Summary
Background and objectives
A negative correlation between the weekly standard Kt/V (urea) and serum cystatin C level (CysC) in functionally anephric dialysis patients has been previously demonstrated. Our objective was to measure the per dialysis CysC reduction ratio (CCRR) and to compare it with other indices of dialytic functions.
Design, setting, participants, & measurements
In a pilot cross-sectional study of 15 functionally anephric patients on conventional high-flux high-efficiency hemodialysis three times per week, CysC levels were drawn pre-, mid-, and postdialysis over 1 week. CCRR was compared with single-pool Kt/V (Sp Kt/V) using urea kinetic modeling, urea reduction ratio (URR), creatinine reduction ratio (CRR), normalized liters processed (LP/kg), and ultrafiltration volume (UF). Normally distributed data (Shapiro-Wilks test) were described as mean ± SD, otherwise as median and interquartile range.
Results
The mean pre- and post-CysC levels were 6.0 ± 1.0 and 4.7 ± 1.1 mg/L. The Sp Kt/V and Std Kt/V were 1.5 ± 0.2 and 2.6. The URR, CRR, and CCRR were 70.2% ± 9.0%, 64.5% ± 8.2%, and 26.1% ± 11.8%, respectively. There was no correlation between the CCRR, and the Sp Kt/V, URR, and CRR, whereas CCRR correlated with LP/kg and UF. Multiple regression analysis with these two parameters provided a model that explained 81% of the variance.
Conclusions
Our data suggest that normalized liters processed and ultrafiltration volume explain most of the variance of CCRR. Therefore, CCRR may be an excellent method to monitor dialysis efficiency of low molecular weight proteins.
Introduction
Serum creatinine and urea are small molecules that are commonly measured to monitor renal function in patients with chronic kidney disease (CKD). Serum creatinine (SCr) is the most commonly used surrogate marker for assessing kidney function in patients with CKD stages I through IV. It has molecular weight of 113 D. It is a metabolic product of creatine and phosphocreatine (1). The use of serum urea (SUr) is recommended by the Kidney Disease Outcomes Quality Improvement (KDOQI) clinical practice guideline to assess dialysis clearance (2). It has molecular weight of 60 D (3).
The preferred assessment of hemodialysis (“dose”) efficiency is by urea kinetic modeling (UKM) calculating the dimensionless parameter Kt/V (urea) (where K = clearance in ml/min, t = time in minutes, and V = volume of distribution in ml). Kt/V values may be given for single-pool (Sp Kt/V) or double-pool (equilibrated or eKt/V) volumes of distribution; they depend upon the urea reduction ratio (URR) over a single hemodialysis treatment (4). To assess dialysis efficiency over a period of 1 week, Gotch derived a new dialysis assessment index named the weekly “standardized” Kt/V (Std Kt/V) (5,6). Std Kt/V allows comparison of different dialysis modalities (e.g., peritoneal versus hemodialysis) and weekly treatment frequencies.
Cystatin C (CysC) is a low molecular weight protein (13 kD, 121 amino acid residues) that is produced by all nucleated cells (7). It is positively charged with an isoelectric point of 9.3. CysC has attractive characteristics as a marker for assessing native kidney or dialysis clearance. Its plasma level is not influenced by age, sex, and body mass index (8). It is distributed mainly extracellularly (9). Its production is relatively constant and it is freely circulating (10). However, it may be affected by conditions that alter cell turnover rate, such as inflammation or thyroid dysfunction (11,12). It remains controversial whether glucocorticoid medications may change the serum CysC level (9,13). Estimated GFR (eGFR) by CysC has shown to be superior to eGFR by SCr with patients with CKD (14–16).
There have been few studies of CysC in dialysis. In peritoneal dialysis patients, the study by Delaney et al. showed that CysC levels are mainly related to the residual renal function, rather than the dialysis clearance (17). Furthermore, the study by Hoek et al. demonstrated a good correlation between 1/CysC and residual renal function (18). To obviate the effect of residual renal function, we examined predialysis or steady state serum CysC levels and found these to be influenced by the dialytic treatment modality and the Std Kt/V and hence were lowest in patients receiving 5 to 7 nights per week of hemodialysis as compared with conventional hemodialysis and peritoneal dialysis (19). There are a few studies using CysC reduction ratio (CCRR) to assess CysC hemodialysis clearance. Thysell et al. demonstrated that with low-flux hemodialysis, CysC concentrations rose after dialysis by 4% ± 6.3% (20). Furthermore, Lindström et al. compared CCRR after hemodiafiltration, hemofiltration, and low-flux hemodialysis (21). The posttreatment CysC concentrations were lowest after hemofiltration and highest after low-flux hemodialysis. A recent study by Park et al. showed a more effective CysC clearance by high-flux hemodialysis compared with low-flux hemodialysis (10). Although these studies have demonstrated the potential value of CysC as an indicator of middle molecule clearance, the variables affecting CCRR were not identified.
In this study, we aimed at assessing the CysC hemodialysis clearance and handling compared with the two small molecules urea and creatinine. Given a largely extracellular distribution of CysC and a presumed slow equilibration between the intravascular and the extravascular volume because of its size, we hypothesized that CysC clearance by high-flux hemodialysis treatment would largely depend on the dialysis dose but could be negatively influenced by the ultrafiltration volume.
Materials and Methods
In this cross-sectional, single-center, open study of patients with ESRD receiving hemodialysis therapy, a total of 15 patients were recruited. All patients provided written informed consent. All patients were on three times weekly high-flux high-efficiency hemodialysis therapies. Only functionally anephric patients, defined as urine output <250 ml per day, were included in the study. Patients were excluded if they did not consent to the study, or if during the previous 3 months hospitalization or dialysis prescription changes occurred. The study was approved by the Ethic Review Board at the University of Western Ontario (HSREB#16599E).
All patients were dialyzed using high-flux high-efficiency polysulphone membrane dialyzers (Optiflux F160NR or F200NR; Fresenius Inc., Toronto, Canada). Either central venous catheters or fistulas served as dialysis access. The blood flow was between 300 and 400 ml/min and the dialysate flow was at 500 ml/min. The blood samples were taken through the patients' dialysis access at the beginning, at the middle, and at the end of their dialysis sessions. The blood samples were taken at all three hemodialysis sessions over a 1-week period. In addition to CysC, SCr, and SUr, the predialysis blood samples prior to the first hemodialysis session included thyroid function (TSH) and C reactive protein (CRP) measurements to exclude their possible influence on CysC levels. Blood samples were taken at mid-dialysis for CysC measurements. Finally, additional blood samples for CysC, SCr, and SUr were taken postdialysis. The postdialysis blood samples utilized the 15-second slow-flow methodology to obviate urea dilution by recirculation (4). For the analyses, the averages of all three pre-, mid-, and postdialysis measurements were used.
CysC was measured by immune nephelometry using an N-latex cystatin C kit (Dade Behring, Mississauga, Canada) on a Behring BN ProSpec analyzer (Dade Behring, Marburg, Germany) at the reference laboratory at the Children's Hospital of Eastern Ontario in Ottawa. The coefficient of variation (CV) of the CysC measurements in blood has been previously established at 3.1% at 1.06 mg/L, 3.5% at 2.04 mg/L, and 6.7% at 5.26 mg/L (22). CysC was reported as an absolute level in mg/L, rather than as eGFR. SCr was measured by modified Jaffe's reaction, using the Synchron System Kits on a Beckman Coulter LX20 Pro (Beckman Coulter Inc., Brea, CA) with a normal adult reference interval of 55 to 120 μmol/L. CRP was measured by immunonephelometry (Dade Behring BN Prospec, Mississauga, Canada) with CV of 4.02% at the level of 12.79 mg/L and 4.48% at 50.87 mg/L. TSH was measured by direct chemiluminescence assay (Bayer Centaur Instrument, Germany).
The single hemodialysis treatment efficacy was taken as the Sp Kt/V calculated by UKM. It was carried out during the second hemodialysis session of the week. The Std Kt/V also calculated from UKM based on Gotch's initial paper (5). Reduction ratios for CysC (CCRR), urea (URR), and creatinine (CRR) were calculated by taking the difference between pre- and postdialysis levels, and divided by predialysis levels. We assumed that the volume of distribution of CysC is different from that of urea and creatinine yet still related to body weight. We also assumed the dialyzer clearance of CysC is mainly related to the dialysis circuit blood flow and total amount removed by time (surface area being similar for all). We, thus, hypothesized that CCRR will be related to the liters of blood processed (LP; L) during dialysis normalized by the target postdialysis weight (LP/kg). LP (L) = dialyzer blood flow (Qb) (ml/min) × time (minutes). LP values were obtained at the end of each dialysis directly from the dialysis machine. The amount of ultrafiltration (L) during dialysis was recorded as it was also felt to influence CCRR by (a) convective removal versus (b) hemoconcentration of CysC.
Statistical Analyses
Statistical analysis was performed using the GraphPad Prism software version 4.03 for Windows (GraphPad Software, San Diego). For the multiple stepwise regression analysis, Medcalc version 11.2.1.0 (Medcalc Software bvba, Mariakerke, Belgium) was used. Contiguous data were analyzed for normal distribution with the Shapiro-Wilk normality test. Mean and SD were reported for normally distributed data; otherwise, median, 25th percentile, and 75th percentile (interquartile range) were given. The paired t test for normally distributed variables and the Mann-Whitney test for non-normally distributed variables were used to compare between the pre- and postdialysis CysC, SCr, and SUr levels. We also compared predialysis levels from the three dialysis sessions to assess the intrapatient variability. Depending on whether or not data were normally distributed, Pearson's correlation or the nonparametric (Spearman's rank) correlation analysis was used to assess the strength of relationship between CCRR, and URR, CRR, Sp Kt/V, Std Kt/V, TSH, and CRP as well as LP/kg and UF. Pearson correlation coefficients were expressed as r values and the significance level of the P value was also recorded. A P value of <0.05 was considered significant. For the multiple regression analysis, we calculated the correlation coefficients r2: this is the proportion of the variation in the dependent variable explained by the regression model. It can range from 0 to 1 and is a measure of the goodness of fit of the model.
Results
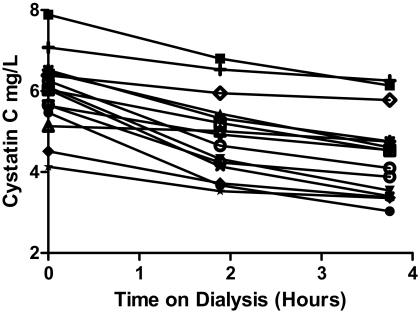
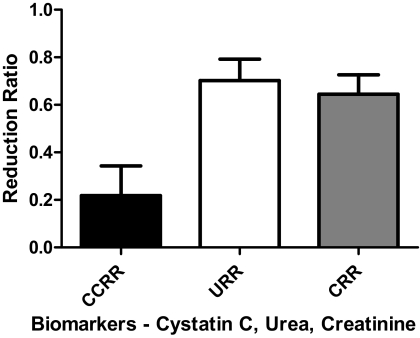
A total of 15 patients were enrolled into the study, all functionally anephric with urine output <250 ml/d. All patients met the inclusion criteria without violating exclusion criteria with unchanged conventional three times weekly in center high-efficiency and high-flux hemodialysis prescription and without hospitalizations over the last 3 months. The mean age ± SD was 67.3 ± 11.2 years. The most common cause of ESRD was diabetes mellitus (53.3%). The median dialysis time was 3.75 (3,4) hours per session. The mean pre- and postdialysis CysC concentrations were 5.96 ± 0.94 and 4.66 ± 1.09 mg/L, respectively (Figure 1). All the patients had nine CysC values over the 1-week interval except one patient who had a single missing postdialysis CysC value. The mean Sp Kt/V was 1.51 ± 0.24, whereas the median Std Kt/V was 2.63 (2.15, 2.71). The median TSH (normal range 0.27 to 4.20 mIU/L) was 1.62 (1.31, 3.16) mIU/L. The mean CRP concentration (normal range ≤5.0 mg/L) was 20.51 ± 15.13 mg/L. The mean LP/kg and UF were 0.89 ± 0.21 L/kg and 2.84 ± 1.06 L. Clinical results are summarized in Table 1. The URR, CRR, and CCRR were 70.2% ± 9.0%, 64.5% ± 8.2%, and 26.1% ± 11.8% (P ≤ 0.002), respectively (Figure 2).
Figure 1.
Mean cystatin C levels during hemodialysis sessions. This figure shows for each of the 15 patients the cystatin C levels at the start, the middle, and the end of dialysis. Each value represents the average of three dialysis treatments.
Table 1.
Baseline characteristics
| Baseline Characteristics | Meana/Medianb/Totalc | SD | Interquartile Range (25% Minimum, 75% Maximum) |
|---|---|---|---|
| Age (years) | 67.33 | 11.20 | |
| Dialysis time (hours) | 3.75 | 3.00, 400 | |
| Sp Kt/V | 1.51 | 0.24 | |
| Std Kt/V | 2.63 | 2.15, 2.71 | |
| Predialysis cystatin C (mg/L) | 5.95 | 0.94 | |
| Postdialysis cystatin C (mg/L) | 4.66 | 1.09 | |
| TSH (mIU/L) | 1.62 | 1.31, 3.16 | |
| CRP (mf/L) | 20.51 | 15.13 | |
| LP/kg (L/kg) | 0.89 | 0.21 | |
| UF (L) | 2.84 | 1.06 | |
| Cause of renal failure: N (%) | |||
| diabetes | 8 (53.3) | NA | |
| hypertension | 1 (6.6) | NA | |
| renal cancer | 1 (6.6) | NA | |
| glomerularnephritis | 2 (13.3) | NA | |
| acute renal failure | 1 (6.6) | NA | |
| polycystic kidney disease | 1 (6.6) | NA | |
| reflux nephropathy | 1 (6.6) | NA |
Expressed as mean if the variable is normally distributed by Shapiro-Wilk normality test.
Expressed as median if the variable is not normally distributed by Shapiro-Wilk normality test.
Expressed in total number and percentage.
Figure 2.
Cystatin C, urea, and creatinine reduction ratios. The reduction ratio for each of the biomarkers is shown. The values for URR, CRR, and CCRR were 70.2% ± 9.0%, 64.5% ± 8.2%, and 26.1% ± 11.8%, respectively. By paired t test, each postdialysis biomarker concentration was significantly lower than the predialysis value (P = 0.002).
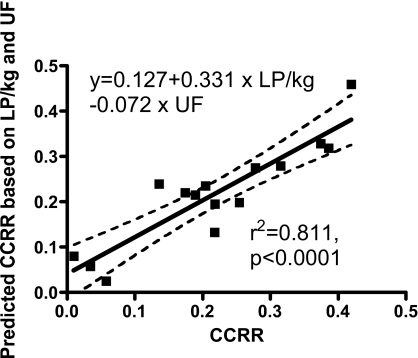
There were no statistically significant correlations between the CCRR and Sp Kt/V, URR, and CRR (P > 0.151). The correlation coefficient between CCRR and LP/kg was r = 0.678 (P = 0.006). There also was a significant but negative correlation between CCRR and UF (r = −0.724, P = 0.002). Multiple regression analysis with these two parameters provided a model that explained 81% of the variance (r2 = 0.811, P < 0.001), CCRR = 0.127 + 0.331 × LP/kg − 0.072 × UF. The correlation between the measured CCRR and that calculated by this model is shown in Figure 3. There was no correlation between predialysis CysC, and Std Kt/V, TSH, and CRP (P > 0.166). As expected, there were strong correlations between Sp Kt/V, and URR (r = 0.770, P < 0.001) and CRR (r = 0.727, P = 0.002). URR and CRR correlated weakly with LP/kg but not with UF. The results of correlation analyses are summarized in Table 2.
Figure 3.
Correlation analysis of the measured CCRR and the calculated CCRR based on a model using the ultrafiltration volume (UF [L]) and the normalized liters processed (LP/kg [L/kg]). The model explained 81% of the variance. This figure shows a highly significant linear correlation between the predicted CCRR and the measured CCRR (r2 = 0.811, P < 0.001).
Table 2.
Correlation analysis and the multivariable analysis between CCRR, URR, and CRR, and other variables
| Variable 1 | Variable 2 | Variable 3 | Correlation Coefficients | P |
|---|---|---|---|---|
| CCRR | Sp Kt/V | 0.212a | 0.447 | |
| CCRR | URR | 0.390a | 0.151 | |
| CCRR | CRR | 0.363a | 0.184 | |
| CCRR | LP/kg | 0.678a | 0.006 | |
| CCRR | UF | −0.724a | 0.002 | |
| UUR | Sp Kt/V | 0.770a | <0.001 | |
| URR | LP/kg | 0.650a | 0.009 | |
| URR | UF | 0.029a | 0.920 | |
| CRR | Sp Kt/V | 0.727a | 0.002 | |
| CRR | LP/kg | 0.641a | 0.010 | |
| CRR | UF | −0.024a | 0.933 | |
| Pre-CysC | Std Kt/V | −0.377a | 0.166 | |
| Pre-CysC | TSH | −0.196a | 0.485 | |
| Pre-CysC | CRP | 0.339a | 0.216 | |
| CCRR | UF | LP/kg | 0.811b | <0.001 |
| URR | UF | LP/Kg | 0.359b | 0.072 |
| CRR | UF | LP/kg | 0.327b | 0.067 |
Pearon correlation coeffecient (r).
Correlation coefficient (r2).
There were no significant differences between the three predialysis CysC levels (paired t test, P > 0.115) of the three dialysis sessions in the 1 week. For the predialysis SUr, however, there was a significant difference between session 1 and session 3 (Mann-Whitney test, P = 0.029). For the predialysis SCr, there were significant differences between session 2 and session 3 (paired t test, P < 0.001), and session 1 and session 3 (paired t test, P = 0.005).
Discussion
The purpose of this study was to evaluate the dialysis clearance and handling of CysC and the variables that affect its clearance in a single high-flux high-efficiency hemodialysis session. It is a continuation of the Al-Malki et al. study (19). There was significant CysC reduction through a single high-flux high-efficiency hemodialysis session. The CCRR was 26.1% ± 11.8%. This is lower than the small solutes clearance, with URR and CRR being 70.2% ± 9.0% and 64.5% ± 8.2%, respectively. There was no significant correlation between CCRR and the small-solute clearance (Sp Kt/V, URR, and CRR). Multiple regression analysis with the LP/kg and UF provided a model that explained 81% of the variance (r2 = 0.811, P < 0.001), CCRR = 0.127 + 0.331 × LP/kg − 0.072 × UF. To the best of our knowledge, this is the first description of the parameters that influence CCRR.
Thysell et al. showed a paradoxical increase in postdialysis CysC level in low-efficiency hemodialysis (20). This was likely due to hemoconcentration and slow equilibration of CysC between intravascular and extravascular spaces. A previous study demonstrated that CysC elimination was more efficient by hemodiafiltration compared with low-flux hemodialysis (21). Park et al. showed a significant difference between low- and high-flux dialyzers in CysC clearance (10). CCRR results were 11.5% ± 16.2% with low-flux dialyzers and 42.4% ± 6.3% with high-flux dialyzers, respectively, with a significant difference in CCRR between dialyzers (P < 0.0001). The lower CCRR of 26% in our study despite very high blood flows may be explained by differences in UF rates, although no details were provided in the Park manuscript. Park et al. also demonstrated a weak correlation between CCRR, and URR and eKt/V. By contrast, there was a strong correlation with CCRR and β2-microglobulin clearance. These studies have demonstrated a significant reduction of CysC through hemodialysis but the variables that affect the CysC clearance were not assessed (10).
The results of our study suggest a very different handling of CysC compared with the small solutes clearances of urea and creatinine, in a single high-flux high-efficiency hemodialysis treatment. All patients were functionally anephric, which eliminates the effects of residual renal function on CysC clearance. Three factors should affect CysC clearance: diffusive clearance, albeit small, convective clearance, and the ultrafiltration volume. So what do we know about cystatin C clearance to explain our findings?
CysC is a middle molecule that distributed mainly extracellularly (9). It is also minimally protein bound with presumed slow redistribution between intravascular space and extravascular space because of its size. Unfortunately, little is known about the equilibration of CysC between the intra- and extravascular space; however, in view of what is known about other middle molecule redistribution, we can assume a slow equilibration (23). Zingraff et al. compared the clearance of radioiodinated serum amyloid P component (125I-SAP), a constituent for systemic amyloidosid deposits, in the healthy subjects and the chronic hemodialysis patients. In the hemodialysis patients, the decline was in a biexponential mode, rather than a single-exponential slope. There was also evidence of “tissue retention” of 125I-SAP in the extravascular space. This was enhanced in patients with symptomatic dialysis–related amyloidosis (23). By contrast, SUr and SCr are distributed both in extracellular (both intra- and extravascular) and intracellular spaces (24), with presumed rapid equilibration between all three compartments during hemodialysis. It is presumed that small molecules are mostly affected by diffusive clearance and relatively unaltered by UF because of rapid equilibration. By contrast, CCRR is affected by a combination of diffusive and convective clearance. Park's data suggest that convective clearance is much more important for CysC (10). Removal of some cystatin C by membrane adbsorbtion as does occur with β2-microglobulin (25) must also be considered. There is, as yet, no published information on this. This possibility needs to be explored.
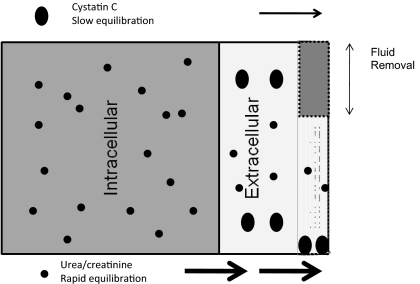
Given these facts and after establishing the inverse correlation between UF and CCRR, we hypothesize that the different volumes of distribution and differing equilibration times between compartments for CysC, creatinine, and urea explain our findings. We hypothesize that urea and even more so creatinine equilibrate quickly between the intra- and extravascular space, thereby remaining unaffected by UF. By contrast, CysC is altered only in the intravascular space by the dialysis, equilibrates slowly, and is largely affected by the sometimes substantial UF observed in our patients (maximum 5 L) in this study. Figure 4 presents a hypothetical model for the different handling of the two molecule classes. This model is highly supported by the fact that we can explain 81% of the variance by UF and liters processed (normalized per kg).
Figure 4.
Kinetic model of creatinine, urea, and cystatin C during hemodialysis. Cystatin C is a middle molecule that is distributed mainly extracellular and minimally protein bound. It was presumed to have slow redistribution between intravascular space and extravascular space because of its size. By contrast, urea and creatinine are distributed both in extracellular (both intra- and extravascular) and intracellular spaces, with presumed rapid equilibration between all three compartments during hemodialysis. As a result, it is presumed that small molecules are mostly affected by diffusive clearance and relatively unaltered by UF because of rapid equilibration. By contrast, CCRR is affected by a combination of diffusive and convective clearance and by ultrafiltration, which may concentrate the intravascular content of cystatin C.
Our study had limitations. It was a small pilot study of only 15 patients. All the patients used high-flux dialyzers. The study results are not applicable to low-flux hemodialysis treatments. The previous study by Al-Malki et al. demonstrated a significant negative correlation between Std Kt/V and predialysis CysC in functionally anephric patients (19). We did not find such a correlation and did not expect to because of the small sample size and the narrow range of Std Kt/V values obtained from patients on identical dialysis modalities. We did not assess CysC rebound posthemodialysis. This was previously demonstrated by the Lindström study; there was a rise in CysC level by 12% in the hemodiafiltration group (21). Further studies comparing rebound after dialysis with no ultrafiltration and ultrafiltration without dialysis are planned. A further limitation is the fact that the cystatin C assay used is not validated for measurements in aqueous solution. Thus, actual clearance or removal measurements could not be made.
Why is CysC an attractive dialysis adequacy marker? By increasing small-molecule clearance beyond that established as thresholds for adequacy in conventional hemodialysis and peritoneal dialysis, the HEMO and the ADEMEX studies have failed to show any mortality benefit (26,27). By contrast, there is evidence that CysC levels associate with clinical outcome. CysC levels have been shown to correlate with cardiac mortality in patients with coronary heart disease (28). In patients with stage III or IV CKD, the CysC level is associated with all-cause and cardiovascular disease mortality (29). If CysC level correlates with clinical outcome in the dialysis population regardless of the residual renal function, it may become an important dialysis adequacy parameter. As a result, further studies remain to assess this association and the target of a satisfactory CysC level.
Conclusions
This study is the first to define the parameters that determine CCRR. The total dialysis dose measured as normalized liters processed plus the ultrafiltration rate are the most important determinants for Cystatin C reduction ratio. This is novel. On the basis of molecular characteristics, we hypothesize on the differences that explain the different handling of SCr and SUr on the one hand and CysC on the other. The current study provides a first model for the kinetics of cystatin C removal by dialysis. Further studies are indicated.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S: Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am J Clin Nutr 37: 478–494, 1983 [DOI] [PubMed] [Google Scholar]
- 2. National Kidney Foundation: 2006 updates clinical practice guidelines and recommendations. Hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis 48[Suppl 1]: S1–S322, 2006. 17045862 [Google Scholar]
- 3. Cheung AK: Hemodilaysis and hemodialtration. In: Primer on kidney diseases, 4th edition, edited by Greenberg A. Philadelphia, Elsevier Saunders, 2005, pp 464–476 [Google Scholar]
- 4. Daugirdas JT: Physiologic principles and urea kinetic modeling. In: Handbook of dialysis, 4th edition, edited by Daugirdas JT, Blake PG, Ing TS. Philadelphia, Lippincott Williams & Wilkins, 2007, pp 25–58 [Google Scholar]
- 5. Suri R, Blake P: Adequacy of hemodialysis. In: Replacement of renal function by dialysis, 5th edition, edited by Horl WH, Koch KM, Lindsay RM, Ronco C. London, Kluwer Academic Publishers, 2004, pp 597–638 [Google Scholar]
- 6. Gotch FA: The current place of urea kinetic modeling with respect to different dialysis modalities. Nephrol Dial Transplant 13[Suppl 6]: 10–14, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Prigent A: Monitoring renal function and limitations of renal function tests. Semin Nucl Med 38: 32–46, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Sharma AP, Kathiravelu A, Nadarajah R, Yasin A, Filler G: Body mass does not have a clinically relevant effect on cystatin C eGFR in children. Nephrol Dial Transplant 24: 470–474, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Filler G, Bökenkamp A, Hofmann W, Le Bricon T, Martínez-Brú C, Grubb A: Cystatin C as a marker of GFR: History, indications, and future research. Rev Clin Biochem 38: 1–8, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Park JS, Kim GH, Kang CM, Lee CH: Application of cystatin C reduction ratio to high-flux hemodialysis as an alternative indicator of the clearance of middle molecules. Korean J Intern Med 25: 77–81, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manetti L, Pardini E, Genovesi M, Compomori A, Grasso L, Morselli LL, Lupi I, Pellegrini G, Bartalona L, Bogazzi F, Martino E: Thyroid function differently affects serum cystatin C and creatinine concentrations. J Endocrinol Invest 4: 346–349, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Wasen E, Isoaho R, Vahlberg T, Kivela SL, Irjala K: Association between markers of renal function and C reactive protein level in the elderly; confounding by functional status. Scan J Clin Lab Invest 68: 484–491, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Foster J, Reisman W, Lepage N, Filler G: Influence of commonly used drugs on the accuracy of cystatin C-derived glomerular filtration rate. Pediatr Nephrol 21: 235–238, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Simonsen O, Grubb A, Thysell H: The blood serum concentration of cystatin C (gamma-trace) as a measure of the glomerular filtration rate. Scand J Clin Lab Invest 45: 97–101, 1985 [DOI] [PubMed] [Google Scholar]
- 15. Löfberg H, Grubb AO: Quantitation of g-trace in human biological fluids: indications for production in the central nervous system. Scand J Clin Lab Invest 39: 619–626, 1979 [DOI] [PubMed] [Google Scholar]
- 16. Dharnidharka V, Kwon C, Stevens G: Cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis 40: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Delaney MP, Stevens PE, Al Hasani M, Stowe HJ, Judge C, Lamb EJ: Relationship of serum cystatin C to peritoneal and renal clearance measures in peritoneal dialysis: A cross-sectional study. Am J Kidney Dis 51: 278–284, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Hoek FJ, Korevaar JC, Dekker FW, Bioeschoten EW, Krediet RT: Estimation of residual glomerular filtration rate in dialysis patients from the plasma cystatin C level. Nephrol Dial Transplant 11: 1633–1638, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Al-Malki N, Heidenheim PA, Filler G, Yasin A, Lindsay RM: Cystatin C levels in functionally anephric patients undergoing dialysis: the effect of different methods and intensities. Clin J Am Soc Nephrol 4: 1606–1610, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thysell H, Grubb A, Lindholm T, Ljunggren L, Mårtensson L: Cystatin C: A new marker of biocompatibility or a good marker for the redistribution of LMW proteins during hemodialysis? ASAIO Trans 34: 202–204, 1988 [PubMed] [Google Scholar]
- 21. Lindström V, Grubb A, Alquist Hegbrant M, Christensson A: Different elimination patterns of β-trace protein, β2-microglobulin and cystatin C in haemodialysis, haemodiafiltration and haemofiltration. Scand J Clin Lab Invest 68: 685–691, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Sambasivan AS, Lepage N, Filler G: Cystatin C intrapatient variability in children with chronic kidney disease is less than serum creatinine. Clin Chem 51: 2215–2216, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Zingraff J, Caillat-Vigneron N, Ureña P, Gagné ER, Bererhi L, Moretti JL, Bardin T, Drüeke TB: Plasma kinetics of 125I-labelled amyloid P component in beta 2M amyloidosis: A possible approach to quantitate disease activity. Nephrol Dial Transplant 10: 223–229, 1995 [PubMed] [Google Scholar]
- 24. Kristensen K, Lindström V, Schmidt C, Blirup-Jensen S, Grubb A, Wide-Swensson D, Strevens H: Temporal changes of the plasma levels of cystatin C, beta-trace protein, beta2-microglobulin, urate and creatinine during pregnancy indicate continuous alterations in the renal filtration process. Scand J Clin Lab Invest 67: 612–618, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Clark WR, Macias WL, Molitoris BA, Wang NH: Membrane adsorbtion of beta 2-microglubulin: Equilibrium and kinetic characterization. Kidney Int 46: 1140–1146, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R: Hemodialysis (HEMO) Study Group: Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S: Mexican Nephrology Collaborative Study Group: Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 13: 1307–1320, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Ix JH, Shlipak MG, Chertow GM, Whooley MA: Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: Data from the Heart and Soul Study. Circulation 115: 173–179, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Menon V, Shlipak MG, Wang X, Coresh J, Greene T, Stevens L, Kusek JW, Beck GH, Collins AJ, Levey AS, Sarnak MJ: Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med 147: 19–27, 2007 [DOI] [PubMed] [Google Scholar]