Summary
Background and objectives
Little is known about the influence of dietary phosphate intake on fibroblast growth factor-23 (FGF23) and its subsequent effects on vitamin D levels. This study addresses changes in intact FGF23 (iFGF23) and C-terminal FGF23 (cFGF23), phosphaturia, and levels of vitamin D on high and low phosphate and calcium intake.
Design, setting, participants, & measurements
Ten healthy subjects adhered to a diet low or high in phosphate and calcium content for 36 hours each with a 1-week interval during which subjects adhered to their usual diet. Serum phosphate, calcium, vitamin D metabolites, parathyroid hormone (PTH), and FGF23 levels (cFGF23 and iFGF23) were measured several times a day. Phosphate, calcium, and creatinine excretion was measured in 24-hour urine on all study days.
Results
Serum phosphate levels and urinary phosphate increased during high dietary phosphate intake (from 1.11 to 1.32 mmol/L, P < 0.0001 and 21.6 to 28.8 mmol/d, P = 0.0005, respectively). FGF23 serum levels increased during high dietary phosphate/calcium intake (cFGF23 from 60 to 72 RU/ml, P < 0.001; iFGF23 from 33 to 37 ng/L, P = 0.003), whereas PTH declined. 1,25-Dihydroxyvitamin D (1,25D) showed an inverse relation with FGF23.
Conclusions
Variation in dietary phosphate and calcium intake induces changes in FGF23 (on top of a circadian rhythm) and 1,25D blood levels as well as in urinary phosphate excretion. These changes are detectable the day after the change in the phosphate content of meals. Higher FGF23 levels are associated with phosphaturia and a decline in 1,25D levels.
Introduction
Phosphorus is an important constituent of nucleotides and is essential for bone mineralization, muscle function, cellular signal transduction, and energy storage. The kidney has a major role in phosphorous homeostasis that is regulated by several hormones such as parathyroid hormone (PTH), 1,25-dihydroxyvitamin D (1,25D), and the more recently identified fibroblast growth factor-23 (FGF23). FGF23 is produced by osteocytes, induces phosphaturia, and is considered to prevent the occurrence of elevated phosphate levels. In patients with chronic kidney disease (CKD), it is independently associated with mortality (1). However, changes in FGF23 levels after a dietary phosphate load are not completely understood.
Previous studies on FGF23 response on phosphate loading have revealed conflicting results because of differences in timing of sample collection, follow-up, and different assays used to determine FGF23 levels (2–5). One study demonstrated an increase in C-terminal FGF23 (cFGF23) on phosphate loading, but it measured FGF23 only after 5 days, thereby precluding any conclusion on the time window before that (3). Several other studies did not find effects of phosphate intake on FGF23, but these were short-term studies (6 to 16 hours) (2,4,6). However, these studies did find an early increase in phosphate excretion, probably due to an early rise in PTH. One study did find an increase of FGF23, but not before 8 hours after the highest phosphate dose (4). Phosphaturia preceded the rise in FGF23 in the latter study.
Therefore, we studied the effects of dietary phosphorus and calcium intake on FGF23 levels using frequent sampling and adequate duration of follow-up. Assays for cFGF23 and intact FGF23 (iFGF23) were used simultaneously.
Materials and Methods
Study Subjects
Ten healthy subjects where recruited among medical students of the VU University Medical Centre in Amsterdam. They all had unremarkable medical histories and were nonsmokers. All subjects had a creatinine clearance >100 ml/min using a 24-hour urine collection. The local medical ethical committee approved this study and all participants gave informed consent.
Study Protocol
This study was conducted as a open-label crossover study. There were two separate study periods of 3 days each with a 1-week interval between periods. The first study period consisted of a low phosphate and calcium diet, whereas subjects adhered to a phosphate- and calcium-enriched rich diet during the second study period. The reason for simultaneously giving high or low calcium and phosphate in the diet was to prevent a phosphate-induced rise in PTH, because that would have made the expected rise in phosphaturia due to FGF23 more difficult to interpret and because this would reflect more real-life conditions because most foods rich in phosphate are also rich in calcium. On the days before the two study periods and on day 1 until the last blood drawing at 4:00 p.m. subjects adhered to their regular diet. After that, the dietary intervention started and was continued until the end of that study period. A dietician prescribed the dietary intervention meals for each study period. On the first day of each study period, baseline fasting measurements of serum phosphate, calcium, urea, creatinine, albumin, PTH, and FGF23 levels were performed at fixed time points (at 8:00 a.m. after fasting and at 12:00 p.m. before lunch and dinner, respectively). 25-Hydroxyvitamin D (25D) and 1,25D levels were measured once a day at 4:00 p.m. Urine was collected for two consecutive 24-hour periods during each study period for the measurement of total calcium and phosphate excretion. On the second day of each study period, blood samples and 24-hour urine collection were taken as described for day 1. On the third day of each study period, a single morning blood sample was taken.
Study Meals
In the first study period of low phosphate and calcium diet, daily intake was restricted to 850 mg of phosphate and 280 mg of calcium. This is well below the amount in a typical Western diet, which contains 1500 mg of phosphate (7–10). During the second study period, dietary phosphate and calcium intake was high: 2880 and 1700 mg, respectively (10). The total energy intake was approximately the same in both study periods: 2000 and 2360 kcal/d, respectively. In healthy U.S. citizens aged 20 to 39 years, dietary phosphate intake is on average 1400 mg and calcium intake is 900 mg (11). In this study, phosphate and calcium intake during the first and second study periods were respectively at the lowest and upper edge of normal phosphate and calcium intake.
Biochemical Analyses
Plasma phosphate (reference range 0.7 to 1.4 mmol/L), calcium (reference range 2.2 to 2.6 mmol/L), albumin (reference range 35 to 52 g/L), urea (reference range 3 to 7.5 mmol/L), and creatinine (reference range 60 to 110 μmol/L) as well as urinary phosphate, calcium, and creatinine were analyzed using a modular system from Roche Diagnostics (Mannheim, Germany). Corrected serum calcium levels were calculated using the formula corrected calcium = measured calcium + (1 − 0.025 × albumin) to correct for serum albumin levels.
Intact PTH (reference range 2 to 11 pmol/L) was analyzed in EDTA-plasma using an immunometric luminescence assay (Architect, Abbott Laboratories, Abbott Park, IL): Intra- and interassay coefficients of variation (CVs) are both 5%. 25D (reference range 25 to 150 nmol/L) was analyzed using an RIA (Diasorin, Stillwater, MN): Intra- and interassay CVs are 7% to 9% and 10%, respectively. 1,25D (reference range 50 to 160 pmol/L) was analyzed in serum using an RIA (IDS, Tyne and Wear, United Kingdom): Intra- and interassay CVs are 8% to 9% and 11%, respectively.
FGF23 was analyzed with two assays. cFGF23 was assessed in EDTA-plasma using a sandwich ELISA (Immutopics, San Clemente, CA) according to the manufacturer's instructions: Intra- and interassay CVs are <5% and <16%, respectively. The iFGF23 was determined in serum using a sandwich ELISA (Kainos Laboratories, Tokio, Japan): Intra- and interassay CVs are <10% and <14%, respectively.
All laboratory measurements were performed in the VU University Medical Centre clinical chemistry department.
Study Endpoints
The primary study endpoint was the change in FGF23 serum levels with dietary phosphate and calcium restriction and high dietary phosphate and calcium intake. Secondary endpoints were the correlation between cFGF23 and iFGF23 and changes in serum phosphate, calcium, PTH, and 1,25D levels and urinary phosphate and calcium excretion.
Statistical Analyses
Statistical analyses were performed for cFGF23 and iFGF23. Data were analyzed with the longitudinal data analysis technique of generalized estimating equations using Stata 11 for Windows. This method is suitable for longitudinal data analysis between a continuous variable and several time-dependent and time-independent covariates (12). iFGF23 and cFGF23 were analyzed as dependent variables using diet (low, normal, and high phosphate content) and time point of day as the independent variables. If appropriate, adjustments were made for differences in baseline values, PTH levels, and creatinine clearance. In the case of skewed data, analyses were performed after log-transformation. P values <0.05 were considered statistically significant.
Results
Study Subjects
Eight women and two men were included. Their mean age was 23.5 ± 1.6 years. They all adhered to the complete study protocol, and there were no missing data.
Biochemical Changes Induced by Diet
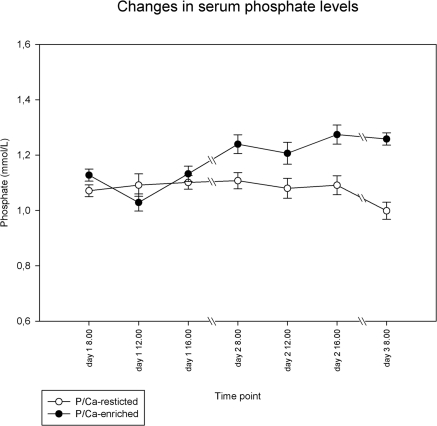
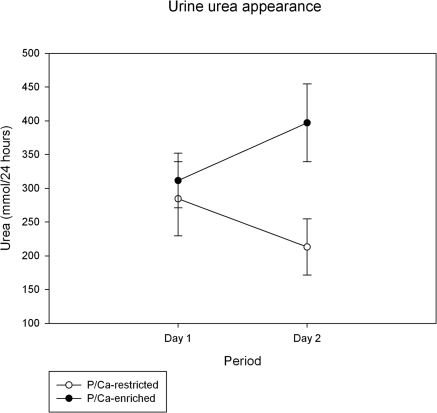
Table 1 shows numerical results for phosphate, calcium, PTH, vitamin D metabolites, urea, albumin, and creatinine serum levels. Creatinine clearance decreased from 125 ± 27 ml/min at baseline to 114 ± 20 ml/min during phosphate/calcium restriction and increased to 131 ± 22 ml/min during phosphate/calcium-enriched meals. Mean fasting serum phosphate levels during the baseline periods were 1.10 ± 0.09 mmol/L. Serum phosphate levels did not change during dietary phosphate/calcium restriction compared with baseline (P = 0.22), but they increased during high dietary phosphate/calcium intake to a maximum of 1.32 mmol/L (P < 0.0001). Serum phosphate levels did not change significantly throughout the course of the day (Figure 1). The two dietary interventions led to significant changes in 24-hour urinary urea content (P = 0.003 for change from regular to phosphate-restricted diet, and P = 0.0006 from regular to phosphate-enriched diet), indicating reasonable separated levels of protein intake (Figure 2). The tight correlation between protein intake and phosphorous intake is well established (10).
Table 1.
Values of laboratory results during the two study periods
| Baseline 1 | Phosphate/Calcium Restricted | Baseline 2 | Phosphate/Calcium Enriched | |
|---|---|---|---|---|
| Phosphate (mmol/L) | 1.09 (0.12) | 1.07 (0.14) | 1.11 (0.13) | 1.25 (0.15) |
| Calcium (mmol/L) | 2.26 (0.08) | 2.24 (0.07) | 2.27 (0.009) | 2.29 (0.07) |
| Albumin (g/L) | 43 (3) | 43 (3) | 42 (3) | 41 (3) |
| Blood urea nitrogen (mmol/L) | 4.1 (0.8) | 3.5 (0.8) | 3.8 (0.6) | 4.8 (0.7) |
| Creatinine (μmol/L) | 72 (9) | 72 (9) | 72 (8) | 71 (9) |
| PTH (pmol/L) | 5.7 (2.6) | 5.4 (2.2) | 5.2 (2.8) | 4.3 (1.6) |
| 25D (nmol/L) | 101.9 (34.27) | 100.2 (34.12) | 96.9 (32.9) | 95.7 (35.30) |
| 1,25D (pmol/L) | 147.7 (47.2) | 156.3 (43.7) | 143.1 (47.8) | 118.2 (27.6) |
Data are shown as mean (SD).
Figure 1.
Change in serum phosphate levels during the two study periods. All measurements on day 1 were done while on a regular diet followed by dietary intervention. Data shown are mean ± SEM. The mean of day 1 (regular diet) did not change on day 2 while on a phosphate/calcium-restricted diet (○). After a phosphate/calcium-enriched diet (●), these means changed from 1.11 to 1.25 mmol/L (P < 0.0001).
Figure 2.
Change in 24-hour urinary urea appearance. Values on day 1 were taken after 24 hours on a regular diet; values on day 2 were taking during 24 hours on a phosphate/calcium-enriched diet or a phosphate/calcium-restricted diet. Data shown are mean ± SD. The decrease after phosphate/calcium restriction (P = 0.003) and the increase on phosphate/calcium-enriched meals (P = 0.0006) were highly significant.
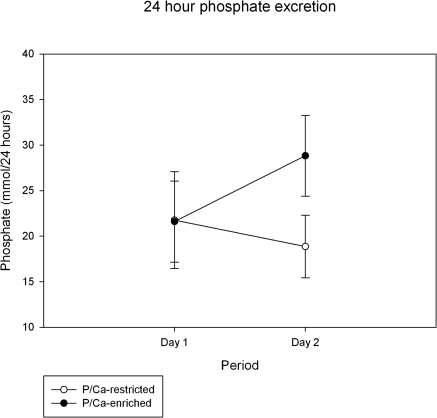
Urinary phosphate excretion tended to decrease (P = 0.09) during phosphate/calcium restriction and increased from 21.6 ± 4.4 to 28.8 ± 4.4 mmol/24 h (P = 0.005) during high dietary phosphate/calcium intake (Figure 3).
Figure 3.
Change in 24-hour phosphate excretion comparing phosphate excretion while on a regular diet (day 1) to that on phosphate/calcium-enriched meals or phosphate/calcium-restricted meals. Data shown are mean ± SD. Phosphaturia decreased nonsignificantly on phosphate/calcium restriction (P = 0.09) and increased while on phosphate/calcium-enriched meals (P = 0.005).
Changes in FGF23 Levels
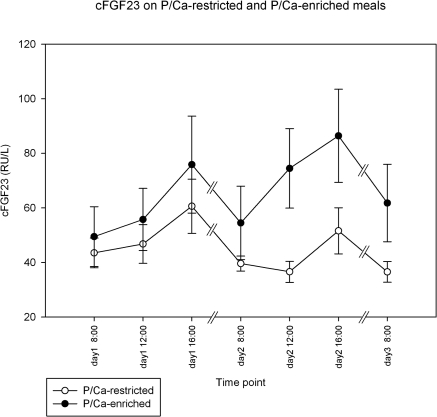
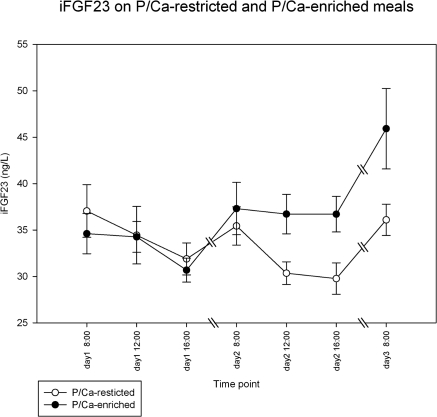
During the two days while subjects consumed their regular meals, cFGF23 increased from 45 ± 27 to 68 ± 45 RU/L (P = 0.002), whereas iFGF23 decreased during the daytime from 36 ± 6 to 31 ± 5 ng/L (P < 0.001) (Figures 4 and 5). To correct for this apparently circadian rhythm, we added the time point of day into our generalized estimating equations model to discern the influence of dietary intervention from daytime fluctuations of FGF23 in subsequent analyses.
Figure 4.
Changes in cFGF23 while on a regular diet (all values on day 1) followed by dietary intervention of a phosphate/calcium-enriched diet or a phosphate/calcium-restricted diet. Data shown are mean ± SEM. See text for the comparison of the different time points on day 1 and the comparison between the subsequent days.
Figure 5.
Changes in iFGF23 while on a regular diet (all values on day 1) followed by dietary intervention of a phosphate/calcium-enriched diet or a phosphate/calcium-restricted diet. Data shown are mean ± SEM. See text for the comparison of the different time points on day 1 and the comparison between the subsequent days.
To compare subsequent days, values for each day are expressed as means for day 1 and day 2, respectively. As compared with regular meals, phosphate/calcium restriction led to a small decrease in cFGF23 from 50.0 to 42.0 RU/L (P = 0.038), whereas phosphate/calcium-enriched meals led to an increase of cFGF23 from 60 to 72 RU/L (P < 0.001) (Figure 4). iFGF23 (Figure 5) did not change significantly on a phosphate/calcium-restricted diet (from 33 to 32 ng/L) but increased from 33 to 37 ng/L on a phosphate/calcium-enriched diet (P = 0.003). Although the entire range of serum phosphate levels was quite small, the univariate analysis showed a statistically significant, positive association between phosphate levels and cFGF23 and iFGF23 (P = 0.001 and 0.005, respectively). Adding the phosphate content in meals to a multivariate model, the association between serum phosphate and FGF23 disappeared, whereas the effect of meal type on FGF23 remained highly significant, probably indicating that serum phosphate is a poor marker for phosphate burden in the meals.
Changes Induced in PTH
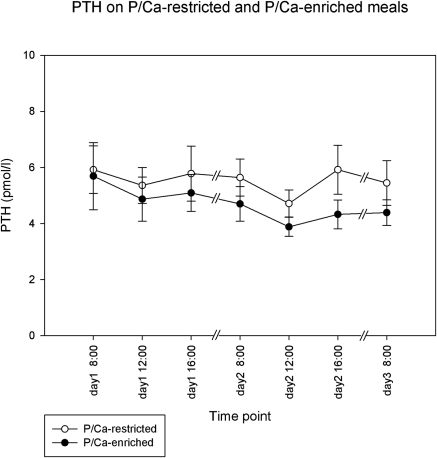
After phosphate/calcium-restricted meals PTH was unchanged: mean of 5.7 ± 2.6 pmol/L during the day while on a regular diet, followed by 5.4 ± 2.2 pmol/L (NS) on the subsequent day while on a phosphate/calcium-restricted diet. However, phosphate/calcium-enriched meals led to a decrease of PTH from 5.2 ± 2.8 to 4.3 ± 1.6 pmol (P = 0.008 after correcting for calcium and phosphate [Figure 6]).
Figure 6.
Effects on PTH after dietary intervention. Only phosphate/calcium loading led to a significant change (P = 0.008) from 5.2 to 4.3 pmol/L (means of days 1 and 2, respectively).
Phosphate Excretion as a Function of FGF23 Levels
Phosphate excretion during a regular diet was 21.8 mmol/24 h and decreased to 18.9 mmol/24 h (NS). However, during phosphate/calcium-enriched meals, phosphate excretion increased from 21.6 to 28.8 mmol/24 h (P < 0.001). As expected, the phosphate/calcium- and protein-enriched diet led to a significant increase in creatinine clearance of 114, 125, and 131 ml/min for low-phosphate/calcium diet, regular diet, and high-phosphate/calcium diet, respectively (P = 0.002). A significant 16% of variance of phosphaturia could be attributed to increased creatinine clearance (P < 0.0001), the remainder 84% being due to diminished tubular reabsorption of phosphate. PTH and FGF23 are known to diminish tubular reabsorption of phosphate. However, we found a significant inverse correlation between PTH and phosphate content of meals (P = 0.006). Multivariate analysis (including time point of the day, PTH, meal used, and baseline values) demonstrated a significant increase in phosphaturia and cFGF23 and iFGF23 levels (P = 0.024 and 0.017, respectively).
Changes in Vitamin D Metabolites Due to FGF23
During the entire experiment there were no changes in the levels of 25D. There was a highly significant negative association between cFGF23 (P < 0,0001) and iFGF23 (P < 0.0001) and 1,25D. Although, as mentioned above, PTH levels declined along with a rise in FGF23, and this lower PTH could have caused the decline in activation of vitamin D, correcting for change in PTH did not at all affect the level of significance between FGF23 and 1,25D. Remarkably, our analysis revealed a significant and independent effect of phosphate/calcium-enriched meals on the levels of 1,25D even after correcting for PTH and FGF23, demonstrating lower 1,25D levels with phosphate/calcium-enriched meals.
Discussion
Our results reveal a circadian rhythm of iFGF23 and cFGF23: iFGF23 peaks in the morning and decreases during the day. This pattern might reflect the circadian rhythm of bone metabolism—unloading during nighttime and loading during daytime—which is possibly driven by the endogenous circadian rhythm of PTH (13). Opposite of iFGF23, cFGF23 rises during the daytime. Because the assay for cFGF23 not only detects full-length FGF23 but also c-terminal fragments, this suggests that these fragments accumulate during the day.
In addition to the abovementioned rhythm, an effect of phosphate content in meals was also observed. cFGF23 and iFGF23 increased within a day after dietary phosphate and calcium loading. Phosphate and calcium restriction led to a decrease in FGF23, albeit NS for iFGF23. The positive association we found between FGF23 and phosphaturia, independent from PTH, supports the assumption that FGF23 is of importance for phosphate homeostasis, at least in the period starting 16 hours after high phosphate (and calcium) intake. Finally we found a negative association between FGF23 level and 1,25D, which is in agreement with previous studies that showed a FGF23-induced downregulation of the 1α-hydroxylase, the enzyme that converts 25D to 1,25D.
Integrating our results with previous studies mentioned in the introduction suggests that on phosphate loading, there is a prompt response of PTH, causing very early phosphaturia (2–4). However, within a time frame of 8 to 16 hours on continued high phosphate intake, FGF23 increases and takes over the phosphaturic effects of PTH. From a homeostatic point of view, it seems advantageous for phosphate homeostasis not to be solely dependent on PTH because that would also increase phosphate levels by activation of vitamin D. Indeed, in early CKD it has been suggested that postprandial hypocalcemia, probably induced by a transient increase in GFR, leads to elevated PTH, thus adding another potential mechanism to the early secondary hyperparathyroidism of CKD (14). In our study we found a decrease of PTH during phosphate-enriched meals, probably due to a simultaneous increase in calcium intake; therefore, PTH cannot explain the change in phosphaturia. The clinical meaning of phosphate-induced increases in FGF23 leading to increased phosphaturia appears to be a two-edged sword. In addition to its protective effect against an increasing phosphate level, animal (15) and human studies (1,16,17) suggest that it might have detrimental effects. The small increase in phosphate levels we demonstrated with our phosphate/calcium-enriched meals could be meaningful in terms of cardiovascular risk given the epidemiologic data correlating phosphate level, even in the normal range in non-CKD patients, to subsequent cardiovascular risk (18), as it does in hemodialysis patients (19).
A clinically important finding of our study is that, although the changes in serum phosphate were rather small and remained within the so-called normal range, they led to important increases in cFGF23 and iFGF23. Therefore, serum phosphate levels seem to be a poor marker of phosphate load. Especially when kidney function is preserved, phosphate levels appear to remain rather stable on phosphate loading, but at the expense of a higher level of FGF23. This higher level of FGF23 is associated with reduced calcitriol level, as has been established in CKD (14) and is confirmed for healthy adults in our study. The stable levels of serum phosphate seen in the study presented here are in contrast with the situation in CKD, in which the phosphaturic effects of FGF23 are limited because of a decrease in the number of functioning nephrons. The subsequent hyperphosphatemia leads to continuous stimulation for FGF23 release. Our data suggest biologic effects of such increases in FGF23 because an association exists between these levels and phosphaturia. Phosphate excretion is accomplished by ultrafiltration and subsequently regulated phosphate reabsorption by PTH and FGF23 at the proximal tubules by the sodium-phosphate exchanger (20). Therefore, when examining phosphaturia, data have to be corrected for changes in GFR. Increases in GFR are induced by higher protein or amino acid intake (21). In the study presented here, it was noticed that a phosphate (and thus protein)-enriched diet led to increases in creatinine clearance. However, in the multivariate analysis, of the 7.2-mmol increase in phosphate excretion, only 1 mmol could be explained by the increase in GFR. The remaining 6.2 mmol was due to reduced tubular reabsorption.
Another well described biologic effect of FGF23 is its inhibition of 1α-hydroxylation of 25D, leading to decreased levels of 1,25D (22). Our data demonstrate for the first time that this phenomenon is also present in healthy subjects already on the first day after initiation of a phosphate/calcium-enriched diet. Although we found a decrease in PTH, which may have caused the decline in 1,25D level, FGF23 remained an independent negative regulator of active vitamin D levels in multivariable analyses. It is remarkable that even after correcting for the lower PTH and higher FGF23 after phosphate/calcium-enriched meals, these meals themselves caused a decline in 1,25D levels. Phosphate itself (23–25), calcium (26), or some other constituent in protein-enriched diets might directly inhibit 1α-hydroxylase not mediated through PTH or FGF23. However, adding phosphate- and albumin-corrected calcium to the model (data not shown) did not change the 1,25D-lowering effects of phosphate-enriched meals. Another possibility is that in our study design we missed an earlier more pronounced decrease in PTH. However, this seems unlikely because previous studies all described an early increase in PTH rather than a decrease on phosphate loading.
Different from several other studies (4) examining the physiologic responses to phosphate loading, we found a decrease in PTH as already mentioned. This is most likely explained by the high calcium content of our phosphate/calcium-enriched diet, or it could be the consequence of the concomitant increase in FGF23 (27). It is also possible that we have missed an initial increase in PTH after phosphate loading as a consequence of our sampling times. However, the aforementioned studies that did measure PTH showed that at 16 hours after phosphate loading PTH was still above baseline levels, whereas we found levels below baseline.
The study presented here shows that the kinetics of C-terminal fragments are different from full-length FGF23. This conclusion is based on the assumption that the C-terminal assay detects cFGF23 and iFGF23, whereas the iFGF23 assay detects only iFGF23 (28). We showed that throughout the day cFGF23 increased, whereas iFGF23 decreased, which suggests accumulation of FGF23 fragments. Some have shown agonistic effects of FGF23 fragments (29), whereas others have found that fragments have competitive inhibitory effects on the Klotho-FGFR1 complex (30) and the FGF23 receptor (31). The reason for the discordant kinetics of iFGF23 and cFGF23 is unclear, but we speculate that there is a circadian rhythm of FGF23 production, being highest early in the day, as reflected by the iFGF23 curve, with a delayed clearance of fragments formed during the day.
Our study has some limitations. First of all, we examined only young healthy volunteers, which limits the applicability of our findings to other individuals, especially those with CKD. However, the assumed major difference would be a much higher FGF23 in the latter because of higher serum phosphate levels. Second, we did not test the early response after the initiation of dietary interventions, so we cannot be sure that observations made from the time point of 16 hours on were the consequence of what happened in that time frame, especially changes in PTH. As mentioned, those studies that did test up to 16 hours after dietary intervention showed that changes in PTH had not been extinguished yet. Furthermore, the effects of PTH on phosphaturia are instantaneous, which is different from FGF23 (32), so the changes in phosphaturia that we found were not likely the effects of unnoticed changes in PTH. Finally, we did not collect urine samples on several occasions each day, precluding drawing any conclusions on changes in fractional phosphate excretion during the day.
In conclusion, FGF23 shows a circadian rhythm that is different for iFGF23 and cFGF23. The former peaks in the morning, whereas the latter peaks late in the afternoon. In addition, phosphate (and calcium) loading increases FGF23. This increase is associated with increased phosphaturia and a reduction in 1,25D levels.
Disclosures
None.
Acknowledgments
We thank Josien Dijkstra-Lagemaat for excellent technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ito N, Fukumoto S, Takeuchi Y, Takeda S, Suzuki H, Yamashita T, Fujita T: Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J Bone Miner Metab 25: 419–422, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Ferrari SL, Bonjour JP, Rizzoli R: Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 90: 1519–1524, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Nishida Y, Taketani Y, Yamanaka-Okumura H, Imamura F, Taniguchi A, Sato T, Shuto E, Nashiki K, Arai H, Yamamoto H, Takeda E: Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int 70: 2141–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Antoniucci DM, Yamashita T, Portale AA: Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 91: 3144–3149, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB: Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64: 2272–2279, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Sherman RA: Dietary phosphate restriction and protein intake in dialysis patients: A misdirected focus. Semin Dial 20: 16–18, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Hruska KA, Mathew S, Lund R, Qiu P, Pratt R: Hyperphosphatemia of chronic kidney disease. Kidney Int 74: 148–157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tonelli M, Pannu N, Manns B: Oral phosphate binders in patients with kidney failure. N Engl J Med 362: 1312–1324, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Kalantar-Zadeh K, Gutekunst L, Mehrotra R, Kovesdy CP, Bross R, Shinaberger CS, Noori N, Hirschberg R, Benner D, Nissenson AR, Kopple JD: Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 519–530, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Ervin RB, Wang CY, Wright JD, Kennedy-Stephenson J: Dietary intake of selected minerals for the United States population 1999–2000: Adv Data 1–5, 2004 [PubMed] [Google Scholar]
- 12. Twisk JW: Longitudinal data analysis. A comparison between generalized estimating equations and random coefficient analysis. Eur J Epidemiol 19: 769–776, 2004 [DOI] [PubMed] [Google Scholar]
- 13. el-Hajj FG, Klerman EB, Brown EN, Choe Y, Brown EM, Czeisler CA: The parathyroid hormone circadian rhythm is truly endogenous—A general clinical research center study. J Clin Endocrinol Metab 82: 281–286, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Juppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 15. El-Abbadi MM, Pai AS, Leaf EM, Yang HY, Bartley BA, Quan KK, Ingalls CM, Liao HW, Giachelli CM: Phosphate feeding induces arterial medial calcification in uremic mice: Role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int 75: 1297–1307, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mirza MA, Larsson A, Lind L, Larsson TE: Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis 205: 385–390, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M: Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119: 2545–2552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RB, Sr, Gaziano JM, Vasan RS: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Liu S, Quarles LD: How fibroblast growth factor 23 works. J Am Soc Nephrol 18: 1637–1647, 2007 [DOI] [PubMed] [Google Scholar]
- 21. ter Wee PM, Geerlings W, Rosman JB, Sluiter WJ, van der GS, Donker AJ: Testing renal reserve filtration capacity with an amino acid solution. Nephron 41: 193–199, 1985 [DOI] [PubMed] [Google Scholar]
- 22. Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T: Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A 98: 6500–6505, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haussler M, Hughes M, Baylink D, Littledike ET, Cork D, Pitt M: Influence of phosphate depletion on the biosynthesis and circulating level of 1α25-dihydroxyvitamin D. Adv Exp Med Biol 81: 233–250, 1977 [DOI] [PubMed] [Google Scholar]
- 24. Tanaka Y, Deluca HF: The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys 154: 566–574, 1973 [DOI] [PubMed] [Google Scholar]
- 25. Tenenhouse HS, Martel J, Gauthier C, Zhang MY, Portale AA: Renal expression of the sodium/phosphate cotransporter gene, Npt2, is not required for regulation of renal 1 alpha-hydroxylase by phosphate. Endocrinology 142: 1124–1129, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Bushinsky DA, Riera GS, Favus MJ, Coe FL: Evidence that blood ionized calcium can regulate serum 1,25(OH)2D3 independently of parathyroid hormone and phosphorus in the rat. J Clin Invest 76: 1599–1604, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J: The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117: 4003–4008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heijboer AC, Levitus M, Vervloet MG, Lips P, ter Wee PM, Dijstelbloem HM, Blankenstein MA: Determination of fibroblast growth factor 23. Ann Clin Biochem 46: 338–340, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Berndt TJ, Craig TA, McCormick DJ, Lanske B, Sitara D, Razzaque MS, Pragnell M, Bowe AE, O'Brien SP, Schiavi SC, Kumar R: Biological activity of FGF-23 fragments. Pflugers Arch 454: 615–623, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goetz R, Nakada Y, Hu MC, Kurosu H, Wang L, Nakatani T, Shi M, Eliseenkova AV, Razzaque MS, Moe OW, Kuro-o M, Mohammadi M: Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci U S A 107: 407–412, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T: FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19: 429–435, 2004 [DOI] [PubMed] [Google Scholar]