Summary
Background and objectives
The objective of this study was to describe the renal and extrarenal findings in patients with recessively inherited familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC) associated with CLDN19 mutations.
Design, setting, participants, & measurements
Medical records of three patients from two French unrelated families with CLDN19 mutations were retrospectively examined.
Results
Direct sequencing of CLDN19 identified a known variant (p.Gly20Asp) in all patients and a new missense mutation (p.Val44Met) in one (compound heterozygous). The patients' renal phenotype closely mimicked CLDN16-related nephropathy: low serum Mg2+ (<0.65 mmol/L) despite oral supplementation, hypercalciuria partly thiazide-sensitive, and progressive renal decline with ESRD reached at age 16 and 22 years in two individuals. Primary characteristics (failure to thrive, recurrent urinary tract infections, or abdominal pain), age at onset (0.8 to 16 years), and rate of renal decline were highly heterogeneous. Ocular involvement was identified in all patients, although two patients did not have visual loss. Additionally, exercise intolerance with pain, weakness, and electromyographical alterations mimicking a Ca2+/K+ channelopathy (pattern V) were observed in two of three individuals. These features persisted despite the normalization of serum K+ and Mg2+ after renal transplantation.
Conclusions
Ocular manifestations, even subtle, and exercise intolerance mimicking mild to moderate periodic paralysis are two symptoms that need to be searched for in patients with FHHNC and may indicate CLDN19 mutations.
Introduction
Nephrocalcinosis is a histologic condition defined by an increase in kidney calcium content, which can be easily identified by an abdominal radiography or a computed tomography scan. It relies on a broad spectrum of etiologies that can be separated into hypercalcemic states of various origin or specific inherited tubular disorders (1).
Among the genetic causes of nephrocalcinosis, familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC) is a rare tubulopathy with recessive inheritance. Most patients with FHHNC harbor a mutation in the CLDN16 gene, which encodes for claudin-16 (OMIM248250) (2–4). Also, mutations of CLDN19, which encodes for claudin-19, have been identified in a subgroup of FHHNC patients with severe ocular involvement (OMIM248190) (5). In mice kidneys, claudin-16 and claudin-19 are expressed in the medullary and cortical thick ascending limb of the loop of Henle (5), where they are colocalized to tight junction proteins and form a cation-selective complex (6). Although their role remains poorly understood (7,8), they probably modulate the passive paracellular magnesium (Mg2+) and calcium (Ca2+) transport in these tubular segments, which is driven by an electrical gradient resulting from potassium (K+) exit across apical membranes through the renal outer-medullary K+ channel and chloride (Cl−) and sodium (Na+) exit across basal membranes through the Cl− channel and Na+-K+-ATPase, respectively (9).
The renal phenotype associated with CLDN19 mimics CLDN16 mutations (5,10). It encompasses renal Mg2+ and Ca2+ wasting, leading to hypomagnesemia, nephrocalcinosis, and progressive renal decline. FHHNC may be disclosed by a wide variety of symptoms, including recurrent urinary tract infection, polyuria and/or polydipsia, nephrolithiasis, or tetanic convulsions (11). In patients with CLDN16 mutations, progression to ESRD may be predicted by the genotype (12). Of note, Konrad et al. emphasized the severe ocular involvement (macular colobomata, nystagmus, myopia, visual loss) shown in CLDN19 contrasting with the mild ocular involvement in some CLDN16 patients (myopia, astygmatism, hypermetropia, strabism) (5,10).
In this study, we describe the renal and extrarenal features from three patients with CLDN19 mutations and suggest that intolerance to muscular exercise mimicking periodic paralysis may be part of the clinical spectrum associated with CLDN19 mutations.
Patients
We report three female patients, from two nonconsanguineous families, with FHHNC and CLDN19 mutations followed in two nephrology units in a university hospital in Toulouse in southwest France. Clinical characteristics and genotypes are presented in Table 1.
Table 1.
Phenotypic characteristics of three individuals with CLDN19 mutation
| Characteristic | Patient |
Normal Values | ||
|---|---|---|---|---|
| F1.3 | F1.4 | F2.3 | ||
| Gender | Female | Female | Female | |
| Mutation | p.Gly20Asp/p.Val44Met | p.Gly20Asp/p.Val44Met | p.Gly20Asp/p.Gly20Asp | |
| Age at renal symptoms onset, years | 10 | 0.9 | 16 | |
| Age at tests, years | 16 | 13 | 16 | |
| Organ involvement | ||||
| kidney | UTI, NL, NC | UTI, NL, NC | NC | |
| eye | PM, Strab, nystagmus | PM | Strab, Myopia, Ir colobomata | |
| nerve | Muscular-exercise intolerance | No | Muscular exercise intolerance | |
| Blood tests | ||||
| SCr, μmol/L | 180 | 200 | 123 | |
| inulin clearance, ml/min per 1.73 m2 | — | — | 48.8 | |
| eGFR (sMDRD), ml/min per 1.73 m2 | 29 | 30 | 53.7 | |
| Mg2+, mmol/L | — | — | 0.59 | 0.73 to 1.06 |
| K+, mmol/L | 3.5 | 3.3 | 3.7 | 3.5 to 5 |
| HCO3−, mmol/L | 23 | 17 | 28 | 21 to 30 |
| Ca2+, mmol/L | 2.1 | 1.84 | 2.35 | 2.2 to 2.6 |
| PO43−, mmol/L | 1.32 | 1.74 | 0.82 | 0.8 to 1.5 |
| 25(OH)vitamin D3, ng/ml | — | — | 13.3 | 9 to 45 |
| 1,25(OH)2 vitamin D3, pg/ml | — | — | 69 | 18 to 60 |
| IPTH, pg/ml | — | 150 | 155 | 15 to 85 |
| alkaline phosphatase, IU/L | 207 | 656 | 208 | 100 to 280 |
| Urinary tests | ||||
| FeMg2+, %a | — | — | 9.9 | <2% |
| Ca2+/Cr ratio, mg/mg | 0.9 | 1.2 | 0.9 | <0.4 |
| TmPO43−, % | — | — | 83.5 | >85 |
| citrate, mg/24 h | 101 | 285 | 362.4 | 400 to 900 |
| oxalate, μmol/24 h | 186 | 163 | 232.8 | 200 to 450 |
| pH (nocturnal) | — | — | 6.36 | |
| proteinuria, mg/24 h | — | — | 510 | <300 |
| hematuria, (0 to 3+) | 0 | 0 | 0 | |
| drug intake | Calcium pyridoxine | Calcium pyridoxine | No | |
| Renal outcome | ||||
| eGFR (sMDRD) | ESRD (at age 22) | ESRD (at age 16) | 51 (at age 17) | |
UTI, urinary-tract infection; NL, nephrolithiasis; NC, nephrocalcinosis; SCr, serum creatinine; PM, pigmentary maculopathy; Strab, strabismus; Ir, Iris; sMDRD, standard Modifications of Diet in Renal Disease equation; TmPO43−, maximum rate of renal tubular reabsorption of phosphate.
FeMg2+ was calculated as follows: urinary/plasma [Mg2+]/urinary/plasma [creatinine].
First symptoms (failure to thrive, urolithiasis with urinary tract infection, and abdominal pain) occurred from 0.9 to 16 years of age. Renal symptoms included recurrent urolithiasis, urinary tract infection, and/or polyuria-polydipsia. Concomitant bilateral nephrocalcinosis with renal atrophy and renal loss of Mg2+ and Ca2+ were found in all patients.
Hypomagnesemia persisted despite oral supplementation and was accompanied by hypokalemia in two individuals. Despite progressive renal failure, calciuria remained high. Although thiazide-diuretic intake lowered calciuria by 50% in one patient, serum Mg2+ remained unchanged. Hypocitraturia was identified in all patients and hyperchloremic acidosis in one. Serum phosphorus was within the normal range in all patients. In two sisters with early diagnosis of nephrocalcinosis, measurements of urinary oxalate level and activity and immunoblotting of alanine-glyoxylate amino-acid transferase from a liver biopsy excluded the hypothesis of primary oxalosis.
Slope of renal decline was heterogeneous among patients. Although one patient had stable estimated GFR (eGFR) between the age 16 and 17 years (51 ml/min per 1.73 m2), two patients reached ESRD at the age of 16 and 22 years after a progressive decrease in eGFR. Renal transplantation was performed in these latter patients. On a calcineurin inhibitor-based immunosuppressive regimen, Mg2+ and Ca2+ renal excretion were normal. Early and severe tertiary hyperparathyroidy occurred in both patients and required surgery. Mild nephrocalcinosis was observed upon 1-year renal biopsy in one patient but was absent in the other 6 years after renal transplantation.
In all patients, various ocular abnormalities of mild to moderate severity were identified, including pigmentary maculopathy, strabismus, iris colobomata, myopia, and nystagmus. However, none had severe visual impairment and asymptomatic pigmentary maculopathy was the only finding in one individual.
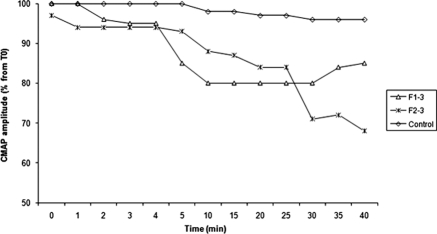
Last, two patients presented neuromuscular disorders (muscular-exercise intolerance characterized by limb stiffness and pain occurring a few minutes after beginning exercise in one and progressive weakness and abundant cramps in the other) having begun at age 14 and 21. A needle electromyography showed neither nerve conduction defect nor myotonic or paramyotonic abnormalities. Muscle-channel dysfunction was thus explored by recording surface compound motor action potential (CMAP) changes after exercise tests (repeating short exercises at room temperature and, after cooling, an extended period of exercise, as described by Fournier et al. [13]). CMAP amplitude correlates with the number of functional muscle fibers. Its decrease is an index of muscle weakness experienced after exercise. In our patients short exercise did not induce significant CMAP changes, whereas longer-duration exercise was followed by a transient 20% to 40% decrease in the amplitude of CMAP starting at 10 minutes after the end of the exercise (Figure 1). In one patient, CMAP decrease occurred while serum Mg2+ and K+ were in the normal range (posttransplant period). Autoimmune disease, viral infection, and drug or heavy metal toxicity were ruled out.
Figure 1.
Electrophysiological characteristics after prolonged exercise in two patients with CLDN19 mutations. The amplitude of CMAP was significantly decreased after exercise that lasted 5 minutes (according to Fournier et al. [13]). The amplitude of CMAP, recorded from the abductor digiti minimi muscle from 0 to 40 minutes after exercise, is expressed as a percentage of the pre-exercise value. In Patient F1.3, tests were performed while serum Mg2+ and K+ levels were normalized.
Parents of patients F1.3 and F1.4 could not be tested but were free of urolithiasis and/or urinary infections. In the parents of F2.3, biologic tests showed normal serum Mg2+ levels (0.78 and 0.85 mmol/L, respectively) but identified hypercalciuria (urinary Ca2+/creatinine ratio of 2.8 and 0.6 mg/mg, respectively [n < 0.4]) and mild chronic kidney disease (eGFR estimated by the simplified Modifications of Diet in Renal Disease formula of 57.8 and 56.1 ml/min per 1.73 m2). No other renal, ocular, or neurologic manifestations were found. All parents declined genetic analysis.
Typical renal features of FHHNC and recessive inheritance of the disease prompted us to test CLDN16 and CLDN19 in both families. Direct sequencing of CLDN19 identified two different heterozygous missense mutations within exon 1 (c.59G>A, p.Gly20Asp and c.130G>A, p.Val44Met) in patient F1.3 and a homozygous missense mutations within exon 1 (c.59G>A, p.Gly20Asp) in patient F2.3. Mutation of CLDN16 was ruled out and genetic counseling was provided. Genotyping of CLDN16 and CLDN19 could not be performed in patient F1.4 but was deduced from her highly suggestive phenotype and the genotyping of her sister (patient F1.3).
Discussion
Herein, we reported on three patients harboring CLDN19 mutations. Mutations in CLDN16 and CLDN19, the genes encoding for two structural proteins of the tight junction (claudin-16 and claudin-19, respectively), have been previously identified in patients with FHHNC (2,3,5). In epithelial cells, claudin-16 (a Na+-channel) and claudin-19 (a Cl−-blocker) interact to form heteromultimers and generate a cation-selective tight junction that regulates paracellular Mg2+ and Ca2+ permeability (6). In mice kidneys, Cldn16 and Cldn19 are mainly expressed in the ascending loop of Henle (and to a lesser extent in the distal convoluted tubule) (5,14).
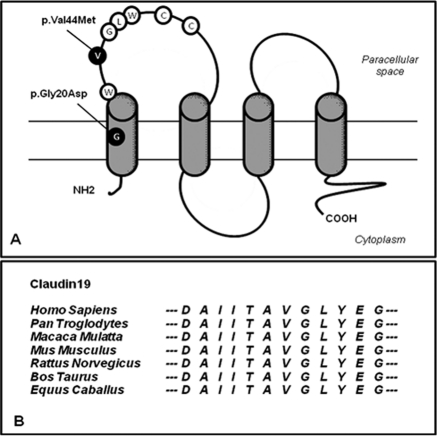
In our patients, direct sequencing of CLDN19 identified a sequence variation previously identified in patients with FHHNC and severe ocular involvement (p.Gly20Asp) and known to be responsible for a mislocalization of the claudin-19 mutant in epithelial cells (5). Whereas one patient harbored a homozygous p.Gly20Asp variant, the two other sisters were compound heterozygous for missense mutations in CLDN19 (Gly20Asp and p.Val44Met). Val44 is a neutral amino acid that has been highly conserved throughout evolution until Fugu fish (see genomic alignment of CLDN19 in seven mammals in Figure 2B from the Ensembl database) and is located within the claudin-specific amino-acid motif W-GLW-C-C (see Figure 2A). Also, the p.Val44Met mutation was not found in 150 control chromosomes from the same geographic origin. In patient F1.3, a concomitant mutation of CLDN16 was ruled out, thus reinforcing the probability of the pathogenic status of the p.Val44Met variant.
Figure 2.
(A) Localization of CLDN19 mutations on the predicted model of claudin-19. The Val44 residue is localized within the claudin-specific W-GLW-C-C motif. (B) Genomic alignment around the Val44 (V) residue of CLDN19 of seven mammalian species (from the Ensembl database).
In this series, blood and urinary tests confirmed the usual features of FHHNC (15) in CLDN19 patients: (1) persistent hypomagnesemia unresponsive to Mg2+ administration, (2) thiazide-sensitive hypercalciuria with normal serum Ca2+ levels and a slightly increased parathormone level, (3) low urinary citrate excretion, and (4) progressive renal failure leading to ESRD. However, one can note some peculiar renal findings. First, although FHHNC is mostly diagnosed during the first years of life (age of diagnosis within the first decade for all patients, within the first year of life for 8 of 12 patients) (5), it can be asymptomatic until the end of the second decade as exemplified by patient F2.3. In patients with CLDN16-related FHHNC, a genotype-phenotype correlation has been established and age at onset and severity may be predicted from the CLDN16 genotype (12). Because of the recent recognition of CLDN19 mutations in humans and their low frequency, this analysis has not yet been performed in patients with CLDN19 mutations. Second, in patient F2.3, calciuria returned to normal values after 1 year of thiazide-diuretic intake, which is an unusual finding (15). Serum Mg2+ level was not modified. Whether thiazide diuretics may counteract renal loss of Ca2+ in CLDN19 patients remains to be clarified. Last, hypokalemia occurred in two patients despite a worsening in GFR (in the absence of diuretic intake, vomiting, or diarrhea). Refractory hypokalemia and K+ depletion are frequently associated with Mg2+ deficiency irrespective of its mechanism and may persist until Mg2+ deficiency is restored (16). It is thus tempting to speculate that the renal loss of K+ in adulthood might be related to chronic Mg2+ deficiency. Alternatively, future studies should test whether expression of genes involved in the molecular mechanisms of renal K+ handling are modified by CLDN19 mutations.
Time from diagnosis of nephrocalcinosis and ESRD was 12 and 16 years. After renal transplantation, Ca2+ and Mg2+ handling was normalized. Renal Ca2+ deposits identified 1 year after renal transplantation in patient F1.3 were probably related to the severe hyperparathyroidism. Moreover, long-term renal biopsy failed to identify nephrocalcinosis confirming the absence of posttransplantation recurrence of FHHNC. It also underlines the need for genetic screening before kidney transplantation in patients with nephrocalcinosis to rule out other causes of congenital nephrocalcinosis such as primary hyperoxaluria. In conclusion, the renal phenotype of CLDN19 mutations closely mimics the kidney involvement observed in CLDN16 patients: Nephrocalcinosis with renal atrophy, renal Mg2+ and Ca2+ wasting, and progressive renal decline, which is sometimes accompanied by incomplete distal tubular acidosis and hypokalemia (10).
In our study, the genotype of parents was unknown, but we could show that individuals F2.1 and F2.2, who were likely to be heterozygous for the CLDN19 variant, had hypercalciuria and moderate chronic kidney disease (stage 3) but no hypomagnesemia. Extrarenal features were absent. These data are consistent with previous reports, and heterozygous mutations of CLDN19 and CLDN16 are now recognized as risk factors for hypercalciuria and kidney stones (5,10,15). Although a common polymorphism in the CLDN14 gene has been associated with the occurrence of kidney stones and increased urinary Ca2+ levels (17), the role of CLDN16 and CLDN19 polymorphisms in hypercalciuria and/or kidney stones in the general population is unknown and remain to be assessed.
In contrast to CLDN16 patients, all CLDN19 patients previously reported had severe ocular involvement associated with near blindness: macular colobomata, myopia, and horizontal nystagmus were identified in 50%, 83%, and 91% of tested patients (5). In the eye of zebrafish, CLDN19 is specifically expressed in the retina, mainly in the retinal pigment epithelium (5). Its function in this epithelium remains unknown, but effective tight junction formation is required during normal retinal development (18). In our study, all patients had ocular involvement; however, its severity was far less significant. Only one patient had mild visual loss (F1.4) and retinal-pigment maculopathy was found incidentally in one. Thus, accurate ophthalmological examination is required in all patients with FHHNC, even in the absence of visual loss. Moreover, genetic testing of CLDN19 should be considered in FHHNC patients with ocular involvement, even if symptoms are subtle.
Herein, we described, for the first time, the electrophysiological characteristics of neuromuscular involvement in CLDN19 patients. Two of the three patients described had similar muscular-exercise intolerance with limb stiffness and cramps. Electrophysiological exploration revealed a similar response after tests: Short exercise at room temperature or after cooling induced no significant changes of CMAP amplitude, whereas CMAP amplitude was significantly decreased 10 to 15 min after sustained exercise. Exercise acts as provocative tests and provides information on the ability of active fibers to depolarize and repolarize. Abnormal CMAP amplitude is observed in approximately 70% to 80% of patients with periodic paralysis (19,20). The electrophysiological pattern observed in our patients (pattern V; CMAP declines after long exercise without preliminary increment) is more frequently associated with mutations in Ca2+ or K+ channel CACNA1A, KCNJ2, and SCN4A genes (13,21,22). Whether isolated neuromuscular defects may be induced by some CLDN19 mutations thus accounting for channelopathies of unknown origin remains to be tested. Of note, these abnormalities were identified in patient F1.3 while serum Mg2+ and K+ levels were normalized (posttransplant period), suggesting that mutations of CLDN19 are directly responsible for the neuromuscular disorders. Last, noninvasive the electromyography techniques that we used failed to detect a decrease in muscle conduction velocities, a frequent finding in Ca2+ channel mutations (23,24). Invalidation of Cldn19 in mice leads to ambulatory disturbances, which is, in part, secondary to the disappearance of tight junctions from myelinated Schwann cells in the peripheral nerves where Cldn19 is expressed (25). The presence of a skeletal muscle channelopathy has not been explored in these mice, and muscular expression of Cldn19 remains unknown. In our patients, no change in nerve conductance was observed. Because accurate assessment of neuromuscular status in CLDN16 patients has not been performed and subtle abnormalities could have been missed, whether this finding is specific of CLDN19 patients needs to be confirmed.
In summary, mutations of CLDN19 lead to recessively inherited hypomagnesemia, hypercalciuria with nephrocalcinosis, and progressive renal decline. Extrarenal features include ocular manifestations, with or without visual impairment, and muscular-exercise intolerance partly mimicking periodic paralysis (i.e., chanellopathies). Age at onset and severity are heterogeneous among families. Further studies are required to assess a potential genotype-phenotype correlation, as has been previously recognized in patients with CLDN16 mutations.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Wrong O: Nephrocalcinosis. In: Oxford Textbook of Clinical Nephrology, edited by Davison AM, Cameron JS, Grunfeld J. Oxford, United Kingdom, Oxford University Press, 2005, pp 1375–1396 [Google Scholar]
- 2. Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP: Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285: 103–106, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Weber S, Hoffmann K, Jeck N, Saar K, Boeswald M, Kuwertz-Broeking E, Meij II, Knoers NV, Cochat P, Sulakova T, Bonzel KE, Soergel M, Manz F, Schaerer K, Seyberth HW, Reis A, Konrad M: Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis maps to chromosome 3q27 and is associated with mutations in the PCLN-1 gene. Eur J Hum Genet 8: 414–422, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Weber S, Schlingmann KP, Peters M, Nejsum LN, Nielsen S, Engel H, Grzeschik KH, Seyberth HW, Grone HJ, Nusing R, Konrad M: Primary gene structure and expression studies of rodent paracellin-1. J Am Soc Nephrol 12: 2664–2672, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S: Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet 79: 949–957, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA: Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest 118: 619–628, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gunzel D, Haisch L, Pfaffenbach S, Krug SM, Milatz S, Amasheh S, Hunziker W, Muller D: Claudin function in the thick ascending limb of Henle's loop. Ann NY Acad Sci 1165: 152–162, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Gunzel D, Yu AS: Function and regulation of claudins in the thick ascending limb of Henle. Pflugers Arch 458: 77–88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alexander RT, Hoenderop JG, Bindels RJ: Molecular determinants of magnesium homeostasis: Insights from human disease. J Am Soc Nephrol 19: 1451–1458, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weber S, Schneider L, Peters M, Misselwitz J, Ronnefarth G, Boswald M, Bonzel KE, Seeman T, Sulakova T, Kuwertz-Broking E, Gregoric A, Palcoux JB, Tasic V, Manz F, Scharer K, Seyberth HW, Konrad M: Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol 12: 1872–1881, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Benigno V, Canonica CS, Bettinelli A, von Vigier RO, Truttmann AC, Bianchetti MG: Hypomagnesaemia-hypercalciuria-nephrocalcinosis: A report of nine cases and a review. Nephrol Dial Transplant 15: 605–610, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Konrad M, Hou J, Weber S, Dotsch J, Kari JA, Seeman T, Kuwertz-Broking E, Peco-Antic A, Tasic V, Dittrich K, Alshaya HO, von Vigier RO, Gallati S, Goodenough DA, Schaller A: CLDN16 genotype predicts renal decline in familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol 19: 171–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fournier E, Arzel M, Sternberg D, Vicart S, Laforet P, Eymard B, Willer JC, Tabti N, Fontaine B: Electromyography guides toward subgroups of mutations in muscle channelopathies. Ann Neurol 56: 650–661, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Angelow S, El-Husseini R, Kanzawa SA, Yu AS: Renal localization and function of the tight junction protein, claudin-19. Am J Physiol Renal Physiol 293: F166–F177, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Praga M, Vara J, Gonzalez-Parra E, Andres A, Alamo C, Araque A, Ortiz A, Rodicio JL: Familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Kidney Int 47: 1419–1425, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Huang C, Kuo E: Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 18: 2649–2652, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, Sulem P, Halldorsson BV, de Vegt F, d'Ancona FC, den Heijer M, Franzson L, Christiansen C, Alexandersen P, Rafnar T, Kristjansson K, Sigurdsson G, Kiemeney LA, Bodvarsson M, Indridason OS, Palsson R, Kong A, Thorsteinsdottir U, Stefansson K: Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet 41: 926–930, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Rahner C, Fukuhara M, Peng S, Kojima S, Rizzolo LJ: The apical and basal environments of the retinal pigment epithelium regulate the maturation of tight junctions during development. J Cell Sci 117: 3307–3318, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Kuntzer T, Flocard F, Vial C, Kohler A, Magistris M, Labarre-Vila A, Gonnaud PM, Ochsner F, Soichot P, Chan V, Monnier G: Exercise test in muscle channelopathies and other muscle disorders. Muscle Nerve 23: 1089–1094, 2000 [DOI] [PubMed] [Google Scholar]
- 20. McManis PG, Lambert EH, Daube JR: The exercise test in periodic paralysis. Muscle Nerve 9: 704–710, 1986 [DOI] [PubMed] [Google Scholar]
- 21. Venance S, Cannon SC, Fialho D, Fontaine B, Hanna MG, Ptacek LJ, Tristani-Firouzi M, Tawil R, Griggs RC; and the CINCH investigators: The primary periodic paralyses: Diagnostics, pathogenesis and treatment. Brain 129: 8–17, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Bendahhou S, Fournier E, Sternberg D, Bassez G, Furby A, Sereni C, Donaldson MR, Larroque MM, Fontaine B, Barhanin J: In vivo and in vitro functional characterization of Andersen's syndrome mutations. J Physiol 565: 731–741, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zwarts MJ, van Weerden TW, Links TP, Haenen HT, Oosterhuis HJ: The muscle fiber conduction velocity and power spectra in familial hypokalemic periodic paralysis. Muscle Nerve 11: 166–173, 1988 [DOI] [PubMed] [Google Scholar]
- 24. Links TP, van der Hoeven JH: Muscle fiber conduction velocity in arg1239his mutation in hypokalemic periodic paralysis. Muscle Nerve 23: 296, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Miyamoto T, Morita K, Takemoto D, Takeuchi K, Kitano Y, Miyakawa T, Nakayama K, Okamura Y, Sasaki H, Miyachi Y, Furuse M, Tsukita S: Tight junctions in Schwann cells of peripheral myelinated axons: A lesson from claudin-19-deficient mice. J Cell Biol 169: 527–538, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]