Summary
Background and objectives
Experimental studies suggest a detrimental role for vasopressin in the pathogenesis of autosomal dominant polycystic kidney disease (ADPKD). However, it is unknown whether endogenous vasopressin concentration is associated with disease severity in patients with ADPKD.
Design, setting, participants, & measurements
Plasma copeptin concentration (a marker of endogenous vasopressin levels) was measured in 102 ADPKD patients (diagnosis based on Ravine criteria) by an immunoassay. Plasma and urinary osmolarity were also measured. To assess disease severity, GFR and effective renal blood flow were measured by continuous infusion of 125I-iothalamate and 131I-hippuran, total renal volume by magnetic resonance imaging, and 24-hour urinary albumin excretion by nephelometry.
Results
In these ADPKD patients, copeptin was associated with the various markers of disease severity in ADPKD (positively with total renal volume [R = 0.47] and albuminuria [R = 0.39] and negatively with GFR [R = −0.58] and effective renal blood flow [R = −0.52], all P < 0.001). These associations were independent of age, gender, and use of diuretics. Copeptin was furthermore associated with plasma osmolarity (P < 0.001) but not with 24-hour urinary volume, 24-hour urinary osmolarity or fractional urea excretion (P = 0.7, 0.9, and 0.3, respectively).
Conclusions
On cross-sectional analysis, copeptin is associated with disease severity in ADPKD patients, supporting the results of experimental studies that suggest that vasopressin antagonists have a renoprotective effect in ADPKD and offering a good prospect for clinical studies with these agents.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common renal hereditary disease (caused in most instances by a mutation in the PKD1 or PKD2 gene [1,2]) with an incidence of 1 in 400 to 1000 live births (3,4) The disease is characterized by progressive cyst formation in both kidneys, often leading to ESRD. Current treatment is not able to inhibit cyst formation or to prevent renal failure.
Vasopressin (VP), also known as antidiuretic hormone, is essential for regulation of water homeostasis and osmoregulation in the body. Its secretion is stimulated in response to an increase in plasma osmolarity or a decrease in blood volume. VP binds to the vasopressin V2 receptors in the collecting duct, which induces the insertion of the molecular water channel aquaporin-2 in the luminal membrane of principal cells. This mediates water resorption, thereby reducing water excretion (5).
Despite its role for normal physiology, VP has also been mentioned to be involved in pathophysiological processes, among others in ADPKD. VP promotes cAMP production by acting on V2 receptors in the distal nephron and collecting ducts. Experimental studies have implicated a central role for cAMP in promoting cyst growth (6). cAMP stimulates cyst formation by promoting chloride-driven fluid secretion and by stimulating activation and proliferation of cyst-derived cells (7). In line with this potential detrimental role of VP are the findings in animal models of polycystic kidney disease, in which blocking the effect of VP (and consequently decreasing cAMP levels) by a pharmacologic agent (8–10) or by drinking more water (11) led to reduction of cyst formation and renal function preservation.
To date, no studies have looked at the association between endogenous VP levels and disease severity in subjects with ADPKD. The paucity of data on this issue may be caused by the fact that measurement of VP is problematic. More than 90% of VP in the circulation is bound to platelets, VP is unstable in isolated plasma (12), and most VP assays have relatively limited sensitivity. Recently, an assay has been developed to measure copeptin, the C-terminal portion of the precursor of VP. Copeptin has been shown to be a reliable marker of VP secretion and a useful substitute for circulating VP concentration in clinical routine (13–15).
Because of the potential detrimental role of VP in the pathogenesis of ADPKD, we aimed to investigate whether endogenous copeptin levels (a surrogate marker of VP) are associated with disease severity in subjects with ADPKD. As markers of disease severity we studied GFR, effective renal blood flow, total renal volume, and albuminuria. To investigate whether copeptin concentration is consistent with normal physiologic regulation and has its normal physiologic effects, we also studied associations with plasma osmolarity, 24-hour urinary volume, 24-hour urinary osmolarity, and fractional urea excretion.
Materials and Methods
ADPKD Patients
One hundred and twenty consecutive patients with ADPKD visiting our outpatient clinic meeting our inclusion and exclusion criteria were asked to participate. Diagnosis of ADPKD was made based on Ravine criteria (16). Subjects were considered ineligible to participate if they received renal replacement therapy; had undergone renal surgery; were unable to undergo magnetic resonance imaging (MRI; as having distorting foreign bodies or aneurysmal clips); had other systemic diseases potentially affecting renal function (as diabetes mellitus and malignancies); or had other medical conditions that included pregnancy, lactation, or who were less than 6 months postpartum. After screening, subjects underwent an extensive medical history. Subjects were scheduled for a 1-day outpatient clinic evaluation. Thirteen patients refused to participate and 2 patients were not eligible. Three patients had a copeptin concentration more than 10 times the interquartile range above the third quartile, although their plasma osmolarity was within normal limits. These subjects were considered outliers (17) and their data were not taken into consideration, leaving 102 patients for analyses.
This study was performed in adherence to the Declaration of Helsinki. All subjects gave written informed consent.
Measurements and Definitions
Blood pressure (BP) was assessed with an automatic device (Dinamap) for 15 minutes during the renal function measurement. Systolic and diastolic BP values were used to calculate mean arterial pressure using the standard formula 2/3 diastolic BP + 1/3 systolic BP. Weight and height were determined. Body mass index was calculated as weight (kg) divided by height (m2). Patients collected a 24-hour urine sample before the outpatient visit. Urinary albumin concentration was determined by immunonephelometry (BNII; Dade Behring Diagnostics, Marburg, Germany). Before renal function measurement, blood samples were drawn for determination of hemoglobin, sodium, creatinine, urea, plasma osmolarity, and copeptin. Concentrations of hemoglobin, sodium, and urea were measured using standard methods. Creatinine was measured with the Roche enzymatic creatinine assay. Plasma and urine osmolarity was measured using freezing point depression. Fractional urea excretion was calculated as [(urinary urea concentration/plasma urea concentration)/(urinary creatinine concentration/plasma creatinine concentration)] × 100.
Copeptin was measured using a new sandwich immunoassay (B.R.A.H.M.S. AG, Hennigsdorf/Berlin, Germany), which was based on the assay described previously (13). The assay was modified as follows: The capture antibody was replaced by a murine monoclonal antibody directed to amino acids 137 to 144 of pro-arginine vasopressin. This modification improved the sensitivity of the assay. The lower detection limit was 0.4 pmol/L and the functional assay sensitivity (20% interassay coefficient of variation) was less than 1 pmol (18).
Renal function measurements were performed using the constant infusion method with 125I-iothalamate to measure GFR and with 131I-hippuran to measure effective renal plasma flow (ERPF) (19–22). Effective renal blood flow (ERBF) was calculated as ERPF/(1 − hematocrit). Hematocrit was measured halfway during the renal function measurement. Patients underwent a standardized abdominal MRI protocol without the use of intravenous contrast. Scanning was performed on a 1.5-T MRI Magnetom Avento (Siemens, Erlangen, Germany) with the use of body matrix and spine matrix coils. Total renal volume (TRV) was measured on T2 weighted coronal images (23) (slice thickness = 4.0 mm) using Analyze Direct 8.0 (AnalyzeDirect, Inc., Overland Park, KS) software.
Statistical Analyses
Analyses were performed with SPSS version 16.0 (SPSS Inc., Chicago, IL). Parametric variables are expressed as mean ± SD, whereas nonparametric variables are given as median (interquartile range). A two-sided P less than 0.05 was considered to indicate statistical significance.
Regression analysis was performed to investigate whether plasma copeptin concentration was correlated with plasma osmolarity, physiologic variables, and variables representing disease severity. In case of non-normal distribution, variables were log transformed, and R and P values are given. GFR and ERBF were normalized for body surface area. To visualize the associations, scatterplots (with males and females depicted separately) were made showing the associations between copeptin and plasma osmolarity and markers of disease severity (TRV, GFR, ERBF, and albuminuria).
To further investigate whether plasma copeptin concentration was associated with markers of disease severity, multivariable regression analysis was performed. Logarithmic transformation of copeptin (and of urinary albumin excretion [UAE] and total renal volume) was applied to fulfill the requirement of equal distribution of the residuals. Associations were investigated crude and after adjustment for age, gender, use of diuretics, and GFR.
Interactions between log copeptin concentration and age and gender were tested for GFR, ERBF, log TRV, and log UAE as the dependent variables.
Results
Characteristics of participating patients are depicted in Table 1. A total of 102 patients (56% male, aged 40 ± 11 years) were analyzed. Median copeptin concentration for the whole group was 7.0 (3.1 to 15.7) pmol/L. Median copeptin concentration was higher in men than in women (10.1 [4.7 to 19.8] versus 3.2 [2.4 to 8.3] pmol/L, P < 0.001).
Table 1.
Patient characteristics (n = 102)
| Variable | |
|---|---|
| Men, n (%) | 57 (56) |
| Age (years) | 40 ± 11 |
| Body mass index (kg/m2) | 26 ± 5 |
| Mean arterial pressure (mmHg) | 96 ± 10 |
| Use of antihypertensive medication, n (%) | 79 (78) |
| Use of diuretics, n (%) | 23 (23) |
| Hemoglobin (mmol/L) | 8.3 ± 0.8 |
| Serum sodium (mmol/L) | 140 ± 2 |
| Plasma osmolarity (mOsm/L) | 290 ± 8 |
| Copeptin (pmol/L) | 7.0 (3.1 to 15.7) |
| Serum creatinine (μmol/L) | 115 ± 70 |
| Serum urea (mmol/L) | 8 ± 4 |
| 24-hour urinary volume (L) | 2.3 ± 0.8 |
| 24-hour urinary osmolarity (mOsm/L) | 425 ± 148 |
| 24-hour UAE (mg/24 h) | 41 (15 to 122) |
| Fractional urea excretion (%) | 42 ± 10 |
| GFR (ml/min/1.73 m2) | 77 ± 31 |
| ERBF (ml/min per 1.73 m2) | 421 ± 170 |
| TRV (L) | 1.50 (0.94 to 2.18) |
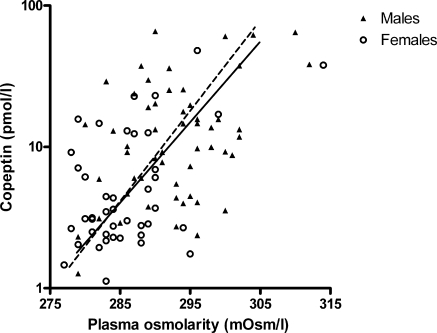
Plasma copeptin concentration, as a surrogate of VP, was significantly correlated with plasma osmolarity (Figure 1, R = 0.53, P < 0.001). This correlation is not different for males when compared with females. No association was found between copeptin and urinary osmolarity (R = 0.01, P = 0.9), copeptin and urinary volume (R = −0.04, P = 0.7), or copeptin and fractional urea excretion (R = −0.10, P = 0.3).
Figure 1.
Association between plasma osmolarity and copeptin (overall R = 0.53, P < 0.001; males ▴ and —, R = 0.40, P = 0.003; and females ○ and – –, R = 0.46, P = 0.002).
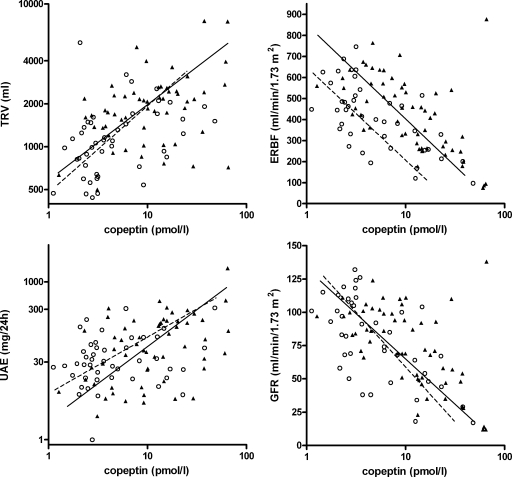
In general, the different markers of disease severity correlated well with each other. Correlation coefficients are depicted in Table 2. Looking into the association between copeptin and markers of disease severity, copeptin was positively associated with total renal volume (overall R = 0.47, P < 0.001, males R = 0.47, P < 0.001, females R = 0.37, P = 0.01) and 24-hour UAE (overall R = 0.39, P < 0.001, males R = 0.30, P = 0.03, females R = 0.29, P = 0.07 for females). These associations remained significant after adjustment for age, gender, and use of diuretics and after adjustment of GFR (Table 3). In Figure 2, these associations are shown. Copeptin was furthermore inversely associated with ERBF (overall R = −0.52, P < 0.001; males R = −0.57, P < 0.001; females R = −0.61, P < 0.001) and with GFR (overall R = −0.58, P < 0.001; males R = −0.58, P < 0.001; females R = −0.61, P < 0.001). These associations remained significant after adjustment for age, gender, and use of diuretics (Table 4). Correlation between ERBF and GFR was very high (R = 0.94, P < 0.001); therefore, adjustment of the association between copeptin and ERBF for GFR was not possible. Figure 2 shows the associations between copeptin and GFR and ERBF. That females have a lower ERBF than males is at least partly caused by the known gender difference in hematocrit (also found in these patients, hematocrit was 0.41 ± 0.03 for males versus 0.38 ± 0.03 for females, P < 0.001) because estimated renal plasma flow is not different between males and females (253 ± 106 versus 256 ± 97 ml/min per 1.73 m2 respectively, P = 0.9).
Table 2.
Associations between the different markers of disease severity
| GFR |
ERBF |
TRV |
||||
|---|---|---|---|---|---|---|
| R | P | R | P | R | P | |
| ERBF | 0.94 | <0.001 | – | – | ||
| TRV | −0.42 | <0.001 | −0.30 | 0.002 | – | – |
| UAE | −0.20 | 0.04 | −0.15 | 0.1 | 0.47 | <0.001 |
Depicted values are Pearson correlation coefficients and P values. Variables are log-transformed in case of non-normal distribution (TRV, UAE).
Table 3.
Associations among copeptin, TRV, and 24-hour UAE as markers of disease severity in ADPKD
| Model | Adjusted for | TRV |
UAE |
||
|---|---|---|---|---|---|
| Standardized β | P | Standardized β | P | ||
| 1 | Crude | 0.47 | <0.001 | 0.39 | <0.001 |
| 2 | As 1 + age and gender | 0.34 | 0.001 | 0.42 | <0.001 |
| 3 | As 2 + use of diuretics | 0.33 | 0.001 | 0.44 | <0.001 |
| 4 | As 2 + GFR | 0.23 | 0.05 | 0.26 | 0.04 |
Independent variable is log copeptin. Dependent variables are log TRV and log albuminuria. A positive standardized β indicates that a high copeptin concentration is associated with a high TRV or UAE, respectively.
Figure 2.
VP in association with markers of disease severity in ADPKD (males ▴ and —; females ○ and – –). On the left, associations between copeptin and TRV (upper left; overall: R = 0.47, P < 0.001; males: R = 0.37, P = 0.006; females: R = 0.37, P = 0.01) and 24-hour UAE (lower left; overall: R = 0.39, P < 0.001; males: R = 0.30, P = 0.03; females: R = 0.29, P = 0.07). On the right, associations between log copeptin and ERBF (upper right; overall R = −0.52, P < 0.001; males: R = −0.57, P < 0.001; females: R = −0.61, P < 0.001) and GFR (lower right; overall R = −0.58, P < 0.001, males: R = −0.58, P < 0.001; females: R = −0.61, P < 0.001).
Table 4.
Associations among copeptin, GFR, and ERBF as markers of disease severity in ADPKD
| Model | Adjusted for | GFR |
ERBF |
||
|---|---|---|---|---|---|
| Standardized β | P | Standardized β | P | ||
| 1 | Crude | −0.58 | <0.001 | −0.52 | <0.001 |
| 2 | As 1 + age and gender | −0.55 | <0.001 | −0.55 | <0.001 |
| 3 | As 2 + use of diuretics | −0.53 | <0.001 | −0.54 | <0.001 |
Independent variable is log copeptin. Dependent variables are GFR and ERBF (both in ml/min per 1.73 m2). A negative standardized β indicates that a high copeptin concentration is associated with a low GFR or ERBF.
Of note, we found no interactions between age and log copeptin concentration or gender and log copeptin concentration on any of the aforementioned markers for disease severity (GFR, ERBF, TRV, and albuminuria). The lack of interaction between gender and copeptin concentration on these markers is also shown in Figures 1 and 2, where the regression lines for males and females all have the same slopes.
Discussion
In this study, we found that in ADPKD patients, plasma osmolarity was associated with copeptin concentration. However, copeptin concentration was not associated with 24-hour urinary volume, 24-hour urinary osmolarity, or fractional urea excretion. Most importantly, we found that copeptin levels were associated with disease severity; higher copeptin levels were associated with lower renal function, lower ERBF, larger kidneys, and more albuminuria. These associations were the same for males and females and were independent of age and use of diuretics.
The markers of disease severity that we studied are acknowledged in literature. TRV is considered a good measure for disease severity because kidney enlargement results from the expansion of cysts in patients with ADPKD. Higher TRV is associated with a more rapid decrease in renal function.24,25 In a large number of animal studies, treatments that inhibited renal enlargement also ameliorated renal function (25). Renal blood flow is also an accepted measure of disease severity. A reduction of renal blood flow parallels the increase in TRV and, importantly, precedes GFR decline and predicts structural and functional disease progression (26). Several studies show that in ADPKD, albuminuria is associated with renal volume (27,28), mean arterial BP, filtration fraction (28), renal growth, and slope of GFR (26). In our study, the different markers for disease severity correlated reasonably well with each other.
Only two studies that we know of, both performed more than 10 years ago, measured VP in ADPKD patients. One study described that VP was increased ADPKD patients (29), the other that VP was increased in hypertensive ADPKD patients compared with nonhypertensive ADPKD patients and healthy controls (30). These studies did not look into potential physiologic regulation and effects of VP. In normal physiology, VP is secreted in response to an increase in plasma osmolarity. We found that also in these ADPKD patients plasma osmolarity was associated with copeptin (as a reliable and stable surrogate of VP). VP mediates urea recirculation and water reabsorption and is therefore under normal circumstances positively associated with urinary osmolarity and inversely with urinary volume and fractional urea excretion. However, in these ADPKD patients we did not find significant associations between copeptin and 24-hour urinary volume, 24-hour urinary osmolarity, or fractional urea excretion. This is in contrast to what is described in healthy subjects (31) and renal transplant recipients (32) and suggests that ADPKD patients do not respond effectively to VP. In line with this assumption is the observation that ADPKD patients have an impaired urinary concentrating capacity that is already present when GFR is still normal (33).
We found that copeptin is associated with the various markers of disease severity in ADPKD. This association is consistent with the hypothesis that VP is involved in disease progression in subjects with ADPKD. This hypothesis is supported by several experimental studies. VP, acting on V2 receptors, is described to increase cAMP in the distal nephron and collecting duct. This promotes chloride-driven fluid secretion and, in polycystic kidney cells, stimulation of the B-RAF/MEK/extracellular signal-regulated pathway for mitogenesis and epithelial cell proliferation (6,34). In line, we found an association between plasma copeptin concentration and urinary cAMP excretion. Furthermore, a V2 receptor antagonist inhibited cyst formation in models for ADPKD (8,9) Moreover, genetic elimination of arginine vasopressin in a rat model of ADPKD yielded animals born with normal kidneys that remained relatively free of cysts unless an exogenous V2 receptor agonist was administered (35). We found copeptin to be higher in males than in females, which is also consistent with the hypothesis that copeptin is involved in disease progression, for it is known that male gender is a risk factor for disease progression in ADPKD (36–38).
Also in other renal conditions than ADPKD, VP is thought to have a potential detrimental role. In non-ADPKD animal models, sustained V2 receptor stimulation results in hyperfiltration (39), which may have deleterious consequences, in particular in diseased kidneys, resulting in renal hypertrophy (40), proteinuria (41), and accelerated renal function decline (42–44). In contrast, in rats with chronic renal failure, increased water intake (resulting in lower VP concentration) or chronic infusion of a V2 receptor antagonist reduced proteinuria and prevented glomerulosclerosis (42,45) and tubulointerstitial fibrosis (46). In humans, we have previously described that higher copeptin levels are associated with microalbuminuria in a population-based cohort (31) and that higher copeptin levels at baseline predict renal function decline in renal transplant recipients (32).
In our ADPKD patients, but also in non-ADPKD subjects (31,47), copeptin concentration was found to be associated with renal function. The fact that copeptin is partly cleared by the kidney (48) is not likely to be an explanation for higher copeptin concentrations in subjects with lower GFR because increased copeptin levels would induce a lowering of plasma osmolarity, which would lead to a reduced copeptin concentration. These mechanisms are difficult to dissect in a cross-sectional study. Alternatively, in subjects with lower GFR, the medullary urea gradient will be impaired because of a loss of functioning nephrons and interstitial fibrosis. Subjects with impaired GFR therefore have lower urinary osmolarity and higher urinary volume than subjects with normal renal function (49,50). Consequently, fluid reabsorption will be more difficult and higher VP levels will be necessary to maintain fluid balance. Furthermore, patients with ADPKD have an additional anatomical disruption of the medullary architecture induced by cyst formation, which will also diminish urinary concentrating capacity. Indeed, in a study including 177 ADPKD patients, the observed renal concentrating defect paralleled the severity of anatomical changes caused by renal cysts (33). Thus, according to this hypothesis, genetically determined progressive cyst formation will lead to higher VP concentration. At the same time, these higher VP levels will lead to disease progression, as reasoned in the aforementioned paragraphs. These two processes are therefore expected to induce a vicious circle in ADPKD, predisposing to cyst growth, distortion of renal anatomy, and renal function decline. This hypothesis may form an explanation why in subjects with ADPKD GFR remains relatively stable for a long period, whereas cyst formation progresses, then enters a phase with accelerated GFR decline (25,51).
We acknowledge that this study has limitations. First, the cross-sectional observational design and the lack of data in noncystic chronic kidney disease do not allow firm conclusions on the possible causal relationship between VP and disease progression. Second, patients were allowed to use their own medication, including diuretics. These drugs may influence plasma osmolarity and consequently copeptin concentration. However, adjustment for diuretic use in our multivariate models did not change our results. Third, we did not perform a water-deprivation test, so we are unable to draw firm conclusions whether ADPKD patients indeed do not respond effectively to VP and have an impaired urinary concentrating capacity.
Strengths of our study are that, as far as we know, this is the first clinical study looking into the association between endogenous VP and disease severity in ADPKD. It provides a rationale for the intervention studies with VP receptor antagonists that are being conducted at this moment in subjects with this disease. Furthermore, we measured various indices of disease severity in ADPKD patients using the gold standards of clearance of iothalamate for GFR, clearance of hippuran for ERBF, MRI for total renal volume, and 24-hour UAE for albuminuria.
What may be the consequences of our findings? First, they may help shed light on the pathophysiology of ADPKD. Second, there may be clinical implications. At the moment, a large-scale randomized clinical trial is being conducted to investigate efficacy of a V2 receptor antagonist in ADPKD (TEMPO ¾ study, NCT00428948). This study is performed in patients with still relatively well preserved renal function (estimated GFR at baseline >60 ml/min), but large renal volume (TRV > 750 ml). Our finding that patients with more severe ADPKD, as assessed by lower GFR, higher TRV, and/or higher albuminuria, have higher copeptin levels suggests that patients with these characteristics may need higher dosages of a VP receptor antagonist to effectively block the hormonal activity of the higher VP levels. Although not studied yet, it is expected that tolvaptan treatment increases copeptin levels by feedback mechanisms and that the increase in copeptin with tolvaptan will reflect adequacy of VP suppression. If so, it is tempting to hypothesize that in patients with ADPKD the increase in copeptin due to tolvaptan could be used as a short-term marker for long-term therapy effectiveness of this drug with respect to renoprotection and that the level of copeptin could be used to determine the effective treatment dose of this drug.
In conclusion, our study shows that in ADPKD, copeptin, a surrogate for VP, is associated with disease severity. This finding supports the results of animal models for ADPKD in which V2 antagonists resulted in renoprotection and offers a good prospect for clinical intervention studies that are currently being conducted with these agents.
Disclosures
None.
Acknowledgments
We greatly acknowledge Lucia Kadijk for her assistance at the outpatient clinic and the technical assistance of Marian Vroom-Dallinga, Roelie Karsten-Barelds, and Dirkina Hesseling for renal function measurements. Furthermore, we acknowledge Mieneke Rook for measurements of TRV and Peter Kappert, Annemarie Tienhoven, and Irene Willeboordse for their technical expertise with the MRIs. Finally, we thank Corrie Nieuwenhout and Annemarie Zantman for their secretarial assistance at the outpatient clinic.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Polycystic kidney disease: The complete structure of the PKD1 gene and its protein. The International Polycystic Kidney Disease Consortium. Cell 81: 289–298, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Peters DJ, Spruit L, Saris JJ, Ravine D, Sandkuijl LA, Fossdal R, Boersma J, van ER, Norby S, Constantinou-Deltas CD: Chromosome 4 localization of a second gene for autosomal dominant polycystic kidney disease. Nat Genet 5: 359–362, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Gabow PA: Autosomal dominant polycystic kidney disease. N Engl J Med 329: 332–342, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Bankir L: Antidiuretic action of vasopressin: Quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc Res 51: 372–390, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Grantham JJ: Lillian Jean Kaplan International Prize for advancement in the understanding of polycystic kidney disease. Understanding polycystic kidney disease: A systems biology approach. Kidney Int 64: 1157–1162, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Belibi FA, Reif G, Wallace DP, Yamaguchi T, Olsen L, Li H, Helmkamp GM, Jr, Grantham JJ: Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int 66: 964–973, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Wang X, Gattone V, Harris PC, Torres VE: Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol 16: 846–851, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH: Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Gattone VH, Wang X, Harris PC, Torres VE: Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Nagao S, Nishii K, Katsuyama M, Kurahashi H, Marunouchi T, Takahashi H, Wallace DP: Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 17: 2220–2227, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Preibisz JJ, Sealey JE, Laragh JH, Cody RJ, Weksler BB: Plasma and platelet vasopressin in essential hypertension and congestive heart failure. Hypertension 5: I129–I138, 1983 [DOI] [PubMed] [Google Scholar]
- 13. Morgenthaler NG, Struck J, Alonso C, Bergmann A: Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52: 112–119, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Szinnai G, Morgenthaler NG, Berneis K, Struck J, Muller B, Keller U, Christ-Crain M: Changes in plasma copeptin, the C-terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J Clin Endocrinol Metab 92: 3973–3978, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Morgenthaler NG, Struck J, Jochberger S, Dunser MW: Copeptin: Clinical use of a new biomarker. Trends Endocrinol Metab 19: 43–49, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM: Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343: 824–827, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Moore DS, McCabe GP: Introduction to the Practice of Statistics, 4th ed, New York, W.H. Freeman, 2002 [Google Scholar]
- 18. Fenske W, Stork S, Blechschmidt A, Maier SG, Morgenthaler NG, Allolio B: Copeptin in the differential diagnosis of hyponatremia. J Clin Endocrinol Metab 94: 123–129, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Donker AJ, van der Hem GK, Sluiter WJ, Beekhuis H: A radioisotope method for simultaneous determination of the glomerular filtration rate and the effective renal plasma flow. Neth J Med 20: 97–103, 1977 [PubMed] [Google Scholar]
- 20. Apperloo AJ, de Zeeuw D, Donker AJ, de Jong PE: Precision of glomerular filtration rate determinations for long-term slope calculations is improved by simultaneous infusion of 125I-iothalamate and 131I-hippuran. J Am Soc Nephrol 7: 567–572, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Zietse R, Blankestijn PJ, Pos B, Balk AH, Derkx FH, Weimar W, Schalekamp MA: Optimising glomerular filtration rate and effective renal plasma flow measurements using a simple pharmacokinetic model. Clin Nephrol 43: 29–34, 1995 [PubMed] [Google Scholar]
- 22. Michels WM, Grootendorst DC, Rozemeijer K, Dekker FW, Krediet RT: Glomerular filtration rate measurements by (125)I-iothalamate should be corrected for inaccurate urine collections with (131)I-hippuran. Clin Nephrol 72: 337–343, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Bae KT, Commean PK, Lee J: Volumetric measurement of renal cysts and parenchyma using MRI: Phantoms and patients with polycystic kidney disease. J Comput Assisted Tomogr 24: 614–619, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr., Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP: Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Grantham JJ, Chapman AB, Torres VE: Volume progression in autosomal dominant polycystic kidney disease: The major factor determining clinical outcomes. Clin J Am Soc Nephrol 1: 148–157, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Torres VE, King BF, Chapman AB, Brummer ME, Bae KT, Glockner JF, Arya K, Risk D, Felmlee JP, Grantham JJ, Guay-Woodford LM, Bennett WM, Klahr S, Meyers CM, Zhang X, Thompson PA, Miller JP: Magnetic resonance measurements of renal blood flow and disease progression in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2: 112–120, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Bae KT, Baumgarten DA, Kenney PJ, King BF, Jr, Glockner JF, Wetzel LH, Brummer ME, O'Neill WC, Robbin ML, Bennett WM, Klahr S, Hirschman GH, Kimmel PL, Thompson PA, Miller JP: Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int 64: 1035–1045, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Chapman AB, Johnson AM, Gabow PA, Schrier RW: Overt proteinuria and microalbuminuria in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 1349–1354, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Michalski A, Grzeszczak W: [The effect of hypervolemia on electrolyte level and level of volume regulating hormones in patients with autosomal dominant polycystic kidney disease]. Pol Arch Med Wewn 96: 329–343, 1996 [PubMed] [Google Scholar]
- 30. Danielsen H, Pedersen EB, Nielsen AH, Herlevsen P, Kornerup HJ, Posborg V: Expansion of extracellular volume in early polycystic kidney disease. Acta Med Scand 219: 399–405, 1986 [DOI] [PubMed] [Google Scholar]
- 31. Meijer E, Bakker SJ, Halbesma N, de Jong PE, Struck J, Gansevoort RT: Copeptin, a surrogate marker of vasopressin, is associated with microalbuminuria in a large population cohort. Kidney Int 77: 29–36, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Meijer E, Bakker SJ, de Jong PE, Homan van der Heide JJ, van Son WJ, Struck J, Lems SP, Gansevoort RT: Copeptin, a surrogate marker of vasopressin, is associated with accelerated renal function decline in renal transplant recipients. Transplantation 88: 561–567, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Gabow PA, Kaehny WD, Johnson AM, Duley IT, Manco-Johnson M, Lezotte DC, Schrier RW: The clinical utility of renal concentrating capacity in polycystic kidney disease. Kidney Int 35: 675–680, 1989 [DOI] [PubMed] [Google Scholar]
- 34. Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, Grantham JJ: Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 63: 1983–1994, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Wang X, Wu Y, Ward CJ, Harris PC, Torres VE: Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19: 102–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gretz N, Zeier M, Geberth S, Strauch M, Ritz E: Is gender a determinant for evolution of renal failure? A study in autosomal dominant polycystic kidney disease. Am J Kidney Dis 14: 178–183, 1989 [DOI] [PubMed] [Google Scholar]
- 37. Gabow PA, Johnson AM, Kaehny WD, Kimberling WJ, Lezotte DC, Duley IT, Jones RH: Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 41: 1311–1319, 1992 [DOI] [PubMed] [Google Scholar]
- 38. Choukroun G, Itakura Y, Albouze G, Christophe JL, Man NK, Grunfeld JP, Jungers P: Factors influencing progression of renal failure in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 6: 1634–1642, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Bouby N, Ahloulay M, Nsegbe E, Dechaux M, Schmitt F, Bankir L: Vasopressin increases glomerular filtration rate in conscious rats through its antidiuretic action. J Am Soc Nephrol 7: 842–851, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Naito A, Hasegawa H, Kurasawa T, Ohtake Y, Matsukawa H, Ezure Y, Koike K, Shigenobu K: Histopathological study of kidney abnormalities in an experimental SIADH rat model and its application to the evaluation of the pharmacologic profile of VP-343, a selective vasopressin V2 receptor antagonist. Biol Pharm Bull 24: 897–901, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Bardoux P, Bichet DG, Martin H, Gallois Y, Marre M, Arthus MF, Lonergan M, Ruel N, Bouby N, Bankir L: Vasopressin increases urinary albumin excretion in rats and humans: Involvement of V2 receptors and the renin-angiotensin system. Nephrol Dial Transplant 18: 497–506, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Bouby N, Bachmann S, Bichet D, Bankir L: Effect of water intake on the progression of chronic renal failure in the 5/6 nephrectomized rat. Am J Physiol 258: F973–F979, 1990 [DOI] [PubMed] [Google Scholar]
- 43. Bouby N, Hassler C, Bankir L: Contribution of vasopressin to progression of chronic renal failure: Study in Brattleboro rats. Life Sci 65: 991–1004, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Bankir, Trinh-Trang-Tan MM: Urea and the kidney. In: The Kidney, 6th ed., edited by Brenner BM. Philadelphia, W.B. Saunders, 2000: 637–679 [Google Scholar]
- 45. Okada H, Suzuki H, Kanno Y, Yamamura Y, Saruta T: Effects of vasopressin V1 and V2 receptor antagonists on progressive renal failure in rats. Clin Sci (Lond) 86: 399–404, 1994 [DOI] [PubMed] [Google Scholar]
- 46. Sugiura T, Yamauchi A, Kitamura H, Matsuoka Y, Horio M, Imai E, Hori M: High water intake ameliorates tubulointerstitial injury in rats with subtotal nephrectomy: Possible role of TGF-beta. Kidney Int 55: 1800–1810, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Bhandari SS, Loke I, Davies JE, Squire IB, Struck J, Ng LL: Gender and renal function influence plasma levels of copeptin in healthy individuals. Clin Sci (Lond) 116: 257–263, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Baumann G, Dingman JF: Distribution, blood transport, and degradation of antidiuretic hormone in man. J Clin Invest 57: 1109–1116, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garcia Nieto VM, Yanes MI, Zamorano MM, Gonzalez MJ, Aros CP, Garin EH: Renal concentrating capacity as a marker for glomerular filtration rate. Acta Paediatr 97: 96–99, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Tannen RL, Regal EM, Dunn MJ, Schrier RW: Vasopressin-resistant hyposthenuria in advanced chronic renal disease. N Engl J Med 280: 1135–1141, 1969 [DOI] [PubMed] [Google Scholar]
- 51. Meijer E, Rook M, Tent H, Navis G, Jagt EJ, Jong PE, Gansevoort RT: Early renal abnormalities in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 5: 1091–1098, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]