Summary
Background and objectives
Because previous studies have not distinguished between intimal (atherosclerotic) and medial vascular calcification, the prevalence and clinical significance of either condition in chronic or end-stage kidney disease (CKD or ESKD) are unknown. We hypothesized that breast arterial calcification (BAC) is exclusively medial and that mammography can serve as a useful marker of generalized medial calcification in CKD and ESKD.
Design, setting, participants, & measurements
Arterial calcification was identified histologically in breast tissue or visually in mammograms and radiographs of extremities from patients with CKD or ESKD.
Results
Medial calcification but no intimal calcification was present in all 16 specimens from patients with CKD or ESKD. In 71 women with ESKD, BAC was present on mammograms in 63% compared with 17% in women without renal insufficiency matched for age, race, and diabetes (P < 0.001). Age and ESKD duration were significant, independent predictors of BAC. BAC was also present in 36% of mammograms from the same patients performed 5.5 ± 0.7 years before the onset of ESKD (P < 0.05 versus control) but in only 14% of patients with stage 3 CKD. Comparison of mammograms and extremity radiographs revealed that BAC was present in over 90% of patients with peripheral arterial calcification (PAC), and PAC was observed in less than 6% of patients without BAC.
Conclusions
BAC is a specific and useful marker of medial vascular calcification in CKD, and its prevalence is markedly increased in ESKD and advanced CKD.
Introduction
Arterial calcification is common in patients with chronic kidney disease (CKD) and end-stage kidney disease (ESKD) and may contribute to the excess cardiovascular mortality in this population (1–4). Calcification can occur either in the intimal layer or medial layer, each with a distinct pathophysiology and possibly a different clinical significance. Intimal, or more precisely neointimal, calcification is associated with atherosclerosis (5) and occurs in the absence of renal failure. Whether it has any clinical significance beyond serving as a marker of advanced atherosclerosis is unclear because calcification may actually stabilize plaques (6,7). Calcification in the medial layer is independent of atherosclerosis or inflammation, occurs in small arteries in addition to large arteries, and is thought to be detrimental by decreasing arterial compliance (8). It is observed specifically in renal failure, diabetes, and aging (9), but its pathophysiology and natural history are otherwise poorly understood.
Investigation of vascular calcification in humans has been hampered by inadequate diagnostic tools. Computed tomography is commonly used to quantify calcification in the aorta, coronary vessels, and other large arteries but cannot reliably distinguish between intimal and medial calcification. Thus, all studies to date have yielded data only on the combined prevalence and clinical significance of these two conditions, without any specific information on either. This is important because the clinical significance and possible therapeutic approach may differ considerably between the two. Furthermore, computed tomography is expensive and exposes the patient to significant radiation. Radiographs of distal extremities have also been used to detect vascular calcification, which is probably entirely medial (10,11), but the sensitivity is unknown. Therefore, there is a need for a simple and safe imaging modality that specifically recognizes medial calcification.
Arterial calcification can be detected on mammograms (12) and, on the basis of the size of these vessels and histologic examination, should be exclusively medial (13,14). However, this has never been established. Breast arterial calcification increases with age (14–19) and is associated with cardiovascular disease (16–21). The prevalence may also be increased in ESKD (22), but this has not been carefully studied or quantified. Because mammography is routinely performed in women over the age of 40, it could be an extremely important tool in understanding the causes and clinical significance of medial vascular calcification in chronic kidney disease.
We hypothesized that breast arterial calcification is a specific marker of generalized medial calcification and that mammography can be used to determine the prevalence and risk factors for medial calcification in chronic kidney disease. To that end, we performed histologic examination of breast tissue, retrospective analysis of mammograms, and correlation with peripheral radiographs in patients with chronic kidney disease. The results will form the basis for future retrospective and prospective studies of the clinical significance of medial vascular calcification in chronic kidney disease.
Materials and Methods
Histology
A computerized search of medical records for patients with a diagnosis of ESKD or CKD and a mastectomy performed in 2007 to 2009 yielded 20 patients, of which 16 had specimens available. Slides prepared from multiple tissue sections at the time of the mastectomy and stained with hematoxylin and eosin were reviewed by a pathologist for the presence of arteries and vascular calcification. Sections showing possible calcification or no calcification were re-examined by von Kossa staining of newly prepared slides from stored tissue blocks.
Mammography
From among 197 female ESKD patients managed by the Renal Division at Emory University between June 2006 and April 2009, we retrospectively identified 71 with screening mammograms performed during the last 10 years within the Emory healthcare system. For each patient, a control subject without renal insufficiency of similar age (within 1 year) was randomly selected from a list of all women undergoing screening mammography in 2007 to 2009, without knowledge of the results of the mammography. The absence of renal insufficiency was defined as a serum creatinine value of less than 1.0 mg/dl performed no earlier than 10 months before the mammogram. Where possible, women were also matched for diabetes status and race, in that order. Women with CKD were identified from a computerized search of all women undergoing mammography from 2007 to 2009 with a diagnosis code of CKD. The stage of CKD was determined from the MDRD2 formula (23) using the serum creatinine value closest in time to the mammogram (but no greater than 2 years). There were a total of 44 women with serum creatinine values available within 2 years of the mammogram. All of the mammograms were reviewed by a single individual, and arterial calcification was scored as either present or absent without knowledge of other characteristics of the subjects. The age, race, and presence of diabetes were determined by review of the electronic medical records. Diabetes mellitus was defined as diabetic medication or diabetes listed as a diagnosis at the time of the mammogram.
Radiographs
The electronic medial records of all subjects with ESKD or CKD with available mammograms were searched for radiographs of the hands, wrists, lower legs, ankles, or feet. The images were reviewed by a single individual unaware of the mammography findings, and medial calcification was identified as diffuse, linear calcification of arteries. For the purpose of this analysis, arterial calcification was assumed to be permanent. Thus, calcification on a mammogram or radiograph was assumed to be present at any later date, and the absence of calcification was assumed to indicate absence of calcification at any prior date. In all other cases, only radiographs within 2 years of the mammogram were included in the analysis.
Statistical Analyses
Univariate analysis was performed using t test for continuous variables and the chi-squared test for categorical variables. Multivariate logistic regression analysis was performed with SPSS 17.0 software (SPSS, Inc., Chicago, Illinois).
Results
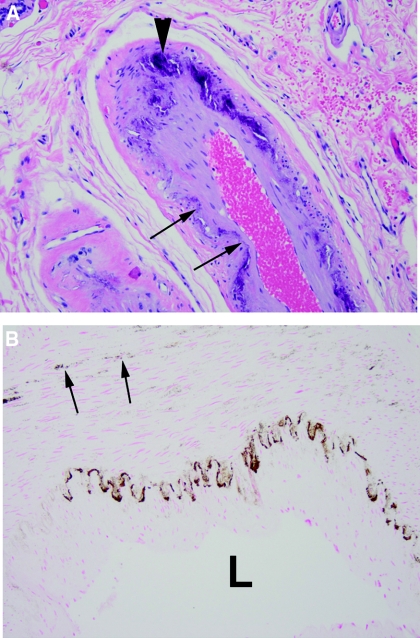
Breast tissue was examined in eight patients with ESKD and eight patients with CKD (serum creatinine, 1.8 to 3.9 mg/dl) undergoing total or partial breast excision. Arterial calcification was present in all 16 patients and apparent by staining with hematoxylin and eosin in 12 (Figure 1). In the remaining specimens, calcification could be detected only by von Kossa staining and was apparent as punctate staining between smooth muscle cells. In all specimens, calcification was limited to the internal elastic lamina and/or the medial layer. Although intimal hyperplasia was common, there was no atherosclerosis and no staining for calcium in the intima.
Figure 1.
Histology of breast arterial calcification. (A) Hematoxylin and eosin stain showing linear calcification of the internal elastic lamina (arrows) and large deposits within the media (arrowheads). (B) von Kossa stain showing linear staining of the internal elastic lamina and granular staining within the media (arrows). L indicates the lumen.
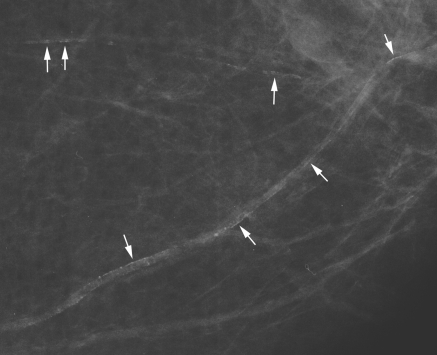
Mammograms were examined from 71 ESKD patients and age-matched subjects without renal insufficiency. As shown in Table 1, the average age of the ESKD patients was 60 years, all but one were African-American, and about half had diabetes. The controls were similar in age and prevalence of diabetes, and all but two were African-American. The mean dialysis duration in the ESKD patients was 64 ± 7 months (range, 1 to 232 months), and the modality was hemodialysis in 67, peritoneal dialysis in one, and peritoneal dialysis followed by hemodialysis in three. Parathyroidectomy had been performed in 24%. An example of breast arterial calcification is shown in Figure 2. The prevalence of breast arterial calcification (BAC) was almost fourfold greater in the ESKD patients. In the ESKD patients with diabetes, the prevalence of BAC was 74% compared with 21% in the control subjects with diabetes.
Table 1.
Clinical characteristics of ESKD patients and control subjects without renal insufficiency
| ESKD | Controls | P | |
|---|---|---|---|
| Number | 71 | 71 | |
| Mean age (years) | 60.0 ± 1.5 (32 to 85) | 60.5 ± 1.5 (35 to 85) | NS |
| Diabetes (%) | 54 | 55 | NS |
| African American (%) | 99 | 97 | NS |
| Breast artery calcification (%) | 63 | 17 | <0.001 |
Figure 2.
A mammogram showing linear calcifications in two breast arteries (arrows).
ESKD patients with breast arterial calcification were significantly older, had a longer duration of ESKD, and were more likely to have diabetes than patients without calcification (Table 2). Parathyroidectomy was more common in patients with BAC but the difference did not reach statistical significance. In a logistic multivariate model, only age and ESKD duration were significant predictors of calcification (Table 3).
Table 2.
Characteristics of ESKD patients with or without breast arterial calcification
| BAC | No BAC | P | |
|---|---|---|---|
| Number | 45 | 26 | |
| Mean age (years) | 62.7 ± 1.6 (37 to 85) | 55.3 ± 2.6 (32 to 84) | 0.013 |
| Dialysis duration (years) | 6.2 ± 0.7 (0.2 to 19) | 3.9 ± 0.9 (0.1 to 19) | 0.024 |
| Diabetes (%) | 62 | 39 | 0.053 |
| Parathyroidectomy (%) | 20 | 12 | NS |
Table 3.
Logistic regression for BAC in ESKD patients
| Odds Ratio | P | |
|---|---|---|
| Age (years) | 1.05 (1.01 to 1.11) | 0.032 |
| Dialysis duration (years) | 1.18 (1.02 to 1.38) | 0.032 |
| Diabetes | 2.70 (0.86 to 8.3) | 0.091 |
95% confidence intervals are given in parentheses.
To determine whether the risk of BAC increases before ESKD, we identified mammograms performed before the onset of ESKD (mean, 5.1 ± 0.7 years) in 25 of the ESKD patients. An additional 44 patients with CKD were identified from a computerized search of patients undergoing mammography. Because only a few CKD patients had advanced disease, analysis was limited to those with stage 3 disease. As shown in Table 4, the prevalence of BAC was 36% in the pre-ESKD mammograms, which was still significantly greater than the control group in Table 1 (P < 0.05), even although the patients at the time of the pre-ESKD mammogram were 5 years younger. Corresponding serum creatinine values were not available. In contrast, the prevalence of BAC in patients with stage 3 CKD was significantly less than in the pre-ESKD patients (P = 0.05) and similar to that in the control group despite the fact that the patients were older. The serum creatinine level in this group was 1.51 ± 0.04 mg/dl (range, 1.2 to 2.1 mg/dl), and the estimated GFR was 41.9 ± 1.3 ml/min per 1.73 m2 (range, 30.5 to 55.0 ml/min per 1.73 m2).
Table 4.
Breast arterial calcification and clinical characteristics in pre-ESKD patients and patients with stage 3 CKD
| Pre-ESKD | CKD Stage 3 | |
|---|---|---|
| Number | 25 | 35 |
| Mean age (years) | 55.3 ± 2.1 (36 to 79) | 66.6 ± 1.7 (48 to 83) |
| Time to ESKD (years) | 5.1 ± 0.7 (0.1 to 12) | |
| Diabetes (%) | 52 | 43 |
| African American (%) | 100 | 83 |
| Breast artery calcification (%) | 36 | 14 |
Arterial calcification on mammograms was compared with peripheral arterial calcification on plain radiographs of extremities to ascertain whether BAC is a marker of generalized medial vascular calcification. Of the CKD and ESKD patients whose mammograms were examined, 62 had also had extremity radiographs (hands, wrists, lower legs, ankles, or feet) that could be included in the analysis (Table 5). Of the 21 that showed diffuse, linear arterial calcification indicative of medial calcification, BAC was also present in 19 (90%). Peripheral arterial calcification was present in only two of 31 patients without BAC (6%). About a third of patients with BAC did not exhibit peripheral arterial calcification, which is likely explained by the greater sensitivity of mammography.
Table 5.
Number of patients with or without BAC or PAC
| PAC Present | PAC Absent | |
|---|---|---|
| BAC present | 19 | 12 |
| BAC absent | 2 | 29 |
Discussion
This is the first detailed examination of breast arterial calcification and its prevalence in chronic kidney disease and is the first study to provide histologic correlation. This correlation yielded several important findings. First, atherosclerosis was absent in breast arteries, and no intimal calcification was seen. Second, the calcification was limited to the internal elastic lamina or the medial layer. Third, early stages of calcification were detected that were not apparent on the specimen radiographs, indicating that the prevalence of medial calcification is greater than that indicated by radiography. The limitation of histologic analyses to surgical specimens could have introduced a selection bias, but there are no data linking breast cancer with arterial calcification. The availability of breast tissue and the ability to identify very early stages of medial calcification will provide the opportunity for detailed histologic studies on the etiology of this disorder in humans.
Because atherosclerosis is limited to large arteries, the vascular calcification identified on plain radiographs of extremities is probably entirely medial. Although this is supported by the diffuse, linear pattern of calcification as well as by histologic studies (10,11), this has not been established with certainty. The fact that this peripheral arterial calcification correlated strongly with breast arterial calcification indicates that BAC is a marker of generalized medial vascular calcification. In fact, mammography is probably more sensitive because almost a third of patients with BAC had no evidence of peripheral arterial calcification on plain radiographs. The possibility that some of the peripheral arterial calcification (PAC) is atherosclerotic cannot be ruled out and may explain the 6% of patients with PAC but no BAC. Because the peripheral radiographs were performed for clinical indications, selection bias could have increased the prevalence of PAC. The results indicate that mammography can be used to assess the prevalence and significance of medial calcification.
Although CKD is known to predispose to medial arterial calcification, the actual prevalence has not been established. Mammography revealed that almost two-thirds of ESKD patients have breast arterial calcification compared with one-sixth of subjects without renal insufficiency matched for age and the presence of diabetes, indicating an almost fourfold risk. The risk of medial calcification does not appear limited to ESKD because an increased prevalence of BAC was noted in the subset of ESKD patients in whom mammography was performed an average of 5 years before the onset of ESKD. Similar prevalences were observed in a prior study but were not compared with an age-matched cohort without kidney disease (24). The prevalence of BAC was not increased in patients with stage 3 CKD, suggesting that the risk of medial calcification develops later in CKD and that preventative strategies may not be necessary in early CKD.
Age is a risk factor for BAC in the general population (14–19) and correlated with BAC in ESKD patients. Duration of ESKD also correlated with BAC independent of age. Whether diabetes also contributes to BAC in ESKD is unclear. The prevalence of diabetes was significantly greater in the patients with BAC, but the effect was diminished in the multivariate model. Some studies have found a link between diabetes and BAC in unselected patients (17–19), whereas others have not (12,16,25–27). Because almost all of the patients were African-American, the results may not necessarily apply to Caucasians with CKD because African Americans have a lower prevalence of coronary artery calcification than Caucasians (28). However, this likely represents atherosclerotic calcification rather than medial calcification, and the difference is much less in women (28). The prevalence of BAC in the general population is similar in Caucasians and African Americans (29), suggesting that the results presented here are applicable across races and that race does not affect the prevalence of medial arterial calcification.
Although this study is limited by the subjective detection of BAC, the small sample sizes, and the retrospective analyses, the results demonstrate the utility of mammography as a specific tool for examining medial vascular calcification in chronic kidney disease. Because most women with CKD are at an age at which yearly mammography is recommended, both retrospective and prospective studies of medial calcification can be performed without additional radiation exposure or imaging costs. Assessment of the prevalence at different stages of CKD, identification of additional risk factors, and determination of the clinical significance will be useful in stratifying risk and determining the timing and necessity of treatment. Currently, evaluation of BAC is constrained by the inability to quantify calcification on mammograms. However, quantification should be possible with the advent of computed tomography of the breast (30), allowing for more precise correlation of BAC with clinical parameters and measurement of progression.
Disclosures
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Sigrist MK, Bungay P, Taal MW, McIntyre CW: Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol Dial Transplant 21: 707–714, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. New Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 3. London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM: Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int 71: 438–441, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Jeziorska M, McCollum C, Woolley DE: Calcification in Atherosclerotic Plaque of Human Carotid Arteries: Associations with Mast Cells and Macrophages. J Pathol 185: 10–17, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Shaalan W, Cheng H, Gewertz B, McKinsey J, Schwartz L, Katz D, Cao D, Desai T, Glagov S, Bassiouny H: Degree of carotid plaque calcification in relation to symptomatic outcome and plaque inflammation. J Vasc Surg 40: 262–269, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT: The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation 103: 1051–1056, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM: Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938–942, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Amann K: Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol 3: 1599–1605, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Shanahan CM, Cary NRB, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME: Medial localization of mineralization-regulating proteins in association with Monckeberg's sclerosis: Evidence for smooth muscle cell-mediated vascular calcification. Circulation 100: 2168–2176, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Chowdhury UK, Airan B, Mishra PK, Kothari SS, Subramaniam GK, Ray R, Singh R, Venugopal P: Histopathology and morphometry of radial artery conduits: Basic study and clinical application. Ann Thorac Surg 78: 1614–1622, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Schmitt EL, Norbeck JM, Threatt B: Incidence of mammary intra-arterial calcification: An age-matched control study. South Med J 78: 1440–1442, 1985 [DOI] [PubMed] [Google Scholar]
- 13. Moore S: Blood vessels and lymphatics. In: Anderson's Pathology, 10th edn., edited by Damjanov I, Linder J. St. Louis, Mosby, 1996, p 1401 [Google Scholar]
- 14. Nielsen BB, Holm NV: Calcification in breast arteries: The frequency and severity of arterial calcification in female breast tissue without malignant changes. Acta Path Microbiol Immunol Scand A 93: 13–16, 1985 [PubMed] [Google Scholar]
- 15. Leinster SJ, Whitehouse GH: Factors which influence the occurrence of vascular calcification in the breast. Br J Radiol 60: 457–458, 1987 [DOI] [PubMed] [Google Scholar]
- 16. Topal U, Kaderli A, Topal NB, Ozdemir B, Yesilbursa D, Cordan J, Ediz B, Aydinlar A: Relationship between the arterial calcification detected in mammography and coronary artery disease. Eur J Radiol 63: 391–395, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Iribarren C, Go AS, Tolstykh I, Sidney S, Johnston SC, Spring DB: Breast vascular calcification and risk of coronary heart disease, stroke, and heart failure. J Womens Health 13: 381–389, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Crystal P, Crystal E, Leor J, Friger M, Katzinovitsh G, Strano S: Breast arterial calcification on routine mammography as a potential marker for increased cardiovascular disease. Am J Cardiol 86: 216–217, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Kemmeren JM, Beijerinck D, van Noord PA, Banga JD, Deurenberg JJ, Pameijer FA, van der Graaf Y: Breast arterial calcifications: Associations with diabetes mellitus and cardiovascular mortality. Radiology 201: 75–78, 1996 [DOI] [PubMed] [Google Scholar]
- 20. van Noord PA, Beijerinck D, Kemmeren JM, van der Graaf Y: Mammograms may convey more than breast cancer risk: Breast arterial calcification and arterio-sclerotic related diseases in women of the DOM cohort. Eur J Cancer Prevent 5: 483–487, 1996 [PubMed] [Google Scholar]
- 21. Yildiz S, Yildiz A, Ertug N, Kaya I, Yilmaz R, Yuksel E, Ziylan SZ: Association of breast arterial calcification and carotid intima-media thickness. Heart Vessels 23: 376–382, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Evans SE, Whitehouse GH: Extensive calcification in the breast in chronic renal failure. Br J Radiol 64: 757–759, 1991 [DOI] [PubMed] [Google Scholar]
- 23. Levey AS, Greene T, Kusek J, Beck GJ, Group MS: A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract]. J Am Soc Nephrol 11: 828A, 2000. 10770960 [Google Scholar]
- 24. Canabal A, Sabate J, Salgueira M, Palma A: Cardiovascular risk in women with chronic renal failure: Mammographic study of vascular calcifications [in Spanish]. Radiologia 50: 54–60, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Reddy J, Bilezikian JP, Smith SJ, Mosca L: Reduced bone mineral density is associated with breast arterial calcification. J Clin Endocrinol Metab 93: 208–211, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sickles EA, Galvin HB: Breast arterial calcification in association with diabetes mellitus: Too weak a correlation to have clinical utility. Radiology 155: 577–579, 1985 [DOI] [PubMed] [Google Scholar]
- 27. Ferreira EMPF, Szejnfeld J, Faintuch S: Correlation between intramammary arterial calcifications and CAD. Acad Radiol 14: 144–150, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF: Ethnic differences in coronary calcification: The Multi-ethnic Study of Atherosclerosis (MESA). Circulation 111: 1313–1320, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Reddy J, Son H, Smith SJ, Paultre F, Mosca L: Prevalence of breast arterial calcifications in an ethnically diverse population of women. Ann Epidem 15: 344–350, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Lindfors KK, Boone JM, Nelson TR, Yang K, Kwan ALC, Miller DF: Dedicated breast CT: Initial clinical experience. Radiology 246: 725–733, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.