher in the dialysis group. VC was significantly more prevalent in the group of patients on dialysis than control subjects, and in both groups the most prevalent pattern of VC was linear calcification located in the intima of the artery wall. Age and undergoing dialysis (with or without previous cardiovascular diseases) were positively and significantly associated with linear calcification. Conversely, the absence of atherosclerosis and low levels of C-reactive protein and phosphorus significantly impeded the development of linear calcification.
Conclusions
VC in large, conduit arteries is more prevalent in patients on dialysis than controls and is predominantly located in a linear fashion in the intima of the arteries.
Introduction
Vascular calcification (VC) is responsible for the higher prevalence of cardiovascular disease (CVD) in chronic kidney disease (CKD) patients (1). Consequently, early detection of VC is relevant and affects incidence of CVD (2). VC has been described as a patchy-like and/or a linear calcification that corresponds to intima (atherosclerosis-related) and media calcification (related to calcium/phosphorus disturbances), respectively (3). Currently, there are several diagnostic techniques for the identification of VC in the clinical practice, including plain x-ray (4,5), coronary calcium score (6), and echocardiography (7). However, these techniques have limitations, and none of them is able to clearly identify the exact location of calcium in the artery wall (calcified atheroma plaque, intima or media). Furthermore, there is a current debate about the location of calcium in the artery wall and their clinical implications (8,9). Recently, Nakamura S et al. (10) reported a study of 102 CKD patients who died of CVD. Calcified plaques and intima calcification were the most prevalent coronary abnormalities. Conversely, only 7 patients out of 102 (6.8%) presented VC in the media, all of them at advanced stages of CKD.
Arterial ultrasound is the only technique able to differentiate the layers of the artery wall and the presence of calcium, and to quantify the burden of atherosclerosis in a noninvasive and reliable way (11–13).
The aim of the work presented here is to study VC and its related factors in dialysis patients using arterial ultrasound (carotid, femoral and brachial arteries) to report the exact location of calcium.
Materials and Methods
Design
This is an observational, cross-sectional, case-control study. We analyzed patients under hemodialysis or peritoneal dialysis from four different dialysis centers. As controls, we used data from a healthy population that was age and sex matched without CVD and normal kidney function (GFR > 60 ml/min) (14). All of the participants in the study underwent the same carotid ultrasound protocol. Images were centrally analyzed offline in the core laboratory.
The local ethics committee of the Hospital Universitari Arnau de Vilanova approved the studies and informed consents.
Procedures
Clinical data and physical examination.
We collated relevant clinical and demographical data. We also included in the database current medications concerning vitamin D, calcium and phosphorus metabolism, statins, and blood pressure (BP)-lowering drugs.
On physical examination, we included BP according to clinical guidelines (15) and body mass index (BMI).
Laboratory variables.
We performed full blood analyses including hemoglobin, total cholesterol and HDL cholesterol, triglycerides, LDL cholesterol (calculated using Friedewald formula), glucose, serum calcium and phosphorus, ferritin, and albumin. C-reactive protein (CRP) was measured following the recommendations of the manufacturer, Roche Diagnostics, using the Cobas analyzer series CRP (wide range). The limit of detection of the Roche assay is 1 mg/L, and the upper limit is 250 mg/L. Normal values in our laboratory are between 1.2 and 6 mg/L. Intact parathyroid hormone (PTH) was measured with EQL Elecsys (Roche Diagnostics), Immulite 2500 intact PTH (Siemens), and Advia Centaur PTH (Bayer) and converted to picograms per milliliter to standardize the measurements according to K/DOQI guidelines (IRMA NICHOLS) (16).
Carotid, femoral and brachial ultrasound.
We used a MicroMaxx SonoSite with a linear transducer (HFL38/13) to 6 MHz. Each sonographer followed the same standard operational procedure as follows:
Carotid ultrasound: This exam was used to measure carotid intima-media thickness (cIMT) to identify the presence of carotid plaques according to the Mannheim consensus (17) and to identify and localize VC. The far wall of the common carotid, bulb, and internal carotid arteries were scanned. We analyzed the presence/absence of calcium in the artery wall (defined by the hyperintensity in the video signal), and the single reader (B.C.) identified the anatomic location of such calcifications (intima, media, type V atheroma plaques). We measured cIMT using the semiautomated U.S. Food and Drug Administration-approved software, SonoCalc IMT.
Femoral ultrasound: In CKD patients, we performed femoral ultrasound with the aim to identify atheromatous plaques and VC. The common and superficial femoral arteries were scanned.
Brachial artery ultrasound: A longitudinal image was recorded aimed at the identification of calcium in the artery wall or atheromatous plaques.
Data from carotid ultrasound were collected from both groups (dialysis and controls), whereas femoral and brachial ultrasounds were performed just in the group of patients in dialysis. Technicians and the expert reader did not have access to clinical, laboratory, or treatment details of participants.
Outcomes and Definitions
CVD.
Presence of coronary heart disease (myocardial infarction, angina, or revascularization), cerebrovascular disease (stroke or transient ischemic attack), and peripheral artery disease were considered as CVD.
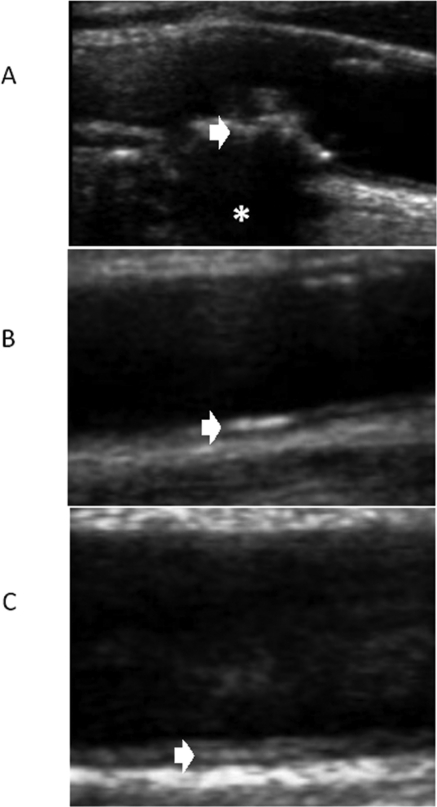
VC (Figure 1).
Figure 1.
Ultrasound exams of different types of VC. (A) Type V atheroma plaque (white arrow) with acoustic shadowing because of plaque calcification (asterisk). (B) Linear hyperechogenicity located in the lumen-intima interphase (white arrow). (C) Linear hyperechogenicity located in the media space (white arrow).
We considered the presence of VC when a type V plaque was identified (highly echogenic plaques producing bright white echoes with shadowing, Figure 1A), or a linear echogenic hyperintensity was observed in the interphase lumen-intima (linear calcification, Figure 1B) or in the interphase media-adventitia (media calcification, Figure 1C). Therefore, we identified four possible VC patterns: type V plaque, linear calcification, media calcification, and no calcification.
Atherosclerosis Assessment
cIMT (mean value of the six territories explored) and the presence of plaques were used to assess atherosclerosis status. cIMT above 1.5 mm was considered as an atheroma plaque according to the Mannheim consensus (17).
Statistical Analyses
Data are expressed as mean (SD) or median and interquartile range when necessary. ANOVA for means or the nonparametric Kruskal–Wallis test for medians was used to compare the quantitative variables among groups of VC. Additionally, the Pearson χ2 test or the Fisher exact test (depending on whether expected frequencies <5 were absent or not, respectively) were used to compare the distribution of frequencies for the qualitative characteristics. Multivariate analysis was performed to identify variables related to the presence of VC. A logistic regression model was applied in which the independent variable was linear calcification and independent variables were identified from the univariate analyses. Those significant variables (P < 0.05) in the ANOVA or the Pearson χ2 test were entered into the model. Furthermore, those variables acting as confounders were also included (i.e., diabetes mellitus, PTH concentration). Among the continuous variables, concentration of phosphorus, CRP, PTH, and cIMT have been categorized according to the locally weighted scatterplot smoothing function using those values closely related to the median value of the variable.
Results
General Characteristics of the Studied Population (Table 1)
Table 1.
General characteristics of the population under study
| Dialysis (n = 232) |
Control (n = 208) | P | ||
|---|---|---|---|---|
| CVD (n = 94) | No CVD (n = 138) | |||
| Age, years | 69 (8) | 61 (14)b | 64 (3)a | <0.001 |
| Sex, male, n (%) | 74 (78.7) | 71 (51.4) | 128 (61.5) | <0.001 |
| BMI, kg/m2 | 26.2 (4.5) | 25.2 (4.7) | 29.4 (4.3)a | <0.001 |
| Systolic BP, mmHg | 135.6 (24) | 135.8 (24) | 143.3 (20)a | 0.003 |
| Diastolic BP, mmHg | 69 (13) | 73 (12)b | 82.5 (11)a | <0.001 |
| Smoking, n (%) | <0.001 | |||
| no | 37 (39.4) | 82 (68.9)b | 108 (51.9) | |
| ex-smokers | 48 (51.1) | 26 (18.8) | 58 (27.9) | |
| current smokers | 9 (9.6) | 30 (21.7) | 42 (20.2) | |
| Time on dialysis, months | 53 (47) | 50 (59) | NA | 0.65 |
| Type 2 diabetes mellitus, n (%) | 45 (47.9) | 30 (21.7) | 67 (32.2) | <0.001 |
| Total cholesterol, mg/dl | 150 (35) | 160 (34) | 213 (41)a | <0.001 |
| HDL cholesterol, mg/dl | 44 (15) | 49 (16)b | 57 (14) | <0.001 |
| LDL cholesterol, mg/dl | 74 (30) | 85 (29)b | 128 (34)a | <0.001 |
| Triglycerides, mg/dl | 165 (113) | 134 (79)b | 146 (79) | 0.03 |
| Glucose, mg/dl | 129 (58) | 106 (43)b | 113 (32)a | <0.001 |
| Calcium, mg/dl | 8.90 (0.54) | 8.84 (0.72) | 9.33 (0.32)a | <0.001 |
| Phosphorus, mg/dl | 4.48 (1.29) | 4.70 (1.22) | 3.43 (0.56)a | <0.001 |
| PTH, pg/ml | 267 (234) | 269 (260) | NA | 0.94 |
| Ferritin, ng/ml | 322 (227) | 274 (190) | NA | 0.21 |
| Albumin, g/dl | 3.74 (0.48) | 3.76 (0.45) | 4.55 (0.24)a | <0.001 |
| CRP, mg/L | 16.64 (26) | 15.3 (30) | 4.66 (7.9)a | <0.001 |
| cIMT, mm | 0.89 (0.17) | 0.79 (0.16)b | 0.83 (0.14)a | <0.001 |
| Carotid plaques, mean (SD) | 2.61 (1.6) | 1.52 (1.3)b | 0.86 (1)a | <0.001 |
NA, not applicable.
P < 0.05 in the Bonferroni post-hoc analysis when comparing controls with dialysis patients.
P < 0.05 in the Bonferroni post-hoc analysis when comparing dialysis patients with and without CVD.
We included 232 patients on dialysis and 208 controls. Demographic, clinical, and laboratory results are displayed in the Table 1 according to group (dialysis versus controls) and, among those on dialysis, whether they suffered from previous CVD or not. Patients and controls were age and sex matched; however, dialysis patients with previous CVD were significantly older and the prevalence of men was significantly higher. Classical cardiovascular risk factors were more prevalent in the group of control subjects than patients on dialysis (higher values of systolic and diastolic BP, BMI, and higher concentrations of total and LDL cholesterol). However, patients on dialysis without previous CVD presented higher diastolic BP, HDL and LDL cholesterol, triglycerides, and glucose than those with previous CVD. In the group of patients on dialysis with previous CVD, the prevalence of diabetes mellitus was significantly higher than in the other groups. Furthermore, the group of dialysis patients presented higher inflammatory-related laboratory values than controls (higher concentration of CRP and lower albumin). cIMT was significantly higher on dialysis patients with previous CVD when compared with those without CVD and controls. Furthermore, the number of carotid plaques was significantly higher in the group with previous CVD than those without.
Main causes of ESRD were unknown (27.6%), diabetes (20.7%), and vasculorenal (17.2%), and we did not find differences in the time on dialysis between groups (with CVD versus no CVD).
Nearly half of the patients on dialysis were receiving statins (103 [44.4%], 50 [53.2%] within the group of CVD and 53 [38.4%] in those without CVD, P = 0.02) and 137 (59.1%) were on BP-lowering drugs. Regarding secondary hyperparathyroidism therapy, 32 (13.8%) patients were receiving paricalcitol, 43 (18.5%) were receiving calcitriol, and 34 (14.7%) were receiving vitamin D supplements. Seventy-two (31%) patients were treated with cinacalcet.
Localization of VC
Carotid, femoral, and brachial ultrasound results are displayed in Table 2. The prevalence of carotid calcification was significantly higher in the group of patients on dialysis (85.3%) than in control subjects (20.3%, P < 0.001). However, in both groups, the most prevalent pattern of carotid calcification was the linear calcification (107 patients out of 232 (46.1%) and 28 (13.8%) of controls). Femoral calcification was paralleled with that observed in the carotids and was the most prominent pattern the linear calcification (121 [60%]). We did not find a significant correlation between carotid and femoral linear calcification (correlation coefficient 0.09, P = 0.16). However, the presence of type V atheroma plaques was highly and significantly correlated between carotid and femoral arteries (correlation coefficient 0.36, P < 0.001). Conversely, the presence of calcification in the brachial artery was significantly reduced, with no type V plaques, 14 patients (7.5%) with linear calcification, and 2 (1.1%) with calcification in the media. When we analyzed separately the results according to the presence of previous CVD, we found a significant increase in type V atheroma plaques and linear calcification in carotids and femorals of those with CVD compared with those without CVD (P < 0.05).
Table 2.
Classification and results of VC in the arteries studied (dialysis n = 232, control n = 202)
| Arterial Territory | Type V Atheroma Plaque |
Linear Calcification |
Medial Calcification |
No Calcification |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dialysis |
Control | Dialysis |
Control | Dialysis |
Control | Dialysis |
Control | |||||
| CVD | No CVD | CVD | No CVD | CVD | No CVD | CVD | No CVD | |||||
| Carotid | 32 (34)c | 37 (26) | 9 (4.5)a | 49 (52)c | 58 (42)b | 28 (13)a | 12 (12) | 10 (7) | 4 (1.9)a | 7 (7) | 27 (19) | 161 (79.7)a |
| Femoral | 17 (18)c | 15 (10) | NA | 60 (63)c | 61 (44) | NA | 10 (10) | 8 (5) | NA | 14 (14) | 16 (11) | NA |
| Brachial | 0 | 0 | NA | 7 (7) | 7 (5) | NA | 1 (1) | 1 (0.7) | NA | 80 (85) | 89 (66) | NA |
Sample size and (percentages) are shown. Percentages in dialysis patients are taken from the total number of patients in each subgroup (CVD [n = 94] versus no CVD [n = 138]). Because of anatomic particularities, we obtained valuable images from 202 of 208 control participants. Similarly, we obtained femoral exams in 201 and brachial exams in 185 dialysis patients.
P < 0.05 when comparing dialysis with controls.
P < 0.05 when separately comparing different types of VC in dialysis and controls.
P < 0.05 when comparing CVD versus no CVD groups in dialysis patients.
Variables Related to Linear Calcification on Dialysis
We evaluated the variables associated with the presence of linear calcification because it was the most prevalent type of VC (Table 3). Patients with linear calcification were significantly older, predominantly male, and had a higher prevalence of previous CVD compared with patients without calcification. There were no differences in time of dialysis and in most of the classical cardiovascular risk factors (diabetes mellitus, BMI, systolic BP, and lipid profile). We did not find significant differences in terms of therapies with the exception for statin and acenocumarol use, which were significantly more prescribed in those patients with linear calcification. Conversely, patients with linear calcification had lower diastolic BP and a significantly higher CRP concentration than patients with no calcification. When we compared the results of atherosclerosis burden, patients with linear calcification presented higher cIMT and a higher number of plaques than those without VC.
Table 3.
Selected characteristics of patients in dialysis according to VC (linear calcification versus no calcification)
| Demographics | Linear Calcification (n = 107) | No Calcification (n = 34) | P |
|---|---|---|---|
| Age, years | 67 (11) | 54 (17) | <0.001 |
| Sex, male, n (%) | 70 (65) | 14 (41) | 0.01 |
| Time on dialysis, months (range) | 44 (0.5–292) | 49 (1.5–343) | 0.63 |
| Diabetes mellitus, n (%) | 39 (36.4) | 7 (21) | 0.07 |
| Smoking, n (%) | 0.04 | ||
| no | 58 (54.2) | 20 (58.8) | |
| ex-smokers | 32 (29.9) | 4 (11.8) | |
| current smokers | 17 (15.9) | 10 (29.4) | |
| Previous CVD, n (%) | 49 (45.8) | 7 (20) | 0.007 |
| BMI, kg/m2 | 26.3 (5) | 25.6 (5) | 0.45 |
| Systolic BP, mmHg | 135 (27) | 136 (22) | 0.84 |
| Diastolic BP, mmHg | 70 (13) | 78 (14) | 0.004 |
| Therapies | |||
| paricalcitol, n (%) | 14 (13.1) | 6 (17.6) | 0.51 |
| calcitriol, n (%) | 18 (16.8) | 8 (23.5) | 0.39 |
| vitamin D supplements, n (%) | 12 (11.2) | 8 (23.5) | 0.08 |
| cinacalcet, n (%) | 32 (29.9) | 10 (29.4) | 0.95 |
| BP, n (%) | 60 (56.1) | 24 (70.6) | 0.12 |
| statin, n (%) | 52 (48.6) | 10 (29.4) | 0.04 |
| acenocumarol, n (%) | 14 (13.1) | 1 (2.9) | 0.02 |
| Laboratory results | |||
| calcium, mg/dl | 8.88 (0.5) | 8.75 (0.6) | 0.28 |
| phosphorus, mg/dl | 4.72 (1.28) | 4.61 (1.22) | 0.66 |
| PTH, pg/ml | 279 (264) | 293 (213) | 0.76 |
| albumin, mg/dl | 3.8 (0.5) | 3.8 (0.39) | 0.83 |
| CRP, mg/dl | 20 (34) | 7.5 (7) | 0.03 |
| total cholesterol, mg/dl | 161 (37) | 162 (34) | 0.88 |
| HDL cholesterol, mg/dl | 48 (17) | 51 (18) | 0.38 |
| LDL cholesterol, mg/dl | 83 (32) | 84 (31) | 0.91 |
| triglycerides, mg/dl | 159 (108) | 150 (122) | 0.68 |
| glucose, mg/dl | 117 (51) | 118 (53) | 0.91 |
| Carotid ultrasound | |||
| intima-media thickness, mm | 0.85 (0.15) | 0.71 (0.14) | <0.001 |
| carotid plaques, mean (SD) | 2.12 (1.4) | 0.44 (0.7) | <0.001 |
Multivariate Analyses of VC (Table 4)
Table 4.
Results of the multivariate analyses
| Linear Calcification |
||||
|---|---|---|---|---|
| Estimate | OR | 95% Confidence Interval | P | |
| Age, years | 0.043 | 1.04 | 1 to 1.08 | 0.04 |
| Sex, male | 0.45 | 1.57 | 0.69 to 3.55 | 0.27 |
| Group | ||||
| Control | – | – | – | – |
| Dialysis, no CVD | 2.05 | 7.77 | 2.81 to 21.4 | <0.001 |
| Dialysis, CVD | 2.09 | 8.09 | 2.67 to 24.5 | <0.001 |
| Diabetes mellitus, no | −0.21 | 0.8 | 0.37 to 1.72 | 0.57 |
| Calcium, mg/dl | 0.16 | 1.17 | 0.4 to 2.8 | 0.70 |
| Phosphorus ≤5 mg/dl | −1.29 | 0.27 | 0.08 to 0.84 | 0.02 |
| CRP ≤ 12 mg/dl | −0.88 | 0.41 | 0.15 to 1.10 | 0.08 |
| PTH ≤ 282 pg/ml | 0.07 | 1.07 | 0.33 to 3.45 | 0.90 |
| Carotid plaque, no | −1.97 | 0.13 | 0.06 to 0.31 | <0.001 |
| cIMT ≤ 0.85 mm | −0.28 | 0.74 | 0.32 to 1.71 | 0.49 |
We then analyzed the variables related to the presence of linear calcification using multivariate analyses. Independent variables were selected from those analyzed with univariate analyses that were significantly related to linear calcification (Table 3; age, sex, previous CVD, smoking status, diastolic BP, CRP, cIMT, carotid plaques, and treatment with statins and acenocumarol). We also included variables that are known to influence VC, such as calcium and phosphorus, PTH concentration, and the diagnoses of diabetes mellitus.
Being on dialysis and with previous CVD was the strongest variable associated with a significantly increased risk of having linear calcification in conduit arteries (odds ratio [OR]: 8.09 [2.67 to 24.5], P < 0.001). Being on dialysis without previous CVD was also significantly associated with a higher risk of linear calcification when compared with controls (OR: 7.77 [2.81 to 21.4], P < 0.001)). Similarly, age was positively associated with linear calcification. However, the multivariate analyses identified several protective variables. The absence of atherosclerosis (no carotid plaques) was significantly associated with a lower risk of linear calcification (OR: 0.13 [0.06 to 0.31], P < 0.001). Similarly, lower concentrations of CRP and serum phosphorus were associated with a lower risk of linear calcification.
Multivariate analyses did not find significant association with sex, diabetes mellitus, calcium, PTH, cIMT, and the considered therapies (statin and acenocumarol; data not shown in the table).
Discussion
Cardiovascular calcification is a strong predictor of cardiovascular events on dialysis patients (18). We have studied the presence of VC in large arteries (carotid, femoral and brachial) using ultrasound with the aim to clearly identify the location of calcium and variables related with VC. Our results showed a significantly higher prevalence of VC in patients on dialysis compared with age- and sex-matched controls with normal kidney function. The most common pattern was a linear calcification located in the lumen-intima interphase in both groups of subjects, although it was more prevalent in dialysis patients than in controls (46.1% versus 13.8%, respectively). Our results showed that age and being on dialysis (with or without previous CVD) were the strongest factors associated with linear calcification. Conversely, lower levels of CRP and phosphorus impeded having VC. Moreover, linear calcification was highly associated with the development of atherosclerosis because the absence of carotid plaques was identified as a protective factor. Indeed, in those arteries with a low prevalence of plaques (i.e., brachial arteries), linear calcification was rarely observed (7.5% of patients on dialysis).
These results are in accordance with previous published studies. In a study of 102 CKD patients who died of CVD, VC was predominantly found in the intima (11). The presence of medial calcification was observed in only 7 of 102 studied patients. Variables related to the intima calcification were advanced stages of CKD, age, smoking, diabetes, calcium-phosphorus product, inflammation, and kidney function.
VC has been historically classified as (1) intima calcification associated with atheroma plaques and (2) medial calcification mediated by a switch of vascular smooth muscle cells (VSMCs) to a procalcification phenotype and associated with CKD and disturbances in the metabolism of calcium, phosphorus, and vitamin D (19). However several potential flaws associated with this traditional view and the data on which it is based have to be considered. First, large arteries (carotid, femoral, brachial, aorta) are “elastic” arteries because their function is merely being a driver of the blood stroke. It implies a poor VSMC content in the media of these arteries (20,21). On the contrary, small, peripheral arteries are aimed at the regulation of blood flow in the tissue according to metabolic demands, and they present a dense layer of VSMCs. Previous studies clearly stated that large arteries are more prone to atherosclerosis development (22), and small vessels (radial and digital) are more easily calcified (23). These previous data support our results because large arteries presented VC in the intima, a process that has been highly related to the development of atherosclerosis.
Second, several research groups have studied the anatomic location of calcium in the artery wall, and they have found a singular pattern that corresponds to the calcification of the internal elastic lamina (IEL; a membrane of elastin and fibers that separate the intima and the media). Age was the strongest variable related to the calcification of the IEL, and kidney function and serum calcium concentration were not significantly associated (24).
Third, previous clinical studies in VC have been performed using x-ray-based techniques (5–7) (plain x-ray, coronary computed tomography). These techniques are not sensitive enough to identify the exact location of calcium; consequently, attributing the calcification to calcified atherosclerosis or IEL/medial calcification is speculative. Conversely, ultrasound is able to identify structures with a different behavior when an ultrasound beam is applied; therefore, ultrasound is able to clearly differentiate the interphase lumen-intima and media-adventitia in the carotid, femoral, and brachial arteries.
Our results demonstrate for the first time that vascular ultrasound is very useful on dialysis patients, especially in the setting of a clear-cut division between the presence of calcium in the artery wall, whether it is located in the intima or in the media. This diagnosis should have clinical implications because the identification of linear calcification (intima calcification), which is related to atherosclerosis and inflammation, should prompt clinicians to strictly control cardiovascular risk factors. In that sense, current results of statins in patients under dialysis are not encouraging because they have not demonstrated a significant reduction in major cardiovascular events in the group of patients receiving atorvastatin (25) or rosuvastatin (26). However, we should acknowledge that a significant proportion of these cardiovascular events are sudden death (160 of 469 in the 4D study), and this may negatively influence the final interpretation. Conversely, in a meta-analysis published in 2008, authors concluded that statins significantly reduce cardiovascular events in CKD-affected patients, although all-cause mortality remained unchanged (27).
Conversely, medial calcification should support the control of metabolic disturbances in terms of calcium, phosphorus, and vitamin D. However, we must highlight that, according to our results, low serum phosphorus concentration is a protective factor for developing linear calcification, and this might be interpreted as the influence of phosphorus on atherosclerosis. According to this hypothesis, a previous study showed that in subjects with normal renal function, serum phosphorus was positively associated with cIMT independently of traditional cardiovascular risk factors (28).
Our work has several limitations. We have performed a cross-sectional study. We need to prospectively analyze the effect of such disturbances (linear calcification) in the incidence of cardiovascular events in this population. Our research protocol is underway, and we have planned to analyze in the near future the effect of the different patterns of VC in cardiovascular events.
Vascular ultrasound is able to clearly differentiate the intima, media, and adventitia of the vascular wall; therefore, we can also localize the abnormalities in these different structures. However, we cannot identify the IEL; therefore, trying to allocate the calcium in the intima or in the IEL might be purely speculative.
Moreover, we have focused our attention on patients on dialysis, and a broader approach, including different stages of CKD, is needed. In that sense, we have started a multicenter, observational study (NEFRONA project: http://www.nefrona.es) planned to recruit 2668 patients at different stages of CKD with a follow-up period of 4 years. Among the aims of the study is the identification, at every stage of CKD, of the significant variables related to the different phenotypes of VC (29).
In summary, VC in large arteries is more prevalent in dialysis patients than controls, and it is predominantly a linear pattern located in the lumen-intima interphase. Age, dialysis, past medical history of CVD, atherosclerosis, and inflammation are variables significantly influencing VC, and further studies assessing the effect in cardiovascular events are necessary.
Disclosures
None.
Acknowledgments
We are especially thankful to all integrants of UDETMA (www.udetma.com). This work has been financially supported by the Instituto de Salud Carlos III (Programa Miguel Servet and FIS). The Instituto de Salud Carlos III funds Blai Coll.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Reslerova M, Moe SM: Vascular calcification in dialysis patients: Pathogenesis and consequences. Am J Kidney Dis 41(Suppl 1): S96–S99, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Okuno S, Ishimura E, Kitatani K, Fujino Y, Kohno K, Maeno Y, Maekawa K, Yamakawa T, Imanishi Y, Inaba M, Nishizawa Y: Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis 49: 417–425, 2007 [DOI] [PubMed] [Google Scholar]
- 3. London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Adragão T, Pires A, Birne R, Curto JD, Lucas C, Gonçalves M, Negrão AP: A plain x-ray vascular calcification score is associated with arterial stiffness and mortality in dialysis patients. Nephrol Dial Transplant 24: 997–1002, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW: New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: A 25-year follow-up study. Atherosclerosis 132: 245–250, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Garland JS, Holden RM, Groome PA, Lam M, Nolan RL, Morton AR, Pickett W: Prevalence and associations of coronary artery calcification in patients with stages 3 to 5 CKD without cardiovascular disease. Am J Kidney Dis 52: 849–858, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Fox CS, Larson MG, Vasan RS, Guo CY, Parise H, Levy D, Leip EP, O'Donnell CJ, D'Agostino RB, Sr, Benjamin EJ: Cross-sectional association of kidney function with valvular and annular calcification: The Framingham heart study. J Am Soc Nephrol 17: 521–527, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Amann K: Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol 3: 1599–1605, 2008 [DOI] [PubMed] [Google Scholar]
- 9. McCullough PA, Agrawal V, Danielewicz E, Abela GS: Accelerated atherosclerotic calcification and Monckeberg's sclerosis: A continuum of advanced vascular pathology in chronic kidney disease. Clin J Am Soc Nephrol 3: 1585–1598, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Nakamura S, Ishibashi-Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y: Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol 4: 1892–1900, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coll B, Feinstein SB: Carotid intima-media thickness measurements: Techniques and clinical relevance. Curr Atheroscler Rep 10: 444–450, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Espeland MA, Craven TE, Riley WA, Corson J, Romont A, Furberg CD: Reliability of longitudinal ultrasonographic measurements of carotid intimal-medial thicknesses. Asymptomatic Carotid Artery Progression Study Research Group. Stroke 27: 480–485, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Coll B, Betriu A, Martinez-Alonso M, Borras M, Craver L, Amoedo ML, Marco MP, Sarro F, Junyent M, Valdivielso JM, Fernandez E: Cardiovascular risk factors underestimate atherosclerotic burden in chronic kidney disease: Usefulness of non-invasive tests in cardiovascular assessment. Nephrol Dial Transplant 25: 3017–3025, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Early detection of atherosclerosis: A randomized trial in the primary prevention of cardiovascular diseases (PRIMARIA). NCT00734123. Available at: http://clinicaltrials.gov/ct2/show/NCT00734123 Accessed September 17, 2010
- 15. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee Seventh report on the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 16. de La Piedra C, Fernández E, González Casaus ML, González Parra E: Different biological functions in PTH molecules. What are we measuring? Nefrologia 28: 123–128, 2008 [PubMed] [Google Scholar]
- 17. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N: Mannheim carotid intima-media thickness consensus (2004–2006). Cerebrovasc Dis 23: 75–80, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM: Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938–942, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Cardús A, Parisi E, Gallego C, Aldea M, Fernández E, Valdivielso JM: 1,25-Dihydroxyvitamin D3 stimulates vascular smooth muscle cell proliferation through a VEGF-mediated pathway. Kidney Int 69: 1377–1384, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Apter JT, Rabinowitz M: Correlation of visco-elastic properties of large arteries with microscopic structure (1). Methods used and their justification (2). Elastin and muscle determined chemically. Circ Res 19: 104–121, 1966 [Google Scholar]
- 21. Greenwald SE: Ageing of the conduit arteries. J Pathol 211: 157–172, 2007. 17200940 [Google Scholar]
- 22. Lekakis JP, Papamichael CM, Cimponeriu AT, Stamatelopoulos KS, Papaioannou TG, Kanakakis J, Alevizaki MK, Papapanagiotou A, Kalofoutis AT, Stamatelopoulos SF: Atherosclerotic changes of extracoronary arteries are associated with the extent of coronary atherosclerosis. Am J Cardiol 85: 949–952, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Ruengsakulrach P, Sinclair R, Komeda M, Raman J, Gordon I, Buxton B. Comparative histopathology of radial artery versus internal thoracic artery and risk factors for development of intimal hyperplasia and atherosclerosis. Circulation 100(Suppl II): 139–144, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Micheletti RG, Fishbein GA, Currier JS, Singer EJ, Fishbein MC: Calcification of the internal elastic lamina of coronary arteries. Modern Pathology 21: 1019–1028, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E: Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Süleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wüthrich RP, Gottlow M, Johnsson E, Zannad F; AURORA Study Group Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 360: 1395–1407, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Strippoli GF, Navaneethan SD, Johnson DW, Perkovic V, Pellegrini F, Nicolucci A, Craig JC: Effects of statins in patients with chronic kidney disease: meta-analysis and meta-regression of randomised controlled trials. BMJ 336: 645–651, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Onufrak SJ, Bellasi A, Shaw LJ, Herzog CA, Cardarelli F, Wilson PW, Vaccarino V, Raggi P: Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis 199: 424–431, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Junyent M, Martínez M, Borrás M, Bertriu A, Coll B, Craver L, Marco MP, Sarró F, Valdivielso JM, Fernández E. Usefulness of imaging techniques and novel biomarkers in the prediction of cardiovascular risk in patients with chronic kidney disease in Spain: The NEFRONA Project. Nefrologia 30: 119–126, 2010 [DOI] [PubMed] [Google Scholar]